Gwangju+Institute+of+Science+and+Technology
-
 A Korean research team develops a new clinical candidate for fatty liver disease
A team of Korean researchers have succeeded in developing a new drug candidate for the treatment of non-alcoholic fatty liver disease (NAFLD) acting on peripheral tissues. To date, there has not been an optimal treatment for non-alcoholic steatohepatitis (NASH), and this discovery is expected to set the grounds for the development of new drugs that can safely suppress both liver fat accumulation and liver fibrosis at the same time.
A joint research team led by Professor Jin Hee Ahn from Gwangju Institute of Science and Technology (GIST) and Professor Hail Kim from the KAIST Graduate School of Medical Science and Engineering developed a new chemical that can suppress disease-specific protein (HTR2A) through years of basic research. The team also revealed to have verified its efficacy and safety through preclinical tests (animal tests) at JD Bioscience Inc., a start-up company founded by Professor Ahn.
Although NAFLD has a prevalence rate as high as 20-30%, and about 5% of the global adult population suffers from NASH, there are no commercial drugs targeting them to date. NAFLD is a chronic disease that starts from the fatty liver and progresses into steatohepatitis, fibrosis, cirrhosis, and liver cancer. The mortality rate of patients increases with accompanied cardiovascular diseases and liver-related complications, and appropriate treatment in the early stage is hence necessary.
< Figure 1. Strategy and history of 5HT2A antagonists. Library and rational design for the development of compound 11c as a potent 5HT2A antagonist. Previous research efforts were discontinued due to limited oral absorption and safety. A therapeutic candidate to overcome this problem was identified and phase 1 clinical trials are currently in progress. >
The new synthetic chemical developed by the joint GIST-KAIST research is an innovative drug candidate that shows therapeutic effects on NASH based on a dual action mechanism that inhibits the accumulation of fat in the liver and liver fibrosis by suppressing the serotonin receptor protein 5HT2A.
The research team confirmed its therapeutic effects in animal models for NAFLD and NASH, in which hepatic steatosis and liver fibrosis* caused by fat accumulation in the liver were suppressed simultaneously by 50-70%.
*fibrosis: stiffening of parts of the liver, also used as a major indicator to track the prognosis of steatosis
The research team explained that the material was designed with optimal polarity and lipid affinity to minimize its permeability across the blood-brain barrier. It therefore does not affect the brain, and causes little side effects in the central nervous system (CNS) such as depression and suicidal ideations, while demonstrating excellent inhibition on its target protein present in tissues outside brain (IC50* = 14 nM). The team also demonstrated its superior efficacy in improving liver fibrosis when compared to similar drugs in the phase 3 clinical trial.
*IC50 (half maximal inhibitory concentration): the concentration at which a chemical suppresses 50% of a particular biological function
< Figure 2. GM-60106 (11c)'s effect on obesity: When GM-60106 was administered to an obese animal model (mice) for 2 months, body weight, body fat mass, and blood sugar were significantly reduced (a-d). In addition, the steatohepatitis level (NAFLD Activity Score) and the expression of genes of the treated mice involved in adipogenesis along with blood/liver fat decreased (e-h) >
Based on the pharmacological data obtained through preclinical trials, the team evaluated the effects of the drug on 88 healthy adults as part of their phase 1 clinical trial, where the side effects and the safe dosage of a drug are tested against healthy adults. Results showed no serious side effects and a good level of drug safety.
In addition, a preliminary efficacy evaluation on eight adults with steatohepatitis is currently underway.
Professor Jin Hee Ahn said, “The aim of this research is to develop a treatment for NASH with little side effects and guaranteed safety by developing a new target. The developed chemical is currently going through phase 1 of the global clinical trial in Australia through JD Bioscience Inc., a bio venture company for innovative drug development.” he added, “The candidate material the research team is currently developing shows not only a high level of safety and preventative effects by suppressing fat accumulation in the liver, but also a direct therapeutic effect on liver fibrosis. This is a strength that distinguishes our material from other competing drugs.”
< Figure 3. Efficacy of GM-60106 (11c) on liver fibrosis: When GM-60106 was administered to a steatohepatitis model (mice) for 3 months, the expression of genes associated with tissue fibrosis was significantly reduced (b-c). As a result of a detailed analysis of the tissues of the animal model, it was confirmed that the rate of tissue fibrosis was reduced and the expression rate of genes related to tissue fibrosis and inflammation was also significantly reduced (e-h). >
Professor Hail Kim from KAIST said, “Until now, this disease did not have a method of treatment other than weight control, and there has been no attempt to develop a drug that can be used for non-obese patients.” He added, “Through this research, we look forward to the development of various treatment techniques targeting a range of metabolic diseases including NASH that do not affect the weight of the patient.”
This study, conducted together by the research teams led by Professor Ahn from GIST and Professor Kim from KAIST, as well as the research team from JD Bioscience Inc., was supported by the Ministry of Science and ICT, and the National New Drug Development Project. The results of this research were published by Nature Communications on January 20.
The team also presented the results of their clinical study on the candidate material coded GM-60106 targeting metabolic abnormality-related MASH* at NASH-TAG Conference 2024, which was held in Utah for three days starting on January 4, which was selected as an excellent abstract.
*MASH (Metabolic Dysfunction-Associated Steatohepatitis): new replacement term for NASH
2024.02.21 View 4557
A Korean research team develops a new clinical candidate for fatty liver disease
A team of Korean researchers have succeeded in developing a new drug candidate for the treatment of non-alcoholic fatty liver disease (NAFLD) acting on peripheral tissues. To date, there has not been an optimal treatment for non-alcoholic steatohepatitis (NASH), and this discovery is expected to set the grounds for the development of new drugs that can safely suppress both liver fat accumulation and liver fibrosis at the same time.
A joint research team led by Professor Jin Hee Ahn from Gwangju Institute of Science and Technology (GIST) and Professor Hail Kim from the KAIST Graduate School of Medical Science and Engineering developed a new chemical that can suppress disease-specific protein (HTR2A) through years of basic research. The team also revealed to have verified its efficacy and safety through preclinical tests (animal tests) at JD Bioscience Inc., a start-up company founded by Professor Ahn.
Although NAFLD has a prevalence rate as high as 20-30%, and about 5% of the global adult population suffers from NASH, there are no commercial drugs targeting them to date. NAFLD is a chronic disease that starts from the fatty liver and progresses into steatohepatitis, fibrosis, cirrhosis, and liver cancer. The mortality rate of patients increases with accompanied cardiovascular diseases and liver-related complications, and appropriate treatment in the early stage is hence necessary.
< Figure 1. Strategy and history of 5HT2A antagonists. Library and rational design for the development of compound 11c as a potent 5HT2A antagonist. Previous research efforts were discontinued due to limited oral absorption and safety. A therapeutic candidate to overcome this problem was identified and phase 1 clinical trials are currently in progress. >
The new synthetic chemical developed by the joint GIST-KAIST research is an innovative drug candidate that shows therapeutic effects on NASH based on a dual action mechanism that inhibits the accumulation of fat in the liver and liver fibrosis by suppressing the serotonin receptor protein 5HT2A.
The research team confirmed its therapeutic effects in animal models for NAFLD and NASH, in which hepatic steatosis and liver fibrosis* caused by fat accumulation in the liver were suppressed simultaneously by 50-70%.
*fibrosis: stiffening of parts of the liver, also used as a major indicator to track the prognosis of steatosis
The research team explained that the material was designed with optimal polarity and lipid affinity to minimize its permeability across the blood-brain barrier. It therefore does not affect the brain, and causes little side effects in the central nervous system (CNS) such as depression and suicidal ideations, while demonstrating excellent inhibition on its target protein present in tissues outside brain (IC50* = 14 nM). The team also demonstrated its superior efficacy in improving liver fibrosis when compared to similar drugs in the phase 3 clinical trial.
*IC50 (half maximal inhibitory concentration): the concentration at which a chemical suppresses 50% of a particular biological function
< Figure 2. GM-60106 (11c)'s effect on obesity: When GM-60106 was administered to an obese animal model (mice) for 2 months, body weight, body fat mass, and blood sugar were significantly reduced (a-d). In addition, the steatohepatitis level (NAFLD Activity Score) and the expression of genes of the treated mice involved in adipogenesis along with blood/liver fat decreased (e-h) >
Based on the pharmacological data obtained through preclinical trials, the team evaluated the effects of the drug on 88 healthy adults as part of their phase 1 clinical trial, where the side effects and the safe dosage of a drug are tested against healthy adults. Results showed no serious side effects and a good level of drug safety.
In addition, a preliminary efficacy evaluation on eight adults with steatohepatitis is currently underway.
Professor Jin Hee Ahn said, “The aim of this research is to develop a treatment for NASH with little side effects and guaranteed safety by developing a new target. The developed chemical is currently going through phase 1 of the global clinical trial in Australia through JD Bioscience Inc., a bio venture company for innovative drug development.” he added, “The candidate material the research team is currently developing shows not only a high level of safety and preventative effects by suppressing fat accumulation in the liver, but also a direct therapeutic effect on liver fibrosis. This is a strength that distinguishes our material from other competing drugs.”
< Figure 3. Efficacy of GM-60106 (11c) on liver fibrosis: When GM-60106 was administered to a steatohepatitis model (mice) for 3 months, the expression of genes associated with tissue fibrosis was significantly reduced (b-c). As a result of a detailed analysis of the tissues of the animal model, it was confirmed that the rate of tissue fibrosis was reduced and the expression rate of genes related to tissue fibrosis and inflammation was also significantly reduced (e-h). >
Professor Hail Kim from KAIST said, “Until now, this disease did not have a method of treatment other than weight control, and there has been no attempt to develop a drug that can be used for non-obese patients.” He added, “Through this research, we look forward to the development of various treatment techniques targeting a range of metabolic diseases including NASH that do not affect the weight of the patient.”
This study, conducted together by the research teams led by Professor Ahn from GIST and Professor Kim from KAIST, as well as the research team from JD Bioscience Inc., was supported by the Ministry of Science and ICT, and the National New Drug Development Project. The results of this research were published by Nature Communications on January 20.
The team also presented the results of their clinical study on the candidate material coded GM-60106 targeting metabolic abnormality-related MASH* at NASH-TAG Conference 2024, which was held in Utah for three days starting on January 4, which was selected as an excellent abstract.
*MASH (Metabolic Dysfunction-Associated Steatohepatitis): new replacement term for NASH
2024.02.21 View 4557 -
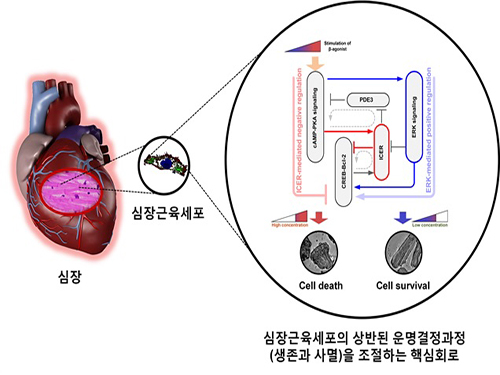 A Key Signal Transduction Pathway Switch in Cardiomyocyte Identified
A KAIST research team has identified the fundamental principle in deciding the fate of cardiomyocyte or heart muscle cells. They have determined that it depends on the degree of stimulus in β-adrenergic receptor signal transduction pathway in the cardiomyocyte to control cells' survival or death. The findings, the team hopes, can be used to treat various heart diseases including heart failure.
The research was led by KAIST Department of Bio and Brain Engineering Chair Professor Kwang-Hyun Cho and conducted by Dr. Sung-Young Shin (lead author) and Ph.D. candidates Ho-Sung Lee and Joon-Hyuk Kang. The research was conducted jointly with GIST (Gwangju Institute of Science and Technology) Department of Biological Sciences Professor Do-Han Kim’s team. The research was supported by the Ministry of Science, ICT and Future Planning, Republic of Korea, and the National Research Foundation of Korea. The paper was published in Nature Communications on December 17, 2014 with the title, “The switching role of β-adrenergic receptor signalling in cell survival or death decision of cardiomyocytes.”
The β-adrenergic receptor signal transduction pathway can promote cell survival (mediated by β2 receptors), but also can result in cell death by inducing toxin (mediated by β1 receptors) that leads to various heart diseases including heart failure. Past attempts to identify the fundamental principle in the fate determining process of cardiomyocyte based on β-adrenergic receptor signalling concluded without much success.
The β-adrenergic receptor is a type of protein on the cell membrane of cardiomyocyte (heart muscle cell) that when stimulated by neurohormones such as epinephrine or norepinephrine would transduce signals making the cardiomyocyte contract faster and stronger.
The research team used large-scale computer simulation analysis and systems biology to identify ERK* and ICER** signal transduction pathways mediated by a feed-forward circuit as a key molecular switch that decides between cell survival and death.
Weak β-adrenergic receptor stimulations activate ERK signal transduction pathway, increasing Bcl-2*** protein expression to promote cardiomyocyte survival. On the other hand, strong β-adrenergic receptor stimulations activate ICER signal transduction pathway, reducing Bcl-2 protein expression to promote cardiomyocyte death.
Researchers used a systems biology approach to identify the mechanism of B-blocker****, a common drug prescribed for heart failure. When cardiomyocyte is treated with β1 inhibitor, strong stimulation on β-adrenergic receptor increases Bcl-2 expression, improving the chance of cardiomyocyte survival, a cell protection effect.
Professor Kwang-Hyun Cho said, “This research used systems biology, an integrated, convergence research of IT (information technology) and BT (biotechnology), to successfully identify the mechanism in deciding the fate of cardiomyocytes based on the β-adrenergic receptor signal transduction pathway for the first time. I am hopeful that this research will enable the control of cardiomyocyte survival and death to treat various heart diseases including heart failure.”
Professor Cho’s team was the first to pioneer a new field of systems biology, especially concerning the complex signal transduction network involved in diseases. Their research is focused on modelling, analyzing simulations, and experimentally proving signal pathways. Professor Cho has published 140 articles in international journals including Cell, Science, and Nature.
* ERK (Extracellular signal-regulated kinases): Signal transduction molecule involved in cell survival
** ICER (Inducible cAMP early repressor): Signal transduction molecule involved in cell death
*** Bcl-2 (B-cell lymphoma 2): Key signal transduction molecule involved in promotion of cell survival
**** β-blocker: Drug that acts as β-adrenergic receptor inhibitor known to slow the progression of heart failure, hence used most commonly in medicine.
Picture: A schematic diagram for the β-AR signalling network
2015.01.05 View 12237
A Key Signal Transduction Pathway Switch in Cardiomyocyte Identified
A KAIST research team has identified the fundamental principle in deciding the fate of cardiomyocyte or heart muscle cells. They have determined that it depends on the degree of stimulus in β-adrenergic receptor signal transduction pathway in the cardiomyocyte to control cells' survival or death. The findings, the team hopes, can be used to treat various heart diseases including heart failure.
The research was led by KAIST Department of Bio and Brain Engineering Chair Professor Kwang-Hyun Cho and conducted by Dr. Sung-Young Shin (lead author) and Ph.D. candidates Ho-Sung Lee and Joon-Hyuk Kang. The research was conducted jointly with GIST (Gwangju Institute of Science and Technology) Department of Biological Sciences Professor Do-Han Kim’s team. The research was supported by the Ministry of Science, ICT and Future Planning, Republic of Korea, and the National Research Foundation of Korea. The paper was published in Nature Communications on December 17, 2014 with the title, “The switching role of β-adrenergic receptor signalling in cell survival or death decision of cardiomyocytes.”
The β-adrenergic receptor signal transduction pathway can promote cell survival (mediated by β2 receptors), but also can result in cell death by inducing toxin (mediated by β1 receptors) that leads to various heart diseases including heart failure. Past attempts to identify the fundamental principle in the fate determining process of cardiomyocyte based on β-adrenergic receptor signalling concluded without much success.
The β-adrenergic receptor is a type of protein on the cell membrane of cardiomyocyte (heart muscle cell) that when stimulated by neurohormones such as epinephrine or norepinephrine would transduce signals making the cardiomyocyte contract faster and stronger.
The research team used large-scale computer simulation analysis and systems biology to identify ERK* and ICER** signal transduction pathways mediated by a feed-forward circuit as a key molecular switch that decides between cell survival and death.
Weak β-adrenergic receptor stimulations activate ERK signal transduction pathway, increasing Bcl-2*** protein expression to promote cardiomyocyte survival. On the other hand, strong β-adrenergic receptor stimulations activate ICER signal transduction pathway, reducing Bcl-2 protein expression to promote cardiomyocyte death.
Researchers used a systems biology approach to identify the mechanism of B-blocker****, a common drug prescribed for heart failure. When cardiomyocyte is treated with β1 inhibitor, strong stimulation on β-adrenergic receptor increases Bcl-2 expression, improving the chance of cardiomyocyte survival, a cell protection effect.
Professor Kwang-Hyun Cho said, “This research used systems biology, an integrated, convergence research of IT (information technology) and BT (biotechnology), to successfully identify the mechanism in deciding the fate of cardiomyocytes based on the β-adrenergic receptor signal transduction pathway for the first time. I am hopeful that this research will enable the control of cardiomyocyte survival and death to treat various heart diseases including heart failure.”
Professor Cho’s team was the first to pioneer a new field of systems biology, especially concerning the complex signal transduction network involved in diseases. Their research is focused on modelling, analyzing simulations, and experimentally proving signal pathways. Professor Cho has published 140 articles in international journals including Cell, Science, and Nature.
* ERK (Extracellular signal-regulated kinases): Signal transduction molecule involved in cell survival
** ICER (Inducible cAMP early repressor): Signal transduction molecule involved in cell death
*** Bcl-2 (B-cell lymphoma 2): Key signal transduction molecule involved in promotion of cell survival
**** β-blocker: Drug that acts as β-adrenergic receptor inhibitor known to slow the progression of heart failure, hence used most commonly in medicine.
Picture: A schematic diagram for the β-AR signalling network
2015.01.05 View 12237 -
 Retirement of Professor Jung-Woong Ra
Retirement of Professor Jung-Woong Ra
The first professor of KAIST Electrical Engineering and Computer Science Department Jung-Woon Ra, who is also the former president of Gwangju Institute of Science and Technology (GIST), retired.
Professor Ra joined KAIST as professor in 1971, the year of KAIST foundation, and established the Department of Electrical Engineering and Computer Science. He has significantly contributed to the development of the department and is recognized for his sincere devotion to the rapid growth of GIST into a specialized research-oriented university as the head of GIST Foundation Committee and the third president of GIST.
For the past 35 years in KAIST, Professor Ra has educated 37 Ph.Ds and 90 masters, and presented 113 papers in domestic and world renowned journals and 93 academic papers.
Particularly, Professor Ra, world-class scholar in the field of electromagnetic wave utilization and application, invented ‘successive electromagnetic wave ground penetrating radar’, with which he discovered the fourth tunnel made by North Korean Military Forces in 1989.
In recognition of his contribution to manpower education and development of science and technology, he won Moran medal of Order of Civil Merit in 1999 and was awarded as a man of merit for electromagnetic wave development in 2005.
Retirement ceremony for Professor Jung was held at Dream Hall in ChungMoonSoul building last Friday, September 29, and Professor Jung was named as Emeritus Professor at the ceremony.
2006.10.10 View 14928
Retirement of Professor Jung-Woong Ra
Retirement of Professor Jung-Woong Ra
The first professor of KAIST Electrical Engineering and Computer Science Department Jung-Woon Ra, who is also the former president of Gwangju Institute of Science and Technology (GIST), retired.
Professor Ra joined KAIST as professor in 1971, the year of KAIST foundation, and established the Department of Electrical Engineering and Computer Science. He has significantly contributed to the development of the department and is recognized for his sincere devotion to the rapid growth of GIST into a specialized research-oriented university as the head of GIST Foundation Committee and the third president of GIST.
For the past 35 years in KAIST, Professor Ra has educated 37 Ph.Ds and 90 masters, and presented 113 papers in domestic and world renowned journals and 93 academic papers.
Particularly, Professor Ra, world-class scholar in the field of electromagnetic wave utilization and application, invented ‘successive electromagnetic wave ground penetrating radar’, with which he discovered the fourth tunnel made by North Korean Military Forces in 1989.
In recognition of his contribution to manpower education and development of science and technology, he won Moran medal of Order of Civil Merit in 1999 and was awarded as a man of merit for electromagnetic wave development in 2005.
Retirement ceremony for Professor Jung was held at Dream Hall in ChungMoonSoul building last Friday, September 29, and Professor Jung was named as Emeritus Professor at the ceremony.
2006.10.10 View 14928