people
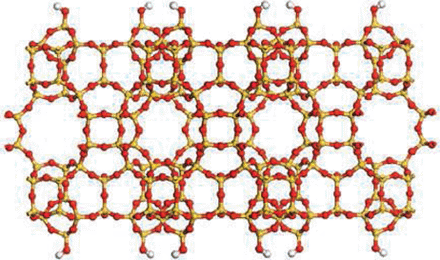
Porous Zeolite Crytals
Science, an internationally renowned scientific journal based in the US, has recently released a special issue of “Breakthrough of the Year, 2011,” dated December 23, 2011. In the issue, the journal introduces ten most important research breakthroughs made this year, and Professor Ryong Ryoo, Department of Chemistry at KAIST, was one of the scientists behind such notable advancements in 2011. Professor Ryoo has been highly regarded internationally for his research on the development of synthetic version of zeolites, a family of porous minerals that is widely used for products such as laundry detergents, cat litters, etc. Below is the article from Science, stating the zeolite research:
For Science’s “Breakthrough of the Year, 2011”, please go to:
-
people Top 10 Emerging Technologies by World Economic Forum
The World Economic Forum’s Meta-Council on Emerging Technologies announced its annual list of breakthrough technologies, the “Top 10 Emerging Technologies of 2016,” on June 23, 2016. The Meta-Council chose the top ten technologies based on the technologies’ potential to improve lives, transform industries, and safeguard the planet. The research field of systems metabolic engineering, founded by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineer
2016-06-27 -
people Two Undergraduate KAIST Students Publish a Book on Health Management
Joonho Suh of the Aerospace Engineering Department and Jiho Suh of the Mechanical Engineering Department are both brothers and undergraduates at KAIST. The Suh brothers, who are three years apart, have recently published a self-help book in English on staying healthy entitled “A Scientific Approach to Building Muscle: Mass Effect.” The book introduces techniques to build muscles, adapting them from an engineering concept called "Active Torque Control (ACT)," the management o
2015-10-26 -
people Seven Graduates of KAIST S+ Convergence AMP Publish a Book, “The First Penguinâ€
Seven graduates of KAIST’s S+ Convergence Advanced Management Program (KAMP) have published a book containing their business success stories, The First Penguin, hoping that in telling their story, they will inspire readers who want to become entrepreneurs. The book is available only in Korean. The title of the book refers to a penguin that enters the water first when other penguins hesitate to dive into the ocean, symbolizing the need to make the first move. The book reflects the experien
2015-05-26 -
people Professor Shim Featured with His Drone System in IEEE Spectrum
The IEEE Spectrum, a technology and science magazine published by the Institute of Electrical and Electronics Engineers (IEEE), featured an article of KAIST’s autonomous unmanned aerial vehicles (UAVs) entitled “South Korea Prepares for Drone vs. Drone Combat,” posted on April 1, 2015. The article introduces the anti-drone defense system being developed by Professor “David” Hyunchul Shim of the Department of Aerospace Engineering at KAIST. With the goal of developi
2015-04-02 -
people Interactions Features KAIST's Human-Computer Interaction Lab
Interactions, a bi-monthly magazine published by the Association for Computing Machinery (ACM), the largest educational and scientific computing society in the world, featured an article introducing Human-Computer Interaction (HCI) Lab at KAIST in the March/April 2015 issue (http://interactions.acm.org/archive/toc/march-april-2015). Established in 2002, the HCI Lab (http://hcil.kaist.ac.kr/) is run by Professor Geehyuk Lee of the Computer Science Department at KAIST. The lab conducts various
2015-03-02