bio
-
 A Single Biological Factor Predicts Distinct Cortical Organizations across Mammalian Species
-A KAIST team’s mathematical sampling model shows that retino-cortical mapping is a prime determinant in the topography of cortical organization.-
Researchers have explained how visual cortexes develop uniquely across the brains of different mammalian species. A KAIST research team led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering has identified a single biological factor, the retino-cortical mapping ratio, that predicts distinct cortical organizations across mammalian species.
This new finding has resolved a long-standing puzzle in understanding visual neuroscience regarding the origin of functional architectures in the visual cortex. The study published in Cell Reports on March 10 demonstrates that the evolutionary variation of biological parameters may induce the development of distinct functional circuits in the visual cortex, even without species-specific developmental mechanisms.
In the primary visual cortex (V1) of mammals, neural tuning to visual stimulus orientation is organized into one of two distinct topographic patterns across species. While primates have columnar orientation maps, a salt-and-pepper type organization is observed in rodents.
For decades, this sharp contrast between cortical organizations has spawned fundamental questions about the origin of functional architectures in the V1. However, it remained unknown whether these patterns reflect disparate developmental mechanisms across mammalian taxa, or simply originate from variations in biological parameters under a universal development process.
To identify a determinant predicting distinct cortical organizations, Professor Paik and his researchers Jaeson Jang and Min Song examined the exact condition that generates columnar and salt-and-pepper organizations, respectively. Next, they applied a mathematical model to investigate how the topographic information of the underlying retinal mosaics pattern could be differently mapped onto a cortical space, depending on the mapping condition.
The research team proved that the retino-cortical feedforwarding mapping ratio appeared to be correlated to the cortical organization of each species. In the model simulations, the team found that distinct cortical circuitries can arise from different V1 areas and retinal ganglion cell (RGC) mosaic sizes. The team’s mathematical sampling model shows that retino-cortical mapping is a prime determinant in the topography of cortical organization, and this prediction was confirmed by neural parameter analysis of the data from eight phylogenetically distinct mammalian species.
Furthermore, the researchers proved that the Nyquist sampling theorem explains this parametric division of cortical organization with high accuracy. They showed that a mathematical model predicts that the organization of cortical orientation tuning makes a sharp transition around the Nyquist sampling frequency, explaining why cortical organizations can be observed in either columnar or salt-and-pepper organizations, but not in intermediates between these two stages.
Professor Paik said, “Our findings make a significant impact for understanding the origin of functional architectures in the visual cortex of the brain, and will provide a broad conceptual advancement as well as advanced insights into the mechanism underlying neural development in evolutionarily divergent species.”
He continued, “We believe that our findings will be of great interest to scientists working in a wide range of fields such as neuroscience, vision science, and developmental biology.”
This work was supported by the National Research Foundation of Korea (NRF).
Image credit: Professor Se-Bum Paik, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Jaeson Jang, Min Song, and Se-Bum Paik. (2020). Retino-cortical mapping ratio predicts columnar and salt-and-pepper organization in mammalian visual cortex. Cell Reports. Volume 30. Issue 10. pp. 3270-3279. Available online at https://doi.org/10.1016/j.celrep.2020.02.038
Profile:
Se-Bum Paik
Assistant Professor
sbpaik@kaist.ac.kr
http://vs.kaist.ac.kr/
VSNN Laboratory
Department of Bio and Brain Engineering
Program of Brain and Cognitive Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile:
Jaeson Jang
Ph.D. Candidate
jaesonjang@kaist.ac.kr
Department of Bio and Brain Engineering, KAIST
Profile:
Min Song
Ph.D. Candidate
night@kaist.ac.kr
Program of Brain and Cognitive Engineering, KAIST
(END)
2020.03.11 View 11665
A Single Biological Factor Predicts Distinct Cortical Organizations across Mammalian Species
-A KAIST team’s mathematical sampling model shows that retino-cortical mapping is a prime determinant in the topography of cortical organization.-
Researchers have explained how visual cortexes develop uniquely across the brains of different mammalian species. A KAIST research team led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering has identified a single biological factor, the retino-cortical mapping ratio, that predicts distinct cortical organizations across mammalian species.
This new finding has resolved a long-standing puzzle in understanding visual neuroscience regarding the origin of functional architectures in the visual cortex. The study published in Cell Reports on March 10 demonstrates that the evolutionary variation of biological parameters may induce the development of distinct functional circuits in the visual cortex, even without species-specific developmental mechanisms.
In the primary visual cortex (V1) of mammals, neural tuning to visual stimulus orientation is organized into one of two distinct topographic patterns across species. While primates have columnar orientation maps, a salt-and-pepper type organization is observed in rodents.
For decades, this sharp contrast between cortical organizations has spawned fundamental questions about the origin of functional architectures in the V1. However, it remained unknown whether these patterns reflect disparate developmental mechanisms across mammalian taxa, or simply originate from variations in biological parameters under a universal development process.
To identify a determinant predicting distinct cortical organizations, Professor Paik and his researchers Jaeson Jang and Min Song examined the exact condition that generates columnar and salt-and-pepper organizations, respectively. Next, they applied a mathematical model to investigate how the topographic information of the underlying retinal mosaics pattern could be differently mapped onto a cortical space, depending on the mapping condition.
The research team proved that the retino-cortical feedforwarding mapping ratio appeared to be correlated to the cortical organization of each species. In the model simulations, the team found that distinct cortical circuitries can arise from different V1 areas and retinal ganglion cell (RGC) mosaic sizes. The team’s mathematical sampling model shows that retino-cortical mapping is a prime determinant in the topography of cortical organization, and this prediction was confirmed by neural parameter analysis of the data from eight phylogenetically distinct mammalian species.
Furthermore, the researchers proved that the Nyquist sampling theorem explains this parametric division of cortical organization with high accuracy. They showed that a mathematical model predicts that the organization of cortical orientation tuning makes a sharp transition around the Nyquist sampling frequency, explaining why cortical organizations can be observed in either columnar or salt-and-pepper organizations, but not in intermediates between these two stages.
Professor Paik said, “Our findings make a significant impact for understanding the origin of functional architectures in the visual cortex of the brain, and will provide a broad conceptual advancement as well as advanced insights into the mechanism underlying neural development in evolutionarily divergent species.”
He continued, “We believe that our findings will be of great interest to scientists working in a wide range of fields such as neuroscience, vision science, and developmental biology.”
This work was supported by the National Research Foundation of Korea (NRF).
Image credit: Professor Se-Bum Paik, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Jaeson Jang, Min Song, and Se-Bum Paik. (2020). Retino-cortical mapping ratio predicts columnar and salt-and-pepper organization in mammalian visual cortex. Cell Reports. Volume 30. Issue 10. pp. 3270-3279. Available online at https://doi.org/10.1016/j.celrep.2020.02.038
Profile:
Se-Bum Paik
Assistant Professor
sbpaik@kaist.ac.kr
http://vs.kaist.ac.kr/
VSNN Laboratory
Department of Bio and Brain Engineering
Program of Brain and Cognitive Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile:
Jaeson Jang
Ph.D. Candidate
jaesonjang@kaist.ac.kr
Department of Bio and Brain Engineering, KAIST
Profile:
Min Song
Ph.D. Candidate
night@kaist.ac.kr
Program of Brain and Cognitive Engineering, KAIST
(END)
2020.03.11 View 11665 -
 Professor Jong Chul Ye Appointed as Distinguished Lecturer of IEEE EMBS
Professor Jong Chul Ye from the Department of Bio and Brain Engineering was appointed as a distinguished lecturer by the International Association of Electrical and Electronic Engineers (IEEE) Engineering in Medicine and Biology Society (EMBS). Professor Ye was invited to deliver a lecture on his leading research on artificial intelligence (AI) technology in medical video restoration. He will serve a term of two years beginning in 2020.
IEEE EMBS's distinguished lecturer program is designed to educate researchers around the world on the latest trends and technology in biomedical engineering. Sponsored by IEEE, its members can attend lectures on the distinguished professor's research subject.
Professor Ye said, "We are at a time where the importance of AI in medical imaging is increasing.” He added, “I am proud to be appointed as a distinguished lecturer of the IEEE EMBS in recognition of my contributions to this field.”
(END)
2020.02.27 View 8206
Professor Jong Chul Ye Appointed as Distinguished Lecturer of IEEE EMBS
Professor Jong Chul Ye from the Department of Bio and Brain Engineering was appointed as a distinguished lecturer by the International Association of Electrical and Electronic Engineers (IEEE) Engineering in Medicine and Biology Society (EMBS). Professor Ye was invited to deliver a lecture on his leading research on artificial intelligence (AI) technology in medical video restoration. He will serve a term of two years beginning in 2020.
IEEE EMBS's distinguished lecturer program is designed to educate researchers around the world on the latest trends and technology in biomedical engineering. Sponsored by IEEE, its members can attend lectures on the distinguished professor's research subject.
Professor Ye said, "We are at a time where the importance of AI in medical imaging is increasing.” He added, “I am proud to be appointed as a distinguished lecturer of the IEEE EMBS in recognition of my contributions to this field.”
(END)
2020.02.27 View 8206 -
 What Fuels a “Domino Effect” in Cancer Drug Resistance?
KAIST researchers have identified mechanisms that relay prior acquired resistance to the first-line chemotherapy to the second-line targeted therapy, fueling a “domino effect” in cancer drug resistance. Their study featured in the February 7 edition of Science Advances suggests a new strategy for improving the second-line setting of cancer treatment for patients who showed resistance to anti-cancer drugs.
Resistance to cancer drugs is often managed in the clinic by chemotherapy and targeted therapy. Unlike chemotherapy that works by repressing fast-proliferating cells, targeted therapy blocks a single oncogenic pathway to halt tumor growth. In many cases, targeted therapy is engaged as a maintenance therapy or employed in the second-line after front-line chemotherapy.
A team of researchers led by Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering and the KAIST Institute for Health Science and Technology (KIHST) has discovered an unexpected resistance signature that occurs between chemotherapy and targeted therapy. The team further identified a set of integrated mechanisms that promotes this kind of sequential therapy resistance.
“There have been multiple clinical accounts reflecting that targeted therapies tend to be least successful in patients who have exhausted all standard treatments,” said the first author of the paper Mark Borris D. Aldonza. He continued, “These accounts ignited our hypothesis that failed responses to some chemotherapies might speed up the evolution of resistance to other drugs, particularly those with specific targets.”
Aldonza and his colleagues extracted large amounts of drug-resistance information from the open-source database the Genomics of Drug Sensitivity in Cancer (GDSC), which contains thousands of drug response data entries from various human cancer cell lines. Their big data analysis revealed that cancer cell lines resistant to chemotherapies classified as anti-mitotic drugs (AMDs), toxins that inhibit overacting cell division, are also resistant to a class of targeted therapies called epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs).
In all of the cancer types analyzed, more than 84 percent of those resistant to AMDs, representatively ‘paclitaxel’, were also resistant to at least nine EGFR-TKIs. In lung, pancreatic, and breast cancers where paclitaxel is often used as a first-line, standard-of-care regimen, greater than 92 percent showed resistance to EGFR-TKIs. Professor Kim said, “It is surprising to see that such collateral resistance can occur specifically between two chemically different classes of drugs.”
To figure out how failed responses to paclitaxel leads to resistance to EGFR-TKIs, the team validated co-resistance signatures that they found in the database by generating and analyzing a subset of slow-doubling, paclitaxel-resistant cancer models called ‘persisters’.
The results demonstrated that paclitaxel-resistant cancers remodel their stress response by first becoming more stem cell-like, evolving the ability to self-renew to adapt to more stressful conditions like drug exposures. More surprisingly, when the researchers characterized the metabolic state of the cells, EGFR-TKI persisters derived from paclitaxel-resistant cancer cells showed high dependencies to energy-producing processes such as glycolysis and glutaminolysis.
“We found that, without an energy stimulus like glucose, these cells transform to becoming more senescent, a characteristic of cells that have arrested cell division. However, this senescence is controlled by stem cell factors, which the paclitaxel-resistant cancers use to escape from this arrested state given a favorable condition to re-grow,” said Aldonza.
Professor Kim explained, “Before this research, there was no reason to expect that acquiring the cancer stem cell phenotype that dramatically leads to a cascade of changes in cellular states affecting metabolism and cell death is linked with drug-specific sequential resistance between two classes of therapies.”
He added, “The expansion of our work to other working models of drug resistance in a much more clinically-relevant setting, perhaps in clinical trials, will take on increasing importance, as sequential treatment strategies will continue to be adapted to various forms of anti-cancer therapy regimens.”
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF-2016R1C1B2009886), and the KAIST Future Systems Healthcare Project (KAISTHEALTHCARE42) funded by the Korean Ministry of Science and ICT (MSIT). Undergraduate student Aldonza participated in this research project and presented the findings as the lead author as part of the Undergraduate Research Participation (URP) Program at KAIST.
< Figure 1. Schematic overview of the study. >
< Figure 2. Big data analysis revealing co-resistance signatures between classes of anti-cancer drugs. >
Publication:
Aldonza et al. (2020) Prior acquired resistance to paclitaxel relays diverse EGFR-targeted therapy persistence mechanisms. Science Advances, Vol. 6, No. 6, eaav7416. Available online at http://dx.doi.org/10.1126/sciadv.aav7416
Profile: Prof. Yoosik Kim, MA, PhD
ysyoosik@kaist.ac.kr
https://qcbio.kaist.ac.kr/
Assistant Professor
Bio Network Analysis Laboratory
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Mark Borris D. Aldonza
borris@kaist.ac.kr
Undergraduate Student
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.10 View 11599
What Fuels a “Domino Effect” in Cancer Drug Resistance?
KAIST researchers have identified mechanisms that relay prior acquired resistance to the first-line chemotherapy to the second-line targeted therapy, fueling a “domino effect” in cancer drug resistance. Their study featured in the February 7 edition of Science Advances suggests a new strategy for improving the second-line setting of cancer treatment for patients who showed resistance to anti-cancer drugs.
Resistance to cancer drugs is often managed in the clinic by chemotherapy and targeted therapy. Unlike chemotherapy that works by repressing fast-proliferating cells, targeted therapy blocks a single oncogenic pathway to halt tumor growth. In many cases, targeted therapy is engaged as a maintenance therapy or employed in the second-line after front-line chemotherapy.
A team of researchers led by Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering and the KAIST Institute for Health Science and Technology (KIHST) has discovered an unexpected resistance signature that occurs between chemotherapy and targeted therapy. The team further identified a set of integrated mechanisms that promotes this kind of sequential therapy resistance.
“There have been multiple clinical accounts reflecting that targeted therapies tend to be least successful in patients who have exhausted all standard treatments,” said the first author of the paper Mark Borris D. Aldonza. He continued, “These accounts ignited our hypothesis that failed responses to some chemotherapies might speed up the evolution of resistance to other drugs, particularly those with specific targets.”
Aldonza and his colleagues extracted large amounts of drug-resistance information from the open-source database the Genomics of Drug Sensitivity in Cancer (GDSC), which contains thousands of drug response data entries from various human cancer cell lines. Their big data analysis revealed that cancer cell lines resistant to chemotherapies classified as anti-mitotic drugs (AMDs), toxins that inhibit overacting cell division, are also resistant to a class of targeted therapies called epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs).
In all of the cancer types analyzed, more than 84 percent of those resistant to AMDs, representatively ‘paclitaxel’, were also resistant to at least nine EGFR-TKIs. In lung, pancreatic, and breast cancers where paclitaxel is often used as a first-line, standard-of-care regimen, greater than 92 percent showed resistance to EGFR-TKIs. Professor Kim said, “It is surprising to see that such collateral resistance can occur specifically between two chemically different classes of drugs.”
To figure out how failed responses to paclitaxel leads to resistance to EGFR-TKIs, the team validated co-resistance signatures that they found in the database by generating and analyzing a subset of slow-doubling, paclitaxel-resistant cancer models called ‘persisters’.
The results demonstrated that paclitaxel-resistant cancers remodel their stress response by first becoming more stem cell-like, evolving the ability to self-renew to adapt to more stressful conditions like drug exposures. More surprisingly, when the researchers characterized the metabolic state of the cells, EGFR-TKI persisters derived from paclitaxel-resistant cancer cells showed high dependencies to energy-producing processes such as glycolysis and glutaminolysis.
“We found that, without an energy stimulus like glucose, these cells transform to becoming more senescent, a characteristic of cells that have arrested cell division. However, this senescence is controlled by stem cell factors, which the paclitaxel-resistant cancers use to escape from this arrested state given a favorable condition to re-grow,” said Aldonza.
Professor Kim explained, “Before this research, there was no reason to expect that acquiring the cancer stem cell phenotype that dramatically leads to a cascade of changes in cellular states affecting metabolism and cell death is linked with drug-specific sequential resistance between two classes of therapies.”
He added, “The expansion of our work to other working models of drug resistance in a much more clinically-relevant setting, perhaps in clinical trials, will take on increasing importance, as sequential treatment strategies will continue to be adapted to various forms of anti-cancer therapy regimens.”
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF-2016R1C1B2009886), and the KAIST Future Systems Healthcare Project (KAISTHEALTHCARE42) funded by the Korean Ministry of Science and ICT (MSIT). Undergraduate student Aldonza participated in this research project and presented the findings as the lead author as part of the Undergraduate Research Participation (URP) Program at KAIST.
< Figure 1. Schematic overview of the study. >
< Figure 2. Big data analysis revealing co-resistance signatures between classes of anti-cancer drugs. >
Publication:
Aldonza et al. (2020) Prior acquired resistance to paclitaxel relays diverse EGFR-targeted therapy persistence mechanisms. Science Advances, Vol. 6, No. 6, eaav7416. Available online at http://dx.doi.org/10.1126/sciadv.aav7416
Profile: Prof. Yoosik Kim, MA, PhD
ysyoosik@kaist.ac.kr
https://qcbio.kaist.ac.kr/
Assistant Professor
Bio Network Analysis Laboratory
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Mark Borris D. Aldonza
borris@kaist.ac.kr
Undergraduate Student
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.10 View 11599 -
 Cancer cell reversion may offer a new approach to colorectal cancer treatment
A novel approach to reverse the progression of healthy cells to malignant ones may offer a more effective way to eradicate colorectal cancer cells with far fewer side effects, according to a team of researchers based in South Korea.
Colorectal cancer, or cancer of the colon, is the third most common cancer in men and the second most common in women worldwide. South Korea has the second highest incident rate of colorectal cancer in the world, topped only by Hungary, according to the World Cancer Research Fund.
Their results were published as a featured cover article on January 2 in Molecular Cancer Research, a journal of the American Association for Cancer Research.
Led by Kwang-Hyun Cho, a professor and associate vice president of research at KAIST , the researchers used a computational framework to analyze healthy colon cells and colorectal cancer cells. They found that some master regulator proteins involved in cellular replication helped healthy colon cells mature, or differentiate into their specific cell type, and remain healthy. One particular protein, called SETDB1, suppressed the helpful proteins, forcing new cells to remain in a state of immaturity with the potential to become cancerous.
“This suggests that differentiated cells have an inherent resistance mechanism against malignant transformation and indicates that cellular reprogramming is indispensable for malignancy,” said Cho. “We speculated that malignant properties might be eradicated if the tissue-specific gene expression is reinstated — if we repress SETDB1 and allow the colon cells to mature and differentiate as they would normally.”
Image credit: Kwang-Hyun Cho, KAIST
Image restriction: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Using human-derived cells, Cho and his team targeted the tissue-specific gene expression programs identified in their computational analysis. These are the blueprints for the proteins that eventually help immature cells differentiate into tissue-specific cell types, such as colon cells. When a person has a genetic mutation, or has exposure to certain environmental factors, this process can go awry, leading to an overexpression of unhelpful proteins, such as SEDTB1.
The researchers specifically reduced the amount of SEDTB1 in these tissue-specific gene expression programs, which allowed the cells to mature and fully differentiate into colon cells.
“Our experiment also shows that SETDB1 depletion combined with cytotoxic drugs might be potentially beneficial to anticancer treatment,” Cho said. Cytotoxic drugs are often used for cancer treatment because the type of medicine contains chemicals that are toxic to cancer cells which can prevent them from replicating or growing. He noted that this combination could be more effective in treating cancer by transforming the cancer cell state into a less malignant or resistant state. He eventually pursues a cancer reversion therapy alone instead of conventional cytotoxic drug therapy since the cancer reversion therapy can provide a much less painful experience for patients with cancer who often have severe side effects from treatments intended to kill off cancerous cells, such as chemotherapy.
The researchers plan to continue studying how to return cancer cells to healthier states, with the ultimate goal of translating their work to therapeutic treatment for patients with colorectal cancer.
“I think our study of cancer reversion would eventually change the current medical practice of treating cancer toward the direction of keeping the patient’s quality of life while minimizing the side effects of current anti-cancer therapies,” Cho said.
###
This work was funded by KAIST and the National Research Foundation of Korea grants funded by the Korean government, the Ministry of Science and Information and Communication Technology.
Other authors include Soobeom Lee, Chae Young Hwang and Dongsan Kim, all of whom are affiliated with the Laboratory for Systems Biology and Bio-Inspired Engineering in the Department of Bio and Brain Engineering at KAIST; Chansu Lee and Sung Noh Hong, both with the Department of Medicine, and Seok-Hyung Kim of the Department of Pathology in the Samsung Medical Center at the Sungkyunkwan University School of Medicine.
-Profile
Professor Kwang-Hyun Cho
ckh@kaist.ac.kr
http://sbie.kaist.ac.kr/
Department of Bio and Brain Engineering
KAIST
https://www.kaist.ac.kr
2020.01.31 View 5837
Cancer cell reversion may offer a new approach to colorectal cancer treatment
A novel approach to reverse the progression of healthy cells to malignant ones may offer a more effective way to eradicate colorectal cancer cells with far fewer side effects, according to a team of researchers based in South Korea.
Colorectal cancer, or cancer of the colon, is the third most common cancer in men and the second most common in women worldwide. South Korea has the second highest incident rate of colorectal cancer in the world, topped only by Hungary, according to the World Cancer Research Fund.
Their results were published as a featured cover article on January 2 in Molecular Cancer Research, a journal of the American Association for Cancer Research.
Led by Kwang-Hyun Cho, a professor and associate vice president of research at KAIST , the researchers used a computational framework to analyze healthy colon cells and colorectal cancer cells. They found that some master regulator proteins involved in cellular replication helped healthy colon cells mature, or differentiate into their specific cell type, and remain healthy. One particular protein, called SETDB1, suppressed the helpful proteins, forcing new cells to remain in a state of immaturity with the potential to become cancerous.
“This suggests that differentiated cells have an inherent resistance mechanism against malignant transformation and indicates that cellular reprogramming is indispensable for malignancy,” said Cho. “We speculated that malignant properties might be eradicated if the tissue-specific gene expression is reinstated — if we repress SETDB1 and allow the colon cells to mature and differentiate as they would normally.”
Image credit: Kwang-Hyun Cho, KAIST
Image restriction: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Using human-derived cells, Cho and his team targeted the tissue-specific gene expression programs identified in their computational analysis. These are the blueprints for the proteins that eventually help immature cells differentiate into tissue-specific cell types, such as colon cells. When a person has a genetic mutation, or has exposure to certain environmental factors, this process can go awry, leading to an overexpression of unhelpful proteins, such as SEDTB1.
The researchers specifically reduced the amount of SEDTB1 in these tissue-specific gene expression programs, which allowed the cells to mature and fully differentiate into colon cells.
“Our experiment also shows that SETDB1 depletion combined with cytotoxic drugs might be potentially beneficial to anticancer treatment,” Cho said. Cytotoxic drugs are often used for cancer treatment because the type of medicine contains chemicals that are toxic to cancer cells which can prevent them from replicating or growing. He noted that this combination could be more effective in treating cancer by transforming the cancer cell state into a less malignant or resistant state. He eventually pursues a cancer reversion therapy alone instead of conventional cytotoxic drug therapy since the cancer reversion therapy can provide a much less painful experience for patients with cancer who often have severe side effects from treatments intended to kill off cancerous cells, such as chemotherapy.
The researchers plan to continue studying how to return cancer cells to healthier states, with the ultimate goal of translating their work to therapeutic treatment for patients with colorectal cancer.
“I think our study of cancer reversion would eventually change the current medical practice of treating cancer toward the direction of keeping the patient’s quality of life while minimizing the side effects of current anti-cancer therapies,” Cho said.
###
This work was funded by KAIST and the National Research Foundation of Korea grants funded by the Korean government, the Ministry of Science and Information and Communication Technology.
Other authors include Soobeom Lee, Chae Young Hwang and Dongsan Kim, all of whom are affiliated with the Laboratory for Systems Biology and Bio-Inspired Engineering in the Department of Bio and Brain Engineering at KAIST; Chansu Lee and Sung Noh Hong, both with the Department of Medicine, and Seok-Hyung Kim of the Department of Pathology in the Samsung Medical Center at the Sungkyunkwan University School of Medicine.
-Profile
Professor Kwang-Hyun Cho
ckh@kaist.ac.kr
http://sbie.kaist.ac.kr/
Department of Bio and Brain Engineering
KAIST
https://www.kaist.ac.kr
2020.01.31 View 5837 -
 New Insights into How the Human Brain Solves Complex Decision-Making Problems
A new study on meta reinforcement learning algorithms helps us understand how the human brain learns to adapt to complexity and uncertainty when learning and making decisions. A research team, led by Professor Sang Wan Lee at KAIST jointly with John O’Doherty at Caltech, succeeded in discovering both a computational and neural mechanism for human meta reinforcement learning, opening up the possibility of porting key elements of human intelligence into artificial intelligence algorithms. This study provides a glimpse into how it might ultimately use computational models to reverse engineer human reinforcement learning.
This work was published on Dec 16, 2019 in the journal Nature Communications. The title of the paper is “Task complexity interacts with state-space uncertainty in the arbitration between model-based and model-free learning.”
Human reinforcement learning is an inherently complex and dynamic process, involving goal setting, strategy choice, action selection, strategy modification, cognitive resource allocation etc. This a very challenging problem for humans to solve owing to the rapidly changing and multifaced environment in which humans have to operate. To make matters worse, humans often need to often rapidly make important decisions even before getting the opportunity to collect a lot of information, unlike the case when using deep learning methods to model learning and decision-making in artificial intelligence applications.
In order to solve this problem, the research team used a technique called 'reinforcement learning theory-based experiment design' to optimize the three variables of the two-stage Markov decision task - goal, task complexity, and task uncertainty. This experimental design technique allowed the team not only to control confounding factors, but also to create a situation similar to that which occurs in actual human problem solving.
Secondly, the team used a technique called ‘model-based neuroimaging analysis.’ Based on the acquired behavior and fMRI data, more than 100 different types of meta reinforcement learning algorithms were pitted against each other to find a computational model that can explain both behavioral and neural data. Thirdly, for the sake of a more rigorous verification, the team applied an analytical method called ‘parameter recovery analysis,’ which involves high-precision behavioral profiling of both human subjects and computational models.
In this way, the team was able to accurately identify a computational model of meta reinforcement learning, ensuring not only that the model’s apparent behavior is similar to that of humans, but also that the model solves the problem in the same way as humans do.
The team found that people tended to increase planning-based reinforcement learning (called model-based control), in response to increasing task complexity. However, they resorted to a simpler, more resource efficient strategy called model-free control, when both uncertainty and task complexity were high. This suggests that both the task uncertainty and the task complexity interact during the meta control of reinforcement learning. Computational fMRI analyses revealed that task complexity interacts with neural representations of the reliability of the learning strategies in the inferior prefrontal cortex.
These findings significantly advance understanding of the nature of the computations being implemented in the inferior prefrontal cortex during meta reinforcement learning as well as providing insight into the more general question of how the brain resolves uncertainty and complexity in a dynamically changing environment. Identifying the key computational variables that drive prefrontal meta reinforcement learning, can also inform understanding of how this process might be vulnerable to break down in certain psychiatric disorders such as depression and OCD. Furthermore, gaining a computational understanding of how this process can sometimes lead to increased model-free control, can provide insights into how under some situations task performance might break down under conditions of high cognitive load.
Professor Lee said, “This study will be of enormous interest to researchers in both the artificial intelligence and human/computer interaction fields since this holds significant potential for applying core insights gleaned into how human intelligence works with AI algorithms.”
This work was funded by the National Institute on Drug Abuse, the National Research Foundation of Korea, the Ministry of Science and ICT, Samsung Research Funding Center of Samsung Electronics.
Figure 1 (modified from the figures of the original paper doi:10.1038/s41467-019-13632-1). Computations implemented in the inferior prefrontal cortex during meta reinforcement learning. (A) Computational model of human prefrontal meta reinforcement learning (left) and the brain areas whose neural activity patterns are explained by the latent variables of the model. (B) Examples of behavioral profiles. Shown on the left is choice bias for different goal types and on the right is choice optimality for task complexity and uncertainty. (C) Parameter recoverability analysis. Compared are the effect of task uncertainty (left) and task complexity (right) on choice optimality.
-Profile
Professor Sang Wan Lee
sangwan@kaist.ac.kr
Department of Bio and Brain Engineering
Director, KAIST Center for Neuroscience-inspired AI
KAIST Institute for Artificial Intelligence (http://aibrain.kaist.ac.kr)
KAIST Institute for Health, Science, and Technology
KAIST (https://www.kaist.ac.kr)
2020.01.31 View 5235
New Insights into How the Human Brain Solves Complex Decision-Making Problems
A new study on meta reinforcement learning algorithms helps us understand how the human brain learns to adapt to complexity and uncertainty when learning and making decisions. A research team, led by Professor Sang Wan Lee at KAIST jointly with John O’Doherty at Caltech, succeeded in discovering both a computational and neural mechanism for human meta reinforcement learning, opening up the possibility of porting key elements of human intelligence into artificial intelligence algorithms. This study provides a glimpse into how it might ultimately use computational models to reverse engineer human reinforcement learning.
This work was published on Dec 16, 2019 in the journal Nature Communications. The title of the paper is “Task complexity interacts with state-space uncertainty in the arbitration between model-based and model-free learning.”
Human reinforcement learning is an inherently complex and dynamic process, involving goal setting, strategy choice, action selection, strategy modification, cognitive resource allocation etc. This a very challenging problem for humans to solve owing to the rapidly changing and multifaced environment in which humans have to operate. To make matters worse, humans often need to often rapidly make important decisions even before getting the opportunity to collect a lot of information, unlike the case when using deep learning methods to model learning and decision-making in artificial intelligence applications.
In order to solve this problem, the research team used a technique called 'reinforcement learning theory-based experiment design' to optimize the three variables of the two-stage Markov decision task - goal, task complexity, and task uncertainty. This experimental design technique allowed the team not only to control confounding factors, but also to create a situation similar to that which occurs in actual human problem solving.
Secondly, the team used a technique called ‘model-based neuroimaging analysis.’ Based on the acquired behavior and fMRI data, more than 100 different types of meta reinforcement learning algorithms were pitted against each other to find a computational model that can explain both behavioral and neural data. Thirdly, for the sake of a more rigorous verification, the team applied an analytical method called ‘parameter recovery analysis,’ which involves high-precision behavioral profiling of both human subjects and computational models.
In this way, the team was able to accurately identify a computational model of meta reinforcement learning, ensuring not only that the model’s apparent behavior is similar to that of humans, but also that the model solves the problem in the same way as humans do.
The team found that people tended to increase planning-based reinforcement learning (called model-based control), in response to increasing task complexity. However, they resorted to a simpler, more resource efficient strategy called model-free control, when both uncertainty and task complexity were high. This suggests that both the task uncertainty and the task complexity interact during the meta control of reinforcement learning. Computational fMRI analyses revealed that task complexity interacts with neural representations of the reliability of the learning strategies in the inferior prefrontal cortex.
These findings significantly advance understanding of the nature of the computations being implemented in the inferior prefrontal cortex during meta reinforcement learning as well as providing insight into the more general question of how the brain resolves uncertainty and complexity in a dynamically changing environment. Identifying the key computational variables that drive prefrontal meta reinforcement learning, can also inform understanding of how this process might be vulnerable to break down in certain psychiatric disorders such as depression and OCD. Furthermore, gaining a computational understanding of how this process can sometimes lead to increased model-free control, can provide insights into how under some situations task performance might break down under conditions of high cognitive load.
Professor Lee said, “This study will be of enormous interest to researchers in both the artificial intelligence and human/computer interaction fields since this holds significant potential for applying core insights gleaned into how human intelligence works with AI algorithms.”
This work was funded by the National Institute on Drug Abuse, the National Research Foundation of Korea, the Ministry of Science and ICT, Samsung Research Funding Center of Samsung Electronics.
Figure 1 (modified from the figures of the original paper doi:10.1038/s41467-019-13632-1). Computations implemented in the inferior prefrontal cortex during meta reinforcement learning. (A) Computational model of human prefrontal meta reinforcement learning (left) and the brain areas whose neural activity patterns are explained by the latent variables of the model. (B) Examples of behavioral profiles. Shown on the left is choice bias for different goal types and on the right is choice optimality for task complexity and uncertainty. (C) Parameter recoverability analysis. Compared are the effect of task uncertainty (left) and task complexity (right) on choice optimality.
-Profile
Professor Sang Wan Lee
sangwan@kaist.ac.kr
Department of Bio and Brain Engineering
Director, KAIST Center for Neuroscience-inspired AI
KAIST Institute for Artificial Intelligence (http://aibrain.kaist.ac.kr)
KAIST Institute for Health, Science, and Technology
KAIST (https://www.kaist.ac.kr)
2020.01.31 View 5235 -
 KAIST Vaccine for Tick-Borne Disease ‘SFTS’ Protects Against Lethal Infection
A KAIST research team reported the development of a DNA vaccine for Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) which completely protects against lethal infection in ferrets. The team confirmed that ferrets immunized with DNA vaccines encoding all SFTSV proteins showed 100% survival rate without detectable viremia and did not develop any clinical symptoms. This study was published in Nature Communications on August 23.
Severe Fever with Thrombocytopenia Syndrome (SFTS) is a newly emerging tick-borne infectious disease. The disease causes fever, severe thrombocytopenia, leukocytopenia as well as vomiting and diarrhea. Severe cases end up with organ system failure often accompanied by hemorrhages, and its mortality rate stands at 10–20%.
The viral disease has been endemic to East Asia but the spread of the tick vector to North America increases the likelihood of potential outbreak beyond the Far East Asia. The World Health Organization (WHO) has also put SFTSV into the priority pathogen requiring urgent attention category. Currently, no vaccine has been available to prevent SFTS.
The research team led by Professor Su-Hyung Park noted that DNA vaccines induce broader immunity to multiple antigens than traditional ones. Moreover, DNA vaccines stimulate both T cell and antibody immunity, which make them suitable for vaccine development.
They constructed DNA vaccines that encode full-length Gn, Gc, N, NS, and RNA polymerase genes based on common sequences of 31 SFTSV strains isolated from patients. Their vaccine candidates induced both neutralizing antibody response and multifunctional SFTSV-specific T cell response in mice and ferrets.
To investigate the vaccine’s efficacy in vivo, the research team applied a recently developed ferret model that recapitulates fatal clinical symptoms in SFTSV infection in humans. Vaccinated ferrets were completely protected from lethal SFTSV challenge without SFTSV detection in their blood, whereas all control ferrets died within 10 days’ post-infection.
The KAIST team found that anti-envelope antibodies play an important role in protective immunity, suggesting that envelope glycoproteins of SFTSV may be the most effective antigens for inducing protective immunity. Moreover, the study revealed that T cell responses specific to non-envelope proteins of SFTSV also can contribute to protection against SFTSV infection.
Professor Park said, “This is the first study demonstrating complete protection against lethal SFTSV challenge using an immunocompetent, middle-sized animal model with clinical manifestations of SFTSV infection. We believe this study provides valuable insights into designing preventive vaccines for SFTSV.”
2020.01.31 View 4635
KAIST Vaccine for Tick-Borne Disease ‘SFTS’ Protects Against Lethal Infection
A KAIST research team reported the development of a DNA vaccine for Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) which completely protects against lethal infection in ferrets. The team confirmed that ferrets immunized with DNA vaccines encoding all SFTSV proteins showed 100% survival rate without detectable viremia and did not develop any clinical symptoms. This study was published in Nature Communications on August 23.
Severe Fever with Thrombocytopenia Syndrome (SFTS) is a newly emerging tick-borne infectious disease. The disease causes fever, severe thrombocytopenia, leukocytopenia as well as vomiting and diarrhea. Severe cases end up with organ system failure often accompanied by hemorrhages, and its mortality rate stands at 10–20%.
The viral disease has been endemic to East Asia but the spread of the tick vector to North America increases the likelihood of potential outbreak beyond the Far East Asia. The World Health Organization (WHO) has also put SFTSV into the priority pathogen requiring urgent attention category. Currently, no vaccine has been available to prevent SFTS.
The research team led by Professor Su-Hyung Park noted that DNA vaccines induce broader immunity to multiple antigens than traditional ones. Moreover, DNA vaccines stimulate both T cell and antibody immunity, which make them suitable for vaccine development.
They constructed DNA vaccines that encode full-length Gn, Gc, N, NS, and RNA polymerase genes based on common sequences of 31 SFTSV strains isolated from patients. Their vaccine candidates induced both neutralizing antibody response and multifunctional SFTSV-specific T cell response in mice and ferrets.
To investigate the vaccine’s efficacy in vivo, the research team applied a recently developed ferret model that recapitulates fatal clinical symptoms in SFTSV infection in humans. Vaccinated ferrets were completely protected from lethal SFTSV challenge without SFTSV detection in their blood, whereas all control ferrets died within 10 days’ post-infection.
The KAIST team found that anti-envelope antibodies play an important role in protective immunity, suggesting that envelope glycoproteins of SFTSV may be the most effective antigens for inducing protective immunity. Moreover, the study revealed that T cell responses specific to non-envelope proteins of SFTSV also can contribute to protection against SFTSV infection.
Professor Park said, “This is the first study demonstrating complete protection against lethal SFTSV challenge using an immunocompetent, middle-sized animal model with clinical manifestations of SFTSV infection. We believe this study provides valuable insights into designing preventive vaccines for SFTSV.”
2020.01.31 View 4635 -
 Scientists Discover the Mechanism of DNA High-Order Structure Formation
(Molecular structures of Abo1 in different energy states (left), Demonstration of an Abo1-assisted histone loading onto DNA by the DNA curtain assay. )
The genetic material of our cells—DNA—exists in a high-order structure called “chromatin”. Chromatin consists of DNA wrapped around histone proteins and efficiently packs DNA into a small volume. Moreover, using a spool and thread analogy, chromatin allows DNA to be locally wound or unwound, thus enabling genes to be enclosed or exposed. The misregulation of chromatin structures results in aberrant gene expression and can ultimately lead to developmental disorders or cancers. Despite the importance of DNA high-order structures, the complexity of the underlying machinery has circumvented molecular dissection.
For the first time, molecular biologists have uncovered how one particular mechanism uses energy to ensure proper histone placement onto DNA to form chromatin. They published their results on Dec. 17 in Nature Communications.
The study focused on proteins called histone chaperones. Histone chaperones are responsible for adding and removing specific histones at specific times during the DNA packaging process. The wrong histone at the wrong time and place could result in the misregulation of gene expression or aberrant DNA replication. Thus, histone chaperones are key players in the assembly and disassembly of chromatin.
“In order to carefully control the assembly and disassembly of chromatin units, histone chaperones act as molecular escorts that prevent histone aggregation and undesired interactions,” said Professor Ji-Joon Song in the Department of Biological Sciences at KAIST. “We set out to understand how a unique histone chaperone uses chemical energy to assemble or disassemble chromatin.”
Song and his team looked to Abo1, the only known histone chaperone that utilizes cellular energy (ATP). While Abo1 is found in yeast, it has an analogous partner in other organisms, including humans, called ATAD2. Both use ATP, which is produced through a cellular process where enzymes break down a molecule’s phosphate bond. ATP energy is typically used to power other cellular processes, but it is a rare partner for histone chaperones.
“This was an interesting problem in the field because all other histone chaperones studied to date do not use ATP,” Song said.
By imaging Abo1 with a single-molecule fluorescence imaging technique known as the DNA curtain assay, the researchers could examine the protein interactions at the single-molecule level. The technique allows scientists to arrange the DNA molecules and proteins on a single layer of a microfluidic chamber and examine the layer with fluorescence microscopy.
The researchers found through real-time observation that Abo1 is ring-shaped and changes its structure to accommodate a specific histone and deposit it on DNA. Moreover, they found that the accommodating structural changes are powered by ADP.
“We discovered a mechanism by which Abo1 accommodates histone substrates, ultimately allowing it to function as a unique energy-dependent histone chaperone,” Song said. “We also found that despite looking like a protein disassembly machine, Abo1 actually loads histone substrates onto DNA to facilitate chromatin assembly.”
The researchers plan to continue exploring how energy-dependent histone chaperones bind and release histones, with the ultimate goal of developing therapeutics that can target cancer-causing misbehavior by Abo1’s analogous human counterpart, ATAD2.
-Profile
Professor Ji-Joon Song
Department of Biological Sciences KI for the BioCentury (https://kis.kaist.ac.kr/index.php?mid=KIB_O) KAIST
2020.01.07 View 8739
Scientists Discover the Mechanism of DNA High-Order Structure Formation
(Molecular structures of Abo1 in different energy states (left), Demonstration of an Abo1-assisted histone loading onto DNA by the DNA curtain assay. )
The genetic material of our cells—DNA—exists in a high-order structure called “chromatin”. Chromatin consists of DNA wrapped around histone proteins and efficiently packs DNA into a small volume. Moreover, using a spool and thread analogy, chromatin allows DNA to be locally wound or unwound, thus enabling genes to be enclosed or exposed. The misregulation of chromatin structures results in aberrant gene expression and can ultimately lead to developmental disorders or cancers. Despite the importance of DNA high-order structures, the complexity of the underlying machinery has circumvented molecular dissection.
For the first time, molecular biologists have uncovered how one particular mechanism uses energy to ensure proper histone placement onto DNA to form chromatin. They published their results on Dec. 17 in Nature Communications.
The study focused on proteins called histone chaperones. Histone chaperones are responsible for adding and removing specific histones at specific times during the DNA packaging process. The wrong histone at the wrong time and place could result in the misregulation of gene expression or aberrant DNA replication. Thus, histone chaperones are key players in the assembly and disassembly of chromatin.
“In order to carefully control the assembly and disassembly of chromatin units, histone chaperones act as molecular escorts that prevent histone aggregation and undesired interactions,” said Professor Ji-Joon Song in the Department of Biological Sciences at KAIST. “We set out to understand how a unique histone chaperone uses chemical energy to assemble or disassemble chromatin.”
Song and his team looked to Abo1, the only known histone chaperone that utilizes cellular energy (ATP). While Abo1 is found in yeast, it has an analogous partner in other organisms, including humans, called ATAD2. Both use ATP, which is produced through a cellular process where enzymes break down a molecule’s phosphate bond. ATP energy is typically used to power other cellular processes, but it is a rare partner for histone chaperones.
“This was an interesting problem in the field because all other histone chaperones studied to date do not use ATP,” Song said.
By imaging Abo1 with a single-molecule fluorescence imaging technique known as the DNA curtain assay, the researchers could examine the protein interactions at the single-molecule level. The technique allows scientists to arrange the DNA molecules and proteins on a single layer of a microfluidic chamber and examine the layer with fluorescence microscopy.
The researchers found through real-time observation that Abo1 is ring-shaped and changes its structure to accommodate a specific histone and deposit it on DNA. Moreover, they found that the accommodating structural changes are powered by ADP.
“We discovered a mechanism by which Abo1 accommodates histone substrates, ultimately allowing it to function as a unique energy-dependent histone chaperone,” Song said. “We also found that despite looking like a protein disassembly machine, Abo1 actually loads histone substrates onto DNA to facilitate chromatin assembly.”
The researchers plan to continue exploring how energy-dependent histone chaperones bind and release histones, with the ultimate goal of developing therapeutics that can target cancer-causing misbehavior by Abo1’s analogous human counterpart, ATAD2.
-Profile
Professor Ji-Joon Song
Department of Biological Sciences KI for the BioCentury (https://kis.kaist.ac.kr/index.php?mid=KIB_O) KAIST
2020.01.07 View 8739 -
 Professor Shin-Hyun Kim Receives the Young Scientist Award
Professor Shin-Hyun Kim from the Department of Chemical and Biomolecular Engineering received the Young Scientist Award from the Korean Academy of Science and Technology.
The Young Scientist Award is presented to a promising young Korean scientist under the age of 40 who shows significant potential, passion, and remarkable achievement.
Professor Kim was lauded for his research of intelligent soft materials. By applying his research, he developed a capsule sensor material that can not only be used for sensors, but also for displays, color aesthetics, anti-counterfeit technology, residual drug detection, and more.
The award ceremony took place on December 14 at the Gwacheon National Science Museum.
The Korean minister of Science and ICT delivered words of encouragement, reminding everyone that “the driving force behind creative performance of scientists is the provision of continuous support.” He added, “Researchers of Korea deserve greater public attention and support.”
(END)
2019.12.21 View 6511
Professor Shin-Hyun Kim Receives the Young Scientist Award
Professor Shin-Hyun Kim from the Department of Chemical and Biomolecular Engineering received the Young Scientist Award from the Korean Academy of Science and Technology.
The Young Scientist Award is presented to a promising young Korean scientist under the age of 40 who shows significant potential, passion, and remarkable achievement.
Professor Kim was lauded for his research of intelligent soft materials. By applying his research, he developed a capsule sensor material that can not only be used for sensors, but also for displays, color aesthetics, anti-counterfeit technology, residual drug detection, and more.
The award ceremony took place on December 14 at the Gwacheon National Science Museum.
The Korean minister of Science and ICT delivered words of encouragement, reminding everyone that “the driving force behind creative performance of scientists is the provision of continuous support.” He added, “Researchers of Korea deserve greater public attention and support.”
(END)
2019.12.21 View 6511 -
 New IEEE Fellow, Professor Jong Chul Ye
Professor Jong Chul Ye from the Department of Bio and Brain Engineering was named a new fellow of the Institute of Electrical and Electronics Engineers (IEEE). IEEE announced this on December 1 in recognition of Professor Ye’s contributions to the development of signal processing and artificial intelligence (AI) technology in the field of biomedical imaging.
As the world’s largest society in the electrical and electronics field, IEEE names the top 0.1% of their members as fellows based on their research achievements.Professor Ye has published more than 100 research papers in world-leading journals in the biomedical imaging field, including those affiliated with IEEE.
He also gave a keynote talk at the yearly conference of the International Society for Magnetic Resonance Imaging (ISMRM) on medical AI technology. In addition, Professor Ye has been appointed to serve as the next chair of the Computational Imaging Technical Committee of the IEEE Signal Processing Society, and the chair of the IEEE Symposium on Biomedical Imaging (ISBI) 2020 to be held in April in Iowa, USA.
Professor Ye said, “The importance of AI technology is developing in the biomedical imaging field. I feel proud that my contributions have been internationally recognized and allowed me to be named an IEEE fellow.”
2019.12.18 View 7824
New IEEE Fellow, Professor Jong Chul Ye
Professor Jong Chul Ye from the Department of Bio and Brain Engineering was named a new fellow of the Institute of Electrical and Electronics Engineers (IEEE). IEEE announced this on December 1 in recognition of Professor Ye’s contributions to the development of signal processing and artificial intelligence (AI) technology in the field of biomedical imaging.
As the world’s largest society in the electrical and electronics field, IEEE names the top 0.1% of their members as fellows based on their research achievements.Professor Ye has published more than 100 research papers in world-leading journals in the biomedical imaging field, including those affiliated with IEEE.
He also gave a keynote talk at the yearly conference of the International Society for Magnetic Resonance Imaging (ISMRM) on medical AI technology. In addition, Professor Ye has been appointed to serve as the next chair of the Computational Imaging Technical Committee of the IEEE Signal Processing Society, and the chair of the IEEE Symposium on Biomedical Imaging (ISBI) 2020 to be held in April in Iowa, USA.
Professor Ye said, “The importance of AI technology is developing in the biomedical imaging field. I feel proud that my contributions have been internationally recognized and allowed me to be named an IEEE fellow.”
2019.12.18 View 7824 -
 Tungsten Suboxide Improves the Efficiency of Platinum in Hydrogen Production
< PhD Candidate Jinkyu Park and Professor Jinwoo Lee >
Researchers presented a new strategy for enhancing catalytic activity using tungsten suboxide as a single-atom catalyst (SAC). This strategy, which significantly improves hydrogen evolution reaction (HER) in metal platinum (pt) by 16.3 times, sheds light on the development of new electrochemical catalyst technologies.
Hydrogen has been touted as a promising alternative to fossil fuels. However, most of the conventional industrial hydrogen production methods come with environmental issues, releasing significant amounts of carbon dioxide and greenhouse gases.
Electrochemical water splitting is considered a potential approach for clean hydrogen production. Pt is one of the most commonly used catalysts to improve HER performance in electrochemical water splitting, but the high cost and scarcity of Pt remain key obstacles to mass commercial applications.
SACs, where all metal species are individually dispersed on a desired support material, have been identified as one way to reduce the amount of Pt usage, as they offer the maximum number of surface exposed Pt atoms.
Inspired by earlier studies, which mainly focused on SACs supported by carbon-based materials, a KAIST research team led by Professor Jinwoo Lee from the Department of Chemical and Biomolecular Engineering investigated the influence of support materials on the performance of SACs.
Professor Lee and his researchers suggested mesoporous tungsten suboxide as a new support material for atomically dispersed Pt, as this was expected to provide high electronic conductivity and have a synergetic effect with Pt.
They compared the performance of single-atom Pt supported by carbon and tungsten suboxide respectively. The results revealed that the support effect occurred with tungsten suboxide, in which the mass activity of a single-atom Pt supported by tungsten suboxide was 2.1 times greater than that of single-atom Pt supported by carbon, and 16.3 times higher than that of Pt nanoparticles supported by carbon.
The team indicated a change in the electronic structure of Pt via charge transfer from tungsten suboxide to Pt. This phenomenon was reported as a result of strong metal-support interaction between Pt and tungsten suboxide.
HER performance can be improved not only by changing the electronic structure of the supported metal, but also by inducing another support effect, the spillover effect, the research group reported. Hydrogen spillover is a phenomenon where adsorbed hydrogen migrates from one surface to another, and it occurs more easily as the Pt size becomes smaller.
The researchers compared the performance of single-atom Pt and Pt nanoparticles supported by tungsten suboxide. The single-atom Pt supported by tungsten suboxide exhibited a higher degree of hydrogen spillover phenomenon, which enhanced the Pt mass activity for hydrogen evolution up to 10.7 times compared to Pt nanoparticles supported by tungsten suboxide.
Professor Lee said, “Choosing the right support material is important for improving electrocatalysis in hydrogen production. The tungsten suboxide catalyst we used to support Pt in our study implies that interactions between the well-matched metal and support can drastically enhance the efficiency of the process.”
This research was supported by the Ministry of Science and ICT and introduced in the International Edition of the German journal Angewandte Chemie.
Figure. Schematic representation of hydrogen evolution reaction (HER) of pseudo single-atom Pt supported by tungsten suboxide
-Publication
Jinkyu Park, Dr. Seonggyu Lee, Hee-Eun Kim, Ara Cho, Seongbeen Kim, Dr. Youngjin Ye, Prof. Jeong Woo Han, Prof. Hyunjoo Lee, Dr. Jong Hyun Jang, and Prof. Jinwoo Lee. 2019. Investigation of the Support Effect in Atomically Dispersed Pt on WO3−x for Utilization of Pt in the Hydrogen Evolution Reaction. International Edition of Angewandte Chemie. Volume No. 58. Issue No. 45. 6 pages. https://doi.org/10.1002/anie.201908122
-ProfileProfessor Jinwoo LeeConvergence of Energy and Nano Science Laboratoryhttp://cens.kaist.ac.kr
Department of Chemical and Biomolecular EngineeringKAIST
2019.10.28 View 18755
Tungsten Suboxide Improves the Efficiency of Platinum in Hydrogen Production
< PhD Candidate Jinkyu Park and Professor Jinwoo Lee >
Researchers presented a new strategy for enhancing catalytic activity using tungsten suboxide as a single-atom catalyst (SAC). This strategy, which significantly improves hydrogen evolution reaction (HER) in metal platinum (pt) by 16.3 times, sheds light on the development of new electrochemical catalyst technologies.
Hydrogen has been touted as a promising alternative to fossil fuels. However, most of the conventional industrial hydrogen production methods come with environmental issues, releasing significant amounts of carbon dioxide and greenhouse gases.
Electrochemical water splitting is considered a potential approach for clean hydrogen production. Pt is one of the most commonly used catalysts to improve HER performance in electrochemical water splitting, but the high cost and scarcity of Pt remain key obstacles to mass commercial applications.
SACs, where all metal species are individually dispersed on a desired support material, have been identified as one way to reduce the amount of Pt usage, as they offer the maximum number of surface exposed Pt atoms.
Inspired by earlier studies, which mainly focused on SACs supported by carbon-based materials, a KAIST research team led by Professor Jinwoo Lee from the Department of Chemical and Biomolecular Engineering investigated the influence of support materials on the performance of SACs.
Professor Lee and his researchers suggested mesoporous tungsten suboxide as a new support material for atomically dispersed Pt, as this was expected to provide high electronic conductivity and have a synergetic effect with Pt.
They compared the performance of single-atom Pt supported by carbon and tungsten suboxide respectively. The results revealed that the support effect occurred with tungsten suboxide, in which the mass activity of a single-atom Pt supported by tungsten suboxide was 2.1 times greater than that of single-atom Pt supported by carbon, and 16.3 times higher than that of Pt nanoparticles supported by carbon.
The team indicated a change in the electronic structure of Pt via charge transfer from tungsten suboxide to Pt. This phenomenon was reported as a result of strong metal-support interaction between Pt and tungsten suboxide.
HER performance can be improved not only by changing the electronic structure of the supported metal, but also by inducing another support effect, the spillover effect, the research group reported. Hydrogen spillover is a phenomenon where adsorbed hydrogen migrates from one surface to another, and it occurs more easily as the Pt size becomes smaller.
The researchers compared the performance of single-atom Pt and Pt nanoparticles supported by tungsten suboxide. The single-atom Pt supported by tungsten suboxide exhibited a higher degree of hydrogen spillover phenomenon, which enhanced the Pt mass activity for hydrogen evolution up to 10.7 times compared to Pt nanoparticles supported by tungsten suboxide.
Professor Lee said, “Choosing the right support material is important for improving electrocatalysis in hydrogen production. The tungsten suboxide catalyst we used to support Pt in our study implies that interactions between the well-matched metal and support can drastically enhance the efficiency of the process.”
This research was supported by the Ministry of Science and ICT and introduced in the International Edition of the German journal Angewandte Chemie.
Figure. Schematic representation of hydrogen evolution reaction (HER) of pseudo single-atom Pt supported by tungsten suboxide
-Publication
Jinkyu Park, Dr. Seonggyu Lee, Hee-Eun Kim, Ara Cho, Seongbeen Kim, Dr. Youngjin Ye, Prof. Jeong Woo Han, Prof. Hyunjoo Lee, Dr. Jong Hyun Jang, and Prof. Jinwoo Lee. 2019. Investigation of the Support Effect in Atomically Dispersed Pt on WO3−x for Utilization of Pt in the Hydrogen Evolution Reaction. International Edition of Angewandte Chemie. Volume No. 58. Issue No. 45. 6 pages. https://doi.org/10.1002/anie.201908122
-ProfileProfessor Jinwoo LeeConvergence of Energy and Nano Science Laboratoryhttp://cens.kaist.ac.kr
Department of Chemical and Biomolecular EngineeringKAIST
2019.10.28 View 18755 -
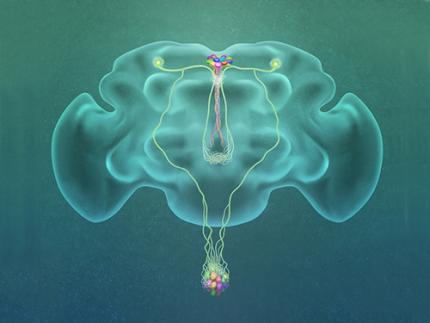 A Single, Master Switch for Sugar Levels?
When a fly eats sugar, a single brain cell sends simultaneous messages to stimulate one hormone and inhibit another to control glucose levels in the body. Further research into this control system with remarkable precision could shed light on the neural mechanisms of diabetes and obesity in humans .
A single neuron appears to monitor and control sugar levels in the fly body, according to research published this week in Nature. This new insight into the mechanisms in the fly brain that maintain a balance of two key hormones controlling glucose levels, insulin and glucagon, can provide a framework for understanding diabetes and obesity in humans.
Neurons that sense and respond to glucose were identified more than 50 years ago, but what they do in our body has remained unclear. Researchers at the Korea Advanced Institute of Science and Technology (KAIST) and New York University School of Medicine have now found a single “glucose-sensing neuron” that appears to be the master controller in Drosophila, the vinegar fly, for maintaining an ideal glucose balance, called homeostasis.
Professor Greg Seong-Bae Suh, Dr. Yangkyun Oh and colleagues identified a key neuron that is excited by glucose, which they called CN neuron. This CN neuron has a unique shape – it has an axon (which is used to transmit information to downstream cells) that is bifurcated. One branch projects to insulin-producing cells, and sends a signal triggering the secretion of the insulin equivalent in flies. The other branch projects to glucagon-producing cells and sends a signal inhibiting the secretion of the glucagon equivalent.
When flies consume food, the levels of glucose in their body increase; this excites the CN neuron, which fires the simultaneous signals to stimulate insulin and inhibit glucagon secretion, thereby maintaining the appropriate balance between the hormones and sugar in the blood. The researchers were able to see this happening in the brain in real time by using a combination of cutting-edge fluorescent calcium imaging technology, as well as measuring hormone and sugar levels and applying highly sophisticated molecular genetic techniques.
When flies were not fed, however, the researchers observed a reduction in the activity of CN neuron, a reduction in insulin secretion and an increase in glucagon secretion. These findings indicate that these key hormones are under the direct control of the glucose-sensing neuron. Furthermore, when they silenced the CN neuron rendering dysfunctional CN neuron in flies, these animals experienced an imbalance, resulting in hyperglycemia – high levels of sugars in the blood, similar to what is observed in diabetes in humans. This further suggests that the CN neuron is critical to maintaining glucose homeostasis in animals.
While further research is required to investigate this process in humans, Suh notes this is a significant step forward in the fields of both neurobiology and endocrinology.
“This work lays the foundation for translational research to better understand how this delicate regulatory process is affected by diabetes, obesity, excessive nutrition and diets high in sugar,” Suh said.
Profile: Greg Seong-Bae Suh
seongbaesuh@kaist.ac.kr
Professor Department of Biological Sciences
KAIST
(Figure: A single glucose-excited CN neuron extends bifurcated axonal branches,
one of which innervates insulin producing cells and stimulates their activity an the other axonal branch projects to glucagon producing cells and inhibits their activity.)
2019.10.24 View 16202
A Single, Master Switch for Sugar Levels?
When a fly eats sugar, a single brain cell sends simultaneous messages to stimulate one hormone and inhibit another to control glucose levels in the body. Further research into this control system with remarkable precision could shed light on the neural mechanisms of diabetes and obesity in humans .
A single neuron appears to monitor and control sugar levels in the fly body, according to research published this week in Nature. This new insight into the mechanisms in the fly brain that maintain a balance of two key hormones controlling glucose levels, insulin and glucagon, can provide a framework for understanding diabetes and obesity in humans.
Neurons that sense and respond to glucose were identified more than 50 years ago, but what they do in our body has remained unclear. Researchers at the Korea Advanced Institute of Science and Technology (KAIST) and New York University School of Medicine have now found a single “glucose-sensing neuron” that appears to be the master controller in Drosophila, the vinegar fly, for maintaining an ideal glucose balance, called homeostasis.
Professor Greg Seong-Bae Suh, Dr. Yangkyun Oh and colleagues identified a key neuron that is excited by glucose, which they called CN neuron. This CN neuron has a unique shape – it has an axon (which is used to transmit information to downstream cells) that is bifurcated. One branch projects to insulin-producing cells, and sends a signal triggering the secretion of the insulin equivalent in flies. The other branch projects to glucagon-producing cells and sends a signal inhibiting the secretion of the glucagon equivalent.
When flies consume food, the levels of glucose in their body increase; this excites the CN neuron, which fires the simultaneous signals to stimulate insulin and inhibit glucagon secretion, thereby maintaining the appropriate balance between the hormones and sugar in the blood. The researchers were able to see this happening in the brain in real time by using a combination of cutting-edge fluorescent calcium imaging technology, as well as measuring hormone and sugar levels and applying highly sophisticated molecular genetic techniques.
When flies were not fed, however, the researchers observed a reduction in the activity of CN neuron, a reduction in insulin secretion and an increase in glucagon secretion. These findings indicate that these key hormones are under the direct control of the glucose-sensing neuron. Furthermore, when they silenced the CN neuron rendering dysfunctional CN neuron in flies, these animals experienced an imbalance, resulting in hyperglycemia – high levels of sugars in the blood, similar to what is observed in diabetes in humans. This further suggests that the CN neuron is critical to maintaining glucose homeostasis in animals.
While further research is required to investigate this process in humans, Suh notes this is a significant step forward in the fields of both neurobiology and endocrinology.
“This work lays the foundation for translational research to better understand how this delicate regulatory process is affected by diabetes, obesity, excessive nutrition and diets high in sugar,” Suh said.
Profile: Greg Seong-Bae Suh
seongbaesuh@kaist.ac.kr
Professor Department of Biological Sciences
KAIST
(Figure: A single glucose-excited CN neuron extends bifurcated axonal branches,
one of which innervates insulin producing cells and stimulates their activity an the other axonal branch projects to glucagon producing cells and inhibits their activity.)
2019.10.24 View 16202 -
 Professor Hyun Gyu Park Appointed as Associate Editor for Biosensors and Bioelectronics
Professor Hyun Gyu Park from the Department of Chemical and Biomolecular Engineering was appointed as an associate editor for Biosensors and Bioelectronics, an international journal published by Elsevier.
Biosensors and Bioelectronics is one of the top SCI journals in the fields of chemistry and analytical science (IF 9.518 as of 2018). Professor Park was recognized and appointed as the associate editor for this journal due to his outstanding research achievements in the fields of nucleic acid engineering, biosensors, and nanobiotechnology.
Professor Park will serve as the associate editor from this October until December 2021.
(END)
2019.10.01 View 5766
Professor Hyun Gyu Park Appointed as Associate Editor for Biosensors and Bioelectronics
Professor Hyun Gyu Park from the Department of Chemical and Biomolecular Engineering was appointed as an associate editor for Biosensors and Bioelectronics, an international journal published by Elsevier.
Biosensors and Bioelectronics is one of the top SCI journals in the fields of chemistry and analytical science (IF 9.518 as of 2018). Professor Park was recognized and appointed as the associate editor for this journal due to his outstanding research achievements in the fields of nucleic acid engineering, biosensors, and nanobiotechnology.
Professor Park will serve as the associate editor from this October until December 2021.
(END)
2019.10.01 View 5766