research
KAIST researchers have discovered that brain somatic mutations in MTOR gene induce intractable epilepsy and suggest a precision medicine to treat epileptic seizures.
Epilepsy is a brain disorder which afflicts more than 50 million people worldwide. Many epilepsy patients can control their symptoms through medication, but about 30% suffer from intractable epilepsy and are unable to manage the disease with drugs. Intractable epilepsy causes multiple seizures, permanent mental, physical, and developmental disabilities, and even death. Therefore, surgical removal of the affected area from the brain has been practiced as a treatment for patients with medically refractory seizures, but this too fails to provide a complete solution because only 60% of the patients who undergo surgery are rendered free of seizures.
A Korean research team led by Professor Jeong Ho Lee of the Graduate School of Medical Science and Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and Professor Dong-Seok Kim of Epilepsy Research Center at Yonsei University College of Medicine has recently identified brain somatic mutations in the gene of mechanistic target of rapamycin (MTOR) as the cause of focal cortical dysplasia type II (FCDII), one of the most important and common inducers to intractable epilepsy, particularly in children. They propose a targeted therapy to lessen epileptic seizures by suppressing the activation of mTOR kinase, a signaling protein in the brain. Their research results were published online in Nature Medicine on March 23, 2015.
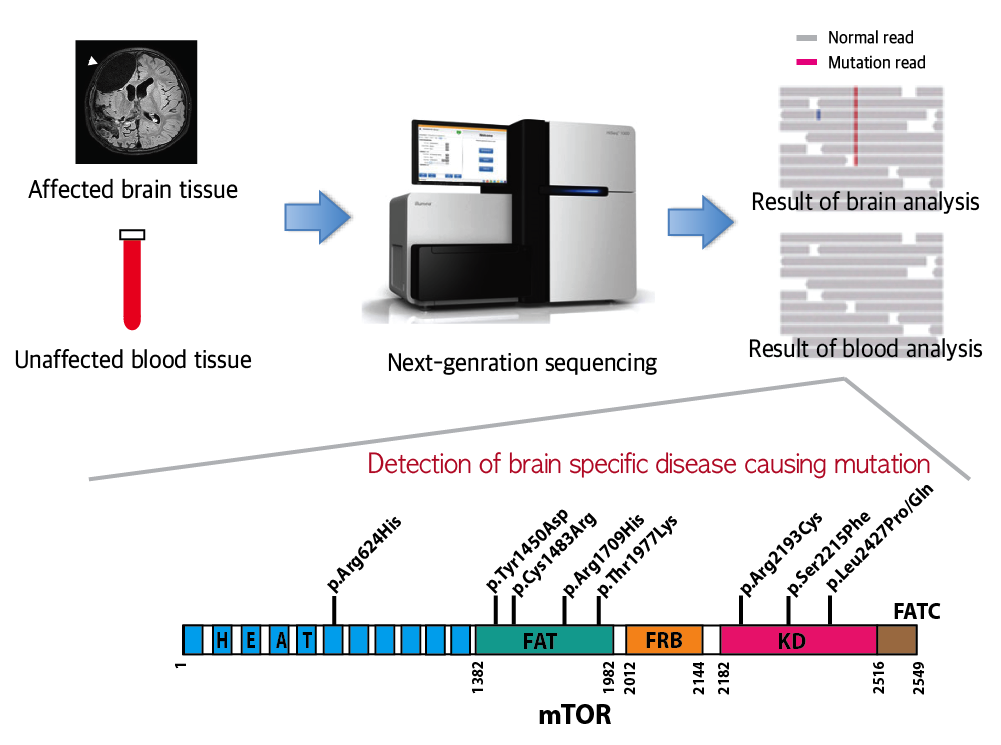
FCDII contributes to the abnormal developments of the cerebral cortex, ranging from cortical disruption to severe forms of cortical dyslamination, balloon cells, and dysplastic neurons. The research team studied 77 FCDII patients with intractable epilepsy who had received a surgery to remove the affected regions from the brain. The researchers used various deep sequencing technologies to conduct comparative DNA analysis of the samples obtained from the patients’ brain and blood, or saliva. They reported that about 16% of the studied patients had somatic mutations in their brain. Such mutations, however, did not take place in their blood or saliva DNA.
Professor Jeong Ho Lee of KAIST said, “This is an important finding. Unlike our previous belief that genetic mutations causing intractable epilepsy exist anywhere in the human body including blood, specific gene mutations incurred only in the brain can lead to intractable epilepsy. From our animal models, we could see how a small fraction of mutations carrying neurons in the brain could affect its entire function.”
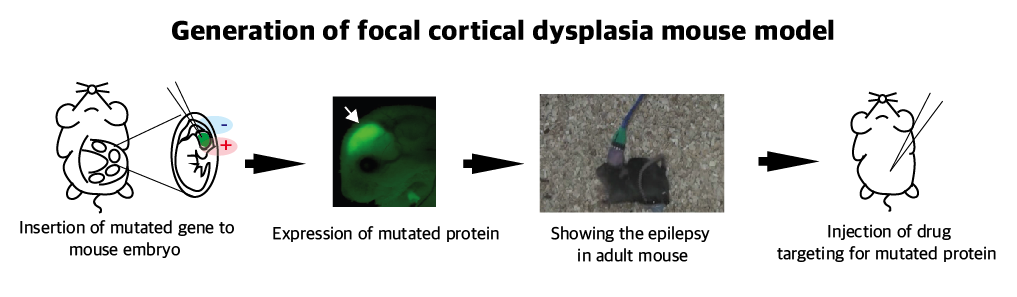
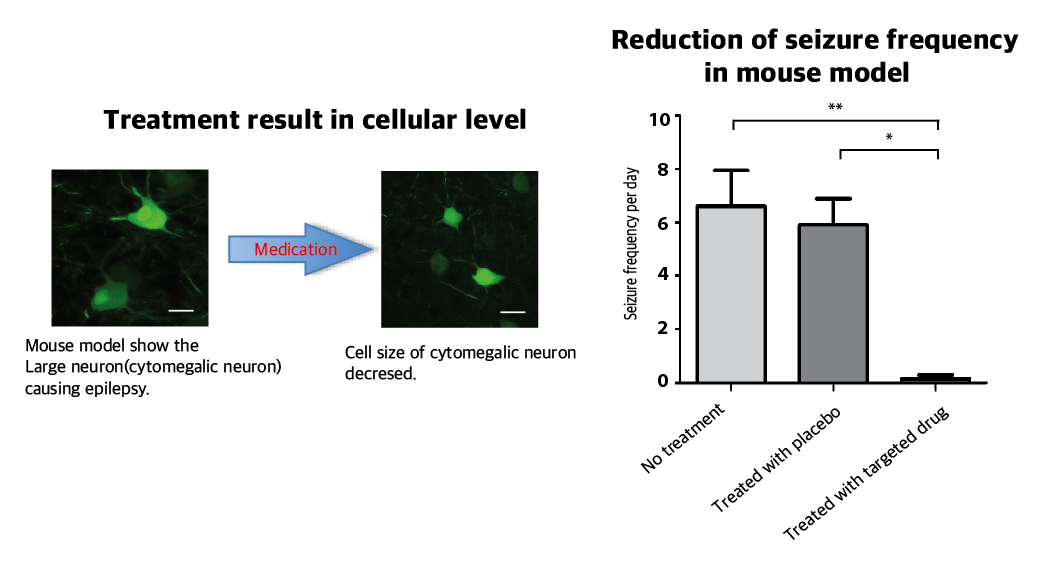
The research team recapitulated the pathogenesis of intractable epilepsy by inducing the focal cortical expression of mutated mTOR in the mouse brain via electroporation method and observed as the mouse develop epileptic symptoms. They then treated these mice with the drug called “rapamycin” to inhibit the activity of mTOR protein and observed that it suppressed the development of epileptic seizures with cytomegalic neurons.
“Our study offers the first evidence that brain-somatic activating mutations in MTOR cause FCDII and identifies mTOR as a treatment target for intractable epilepsy,” said co-author Dr. Dong-Seok Kim, a neurosurgeon at Yonsei Medical Center with the country’s largest surgical experiences in treating patients with this condition.
The research paper is titled “Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy.” (Digital Object Identifier #: 10.1038/nm.3824)
Picture 1: A schematic image to show how to detect brain specific mutation using next-generation sequencing technology with blood-brain paired sample. Simple comparison of non-overlapping mutations between affected and unaffected tissues is able to detect brain specific mutations.
Picture 2: A schematic image to show how to generate focal cortical dysplasia mouse model. This mouse model open the new window of drug screening for seizure patients.
Picture 3: Targeted medicine can rescue the focal cortical dysplasia symptoms including cytomegalic neuron & intractable epilepsy.
-
research Unraveling Mitochondrial DNA Mutations in Human Cells
Throughout our lifetime, cells accumulate DNA mutations, which contribute to genetic diversity, or “mosaicism”, among cells. These genomic mutations are pivotal for the aging process and the onset of various diseases, including cancer. Mitochondria, essential cellular organelles involved in energy metabolism and apoptosis, possess their own DNA, which are susceptible to mutations. However, studies on mtDNA mutations and mosaicism have been limited due to a variety of technical challe
2024-07-24 -
research A KAIST-SNUH Team Devises a Way to Make Mathematical Predictions to find Metabolites Related to Somatic Mutations in Cancers
Cancer is characterized by abnormal metabolic processes different from those of normal cells. Therefore, cancer metabolism has been extensively studied to develop effective diagnosis and treatment strategies. Notable achievements of cancer metabolism studies include the discovery of oncometabolites* and the approval of anticancer drugs by the U.S. Food and Drug Administration (FDA) that target enzymes associated with oncometabolites. Approved anticancer drugs such as ‘Tibsovo (active ingre
2024-03-18 -
research Genome Sequencing Unveils Mutational Impacts of Radiation on Mammalian Cells
Recent release of the waste water from Japan's Fukushima nuclear disaster stirred apprehension regarding the health implications of radiation exposure. Classified as a Group 1 carcinogen, ionizing radiation has long been associated with various cancers and genetic disorders, as evidenced by survivors and descendants of atomic bombings and the Chernobyl disaster. Despite much smaller amount, we remain consistently exposed to low levels of radiation in everyday life and medical procedures. Radi
2024-02-15 -
research 'Jumping Genes' Found to Alter Human Colon Genomes, Offering Insights into Aging and Tumorigenesis
The Korea Advanced Institute of Science and Technology (KAIST) and their collaborators have conducted a groundbreaking study targeting 'jumping genes' in the entire genomes of the human large intestine. Published in Nature on May 18 2023, the research unveils the surprising activity of 'Long interspersed nuclear element-1 (L1),' a type of jumping gene previously thought to be mostly dormant in human genomes. The study shows that L1 genes can become activated and disrupt genomic functions through
2023-05-22 -
research Genomic Data Reveals New Insights into Human Embryonic Development
KAIST researchers have used whole-genome sequencing to track the development from a single fertilized-egg to a human body Genomic scientists at KAIST have revealed new insights into the process of human embryonic development using large-scale, whole-genome sequencing of cells and tissues from adult humans. The study, published in Nature on Aug.25, is the first to analyse somatic mutations in normal tissue across multiple organs within and between humans. An adult human body comprises trilli
2021-08-31