research
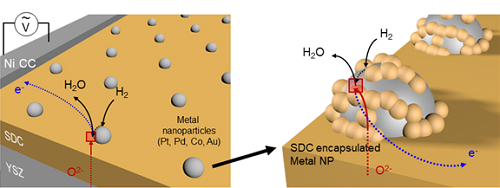
A KAIST team presented an ideal electrode design to enhance the performance of high-temperature fuel cells. The new analytical platform with advanced nanoscale patterning method quantitatively revealed the electrochemical value of metal nanoparticles dispersed on the oxide electrode, thus leading to electrode design directions that can be used in a variety of eco-friendly energy technologies.
The team, working under Professor WooChul Jung and Professor Sang Ouk Kim at the Department of Materials Science and Engineering, described an accurate analysis of the reactivity of oxide electrodes boosted by metal nanoparticles, where all particles participate in the reaction. They identified how the metal catalysts activate hydrogen electro-oxidation on the ceria-based electrode surface and quantify how rapidly the reaction rate increases with the proper choice of metals.
Metal nanoparticles with diameters of 10 nanometers or less have become a key component in high-performance heterogeneous catalysts, primarily serving as a catalytic activator. Recent experimental and theoretical findings suggest that the optimization of the chemical nature at the metal and support interfaces is essential for performance improvement.
However, the high cost associated with cell fabrication and operation as well as poorer stability of metal nanoparticles at high temperatures have been a long-standing challenge. To solve this problem, the team utilized a globally recognized metal nano patterning technology that uses block copolymer self-assembled nano templates and succeeded in uniformly synthesizing metal particles 10 nanometers in size on the surface of oxide fuel cell electrodes. They also developed a technology to accurately analyze the catalyst characteristics of single particles at high temperatures and maximize the performance of a fuel cell with minimal catalyst use.
The research team confirmed that platinum, which is a commonly used metal catalyst, could boost fuel cell performance by as much as 21 times even at an amount of 300 nanograms, which only costs about 0.015 KRW.
The team quantitatively identified and compared the characteristics of widely used metal catalysts other than platinum, such as palladium, gold, and cobalt, and also elucidated the precise principle of catalyst performance through theoretical analysis.
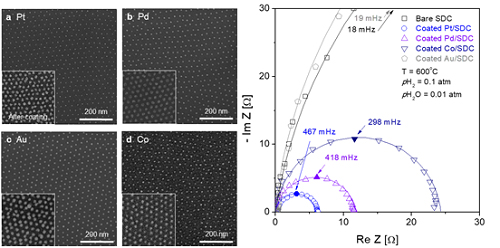
(Figure 2. Comparison of the electrochemical catalytic characteristics for various 10nm metal nanoparticles (platinum, palladium, cobalt, gold) at a high temperature.)
Professor Jung said, "We have broken the conventional methods of increasing the amount of catalyst which have deemed inefficient and expensive. Our results suggest a clear idea for high performance fuel cells using very small amounts of nanoparticles. This technology can be applied to many different industrial fields, advancing the commercialization of eco-friendly energy technologies such as fuel cells that generate electricity and electrolytic cells that produce hydrogen from water.”
The research has been published as the cover article of Nature Nanotechnology in the March issue. This research was carried out with support from the Nano-Material Technology Development Program through the National Research Foundation of Korea.
-
research Tungsten Suboxide Improves the Efficiency of Platinum in Hydrogen Production
< PhD Candidate Jinkyu Park and Professor Jinwoo Lee > Researchers presented a new strategy for enhancing catalytic activity using tungsten suboxide as a single-atom catalyst (SAC). This strategy, which significantly improves hydrogen evolution reaction (HER) in metal platinum (pt) by 16.3 times, sheds light on the development of new electrochemical catalyst technologies. Hydrogen has been touted as a promising alternative to fossil fuels. However, most of the conventional industrial h
2019-10-28 -
research A Hole in One for Holographic Display
(Professor YongKeun Park) Researchers have designed an ultrathin display that can project dynamic, multi-coloured, 3D holographic images, according to a study published in Nature Communications. The system’s critical component is a thin film of titanium filled with tiny holes that precisely correspond with each pixel in a liquid crystal display (LCD) panel. This film acts as a ‘photon sieve’ – each pinhole diffracts light emerging from them widely, resulting in a
2019-04-18 -
research New Catalyst for Synthesizing Chiral Molecules Selectively
(from left: Dr. Yoonsu Park and Professor Sukbok Chang from the Department of Chemistry) Molecules in nature often have “twin” molecules that look identical. In particular, the twin molecules that look like mirror images to each other are called enantiomers. However, even though they have the same type and number of elements, these twin molecules exhibit completely different properties. Professor Sukbok Chang and Dr. Yoonsu Park from the Department of Chemistry
2019-03-05 -
research New LSB with Theoretical Capacity over 90%
(Professor Hee-Tak Kim and Hyunwon Chu) A KAIST research team has developed a lithium sulfur battery (LSB) that realizes 92% of the theoretical capacity and an areal capacity of 4mAh/cm2. LSBs are gaining a great deal of attention as an alternative for lithium ion batteries (LIBs) because they have a theoretical energy density up to six to seven times higher than that of LIBs, and can be manufactured in a more cost-effective way. However, LSBs face the obstacle of
2019-02-11 -
research Noninvasive Light-Sensitive Recombinase for Deep Brain Genetic Manipulation
A KAIST team presented a noninvasive light-sensitive photoactivatable recombinase suitable for genetic manipulation in vivo. The highly light-sensitive property of photoactivatable Flp recombinase will be ideal for controlling genetic manipulation in deep mouse brain regions by illumination with a noninvasive light-emitting diode. This easy-to-use optogenetic module made by Professor Won Do Heo and his team will provide a side-effect free and expandable genetic manipulation tool for neurosci
2019-01-22