Bio-Integrated+Electronics+and+Systems+Research+Group
-
 KAIST Research Team Develops Sweat-Resistant Wearable Robot Sensor
New electromyography (EMG) sensor technology that allows the long-term stable control of wearable robots and is not affected by the wearer’s sweat and dead skin has gained attention recently. Wearable robots are devices used across a variety of rehabilitation treatments for the elderly and patients recovering from stroke or trauma.
A joint research team led by Professor Jae-Woong Jung from the KAIST School of Electrical Engineering (EE) and Professor Jung Kim from the KAIST Department of Mechanical Engineering (ME) announced on January 23rd that they have successfully developed a stretchable and adhesive microneedle sensor that can electrically sense physiological signals at a high level without being affected by the state of the user’s skin.
For wearable robots to recognize the intentions behind human movement for their use in rehabilitation treatment, they require a wearable electrophysiological sensor that gives precise EMG measurements. However, existing sensors often show deteriorating signal quality over time and are greatly affected by the user’s skin conditions. Furthermore, the sensor’s higher mechanical hardness causes noise since the contact surface is unable to keep up with the deformation of the skin. These shortcomings limit the reliable, long-term control of wearable robots.
< Figure 1. Design and working concept of the Stretchable microNeedle Adhesive Patch (SNAP). (A) Schematic illustration showing the overall system configuration and application of SNAP. (B) Exploded view schematic diagram of a SNAP, consisting of stretchable serpentine interconnects, Au-coated Si microneedle, and ECA made of Ag flakes–silicone composite. (C) Optical images showing high mechanical compliance of SNAP. >
However, the recently developed technology is expected to allow long-term and high-quality EMG measurements as it uses a stretchable and adhesive conducting substrate integrated with microneedle arrays that can easily penetrate the stratum corneum without causing discomfort. Through its excellent performance, the sensor is anticipated to be able to stably control wearable robots over a long period of time regardless of the wearer’s changing skin conditions and without the need for a preparation step that removes sweat and dead cells from the surface of their skin.
The research team created a stretchable and adhesive microneedle sensor by integrating microneedles into a soft silicon polymer substrate. The hard microneedles penetrate through the stratum corneum, which has high electrical resistance. As a result, the sensor can effectively lower contact resistance with the skin and obtain high-quality electrophysiological signals regardless of contamination. At the same time, the soft and adhesive conducting substrate can adapt to the skin’s surface that stretches with the wearer’s movement, providing a comfortable fit and minimizing noise caused by movement.
< Figure 2. Demonstration of the wireless Stretchable microNeedle Adhesive Patch (SNAP) system as an Human-machine interfaces (HMI) for closed-loop control of an exoskeleton robot. (A) Illustration depicting the system architecture and control strategy of an exoskeleton robot. (B) The hardware configuration of the pneumatic back support exoskeleton system. (C) Comparison of root mean square (RMS) of electromyography (EMG) with and without robotic assistance of pretreated skin and non-pretreated skin. >
To verify the usability of the new patch, the research team conducted a motion assistance experiment using a wearable robot. They attached the microneedle patch on a user’s leg, where it could sense the electrical signals generated by the muscle. The sensor then sent the detected intention to a wearable robot, allowing the robot to help the wearer lift a heavy object more easily.
Professor Jae-Woong Jung, who led the research, said, “The developed stretchable and adhesive microneedle sensor can stability detect EMG signals without being affected by the state of a user’s skin. Through this, we will be able to control wearable robots with higher precision and stability, which will help the rehabilitation of patients who use robots.”
The results of this research, written by co-first authors Heesoo Kim and Juhyun Lee, who are both Ph.D. candidates in the KAIST School of EE, were published in Science Advances on January 17th under the title “Skin-preparation-free, stretchable microneedle adhesive patches for reliable electrophysiological sensing and exoskeleton robot control”.
This research was supported by the Bio-signal Sensor Integrated Technology Development Project by the National Research Foundation of Korea, the Electronic Medicinal Technology Development Project, and the Step 4 BK21 Project.
2024.01.30 View 4081
KAIST Research Team Develops Sweat-Resistant Wearable Robot Sensor
New electromyography (EMG) sensor technology that allows the long-term stable control of wearable robots and is not affected by the wearer’s sweat and dead skin has gained attention recently. Wearable robots are devices used across a variety of rehabilitation treatments for the elderly and patients recovering from stroke or trauma.
A joint research team led by Professor Jae-Woong Jung from the KAIST School of Electrical Engineering (EE) and Professor Jung Kim from the KAIST Department of Mechanical Engineering (ME) announced on January 23rd that they have successfully developed a stretchable and adhesive microneedle sensor that can electrically sense physiological signals at a high level without being affected by the state of the user’s skin.
For wearable robots to recognize the intentions behind human movement for their use in rehabilitation treatment, they require a wearable electrophysiological sensor that gives precise EMG measurements. However, existing sensors often show deteriorating signal quality over time and are greatly affected by the user’s skin conditions. Furthermore, the sensor’s higher mechanical hardness causes noise since the contact surface is unable to keep up with the deformation of the skin. These shortcomings limit the reliable, long-term control of wearable robots.
< Figure 1. Design and working concept of the Stretchable microNeedle Adhesive Patch (SNAP). (A) Schematic illustration showing the overall system configuration and application of SNAP. (B) Exploded view schematic diagram of a SNAP, consisting of stretchable serpentine interconnects, Au-coated Si microneedle, and ECA made of Ag flakes–silicone composite. (C) Optical images showing high mechanical compliance of SNAP. >
However, the recently developed technology is expected to allow long-term and high-quality EMG measurements as it uses a stretchable and adhesive conducting substrate integrated with microneedle arrays that can easily penetrate the stratum corneum without causing discomfort. Through its excellent performance, the sensor is anticipated to be able to stably control wearable robots over a long period of time regardless of the wearer’s changing skin conditions and without the need for a preparation step that removes sweat and dead cells from the surface of their skin.
The research team created a stretchable and adhesive microneedle sensor by integrating microneedles into a soft silicon polymer substrate. The hard microneedles penetrate through the stratum corneum, which has high electrical resistance. As a result, the sensor can effectively lower contact resistance with the skin and obtain high-quality electrophysiological signals regardless of contamination. At the same time, the soft and adhesive conducting substrate can adapt to the skin’s surface that stretches with the wearer’s movement, providing a comfortable fit and minimizing noise caused by movement.
< Figure 2. Demonstration of the wireless Stretchable microNeedle Adhesive Patch (SNAP) system as an Human-machine interfaces (HMI) for closed-loop control of an exoskeleton robot. (A) Illustration depicting the system architecture and control strategy of an exoskeleton robot. (B) The hardware configuration of the pneumatic back support exoskeleton system. (C) Comparison of root mean square (RMS) of electromyography (EMG) with and without robotic assistance of pretreated skin and non-pretreated skin. >
To verify the usability of the new patch, the research team conducted a motion assistance experiment using a wearable robot. They attached the microneedle patch on a user’s leg, where it could sense the electrical signals generated by the muscle. The sensor then sent the detected intention to a wearable robot, allowing the robot to help the wearer lift a heavy object more easily.
Professor Jae-Woong Jung, who led the research, said, “The developed stretchable and adhesive microneedle sensor can stability detect EMG signals without being affected by the state of a user’s skin. Through this, we will be able to control wearable robots with higher precision and stability, which will help the rehabilitation of patients who use robots.”
The results of this research, written by co-first authors Heesoo Kim and Juhyun Lee, who are both Ph.D. candidates in the KAIST School of EE, were published in Science Advances on January 17th under the title “Skin-preparation-free, stretchable microneedle adhesive patches for reliable electrophysiological sensing and exoskeleton robot control”.
This research was supported by the Bio-signal Sensor Integrated Technology Development Project by the National Research Foundation of Korea, the Electronic Medicinal Technology Development Project, and the Step 4 BK21 Project.
2024.01.30 View 4081 -
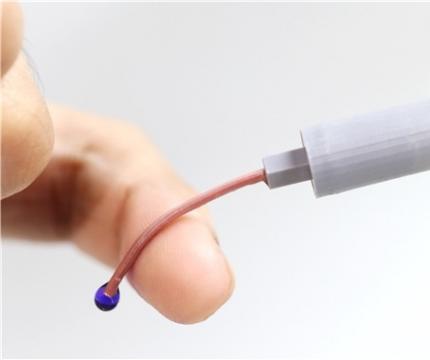 An intravenous needle that irreversibly softens via body temperature on insertion
- A joint research team at KAIST developed an intravenous (IV) needle that softens upon insertion, minimizing risk of damage to blood vessels and tissues.
- Once used, it remains soft even at room temperature, preventing accidental needle stick injuries and unethical multiple use of needle.
- A thin-film temperature sensor can be embedded with this needle, enabling real-time monitoring of the patient's core body temperature, or detection of unintended fluid leakage, during IV medication.
Intravenous (IV) injection is a method commonly used in patient’s treatment worldwide as it induces rapid effects and allows treatment through continuous administration of medication by directly injecting drugs into the blood vessel. However, medical IV needles, made of hard materials such as stainless steel or plastic which do not mechanically match the soft biological tissues of the body, can cause critical problems in healthcare settings, starting from minor tissue damages in the injection sites to serious inflammations.
The structure and dexterity of rigid medical IV devices also enable unethical reuse of needles for reduction of injection costs, leading to transmission of deadly blood-borne disease infections such as human immunodeficiency virus (HIV) and hepatitis B/C viruses. Furthermore, unintended needlestick injuries are frequently occurring in medical settings worldwide, that are viable sources of such infections, with IV needles having the greatest susceptibility of being the medium of transmissible diseases. For these reasons, the World Health Organization (WHO) in 2015 launched a policy on safe injection practices to encourage the development and use of “smart” syringes that have features to prevent re-use, after a tremendous increase in the number of deadly infectious disease worldwide due to medical-sharps related issues.
KAIST announced on the 13th that Professor Jae-Woong Jeong and his research team of its School of Electrical Engineering succeeded in developing the Phase-Convertible, Adapting and non-REusable (P-CARE) needle with variable stiffness that can improve patient health and ensure the safety of medical staff through convergent joint research with another team led by Professor Won-Il Jeong of the Graduate School of Medical Sciences.
The new technology is expected to allow patients to move without worrying about pain at the injection site as it reduces the risk of damage to the wall of the blood vessel as patients receive IV medication. This is possible with the needle’s stiffness-tunable characteristics which will make it soft and flexible upon insertion into the body due to increased temperature, adapting to the movement of thin-walled vein. It is also expected to prevent blood-borne disease infections caused by accidental needlestick injuries or unethical re-using of syringes as the deformed needle remains perpetually soft even after it is retracted from the injection site.
The results of this research, in which Karen-Christian Agno, a doctoral researcher of the School of Electrical Engineering at and Dr. Keungmo Yang of the Graduate School of Medical Sciences participated as co-first authors, was published in Nature Biomedical Engineering on October 30. (Paper title: A temperature-responsive intravenous needle that irreversibly softens on insertion)
< Figure 1. Disposable variable stiffness intravenous needle. (a) Conceptual illustration of the key features of the P-CARE needle whose mechanical properties can be changed by body temperature, (b) Photograph of commonly used IV access devices and the P-CARE needle, (c) Performance of common IV access devices and the P-CARE needle >
“We’ve developed this special needle using advanced materials and micro/nano engineering techniques, and it can solve many global problems related to conventional medical needles used in healthcare worldwide”, said Jae-Woong Jeong, Ph.D., an associate professor of Electrical Engineering at KAIST and a lead senior author of the study.
The softening IV needle created by the research team is made up of liquid metal gallium that forms the hollow, mechanical needle frame encapsulated within an ultra-soft silicone material. In its solid state, gallium has sufficient hardness that enables puncturing of soft biological tissues. However, gallium melts when it is exposed to body temperature upon insertion, and changes it into a soft state like the surrounding tissue, enabling stable delivery of the drug without damaging blood vessels. Once used, a needle remains soft even at room temperature due to the supercooling phenomenon of gallium, fundamentally preventing needlestick accidents and reuse problems.
Biocompatibility of the softening IV needle was validated through in vivo studies in mice. The studies showed that implanted needles caused significantly less inflammation relative to the standard IV access devices of similar size made of metal needles or plastic catheters. The study also confirmed the new needle was able to deliver medications as reliably as commercial injection needles.
< Photo 1. Photo of the P-CARE needle that softens with body temperature. >
Researchers also showed possibility of integrating a customized ultra-thin temperature sensor with the softening IV needle to measure the on-site temperature which can further enhance patient’s well-being. The single assembly of sensor-needle device can be used to monitor the core body temperature, or even detect if there is a fluid leakage on-site during indwelling use, eliminating the need for additional medical tools or procedures to provide the patients with better health care services.
The researchers believe that this transformative IV needle can open new opportunities for wide range of applications particularly in clinical setups, in terms of redesigning other medical needles and sharp medical tools to reduce muscle tissue injury during indwelling use. The softening IV needle may become even more valuable in the present times as there is an estimated 16 billion medical injections administered annually in a global scale, yet not all needles are disposed of properly, based on a 2018 WHO report.
< Figure 2. Biocompatibility test for P-CARE needle: Images of H&E stained histology (the area inside the dashed box on the left is provided in an expanded view in the right), TUNEL staining (green), DAPI staining of nuclei (blue) and co-staining (TUNEL and DAPI) of muscle tissue from different organs. >
< Figure 3. Conceptual images of potential utilization for temperature monitoring function of P-CARE needle integrated with a temperature sensor. >
(a) Schematic diagram of injecting a drug through intravenous injection into the abdomen of a laboratory mouse (b) Change of body temperature upon injection of drug (c) Conceptual illustration of normal intravenous drug injection (top) and fluid leakage (bottom) (d) Comparison of body temperature during normal drug injection and fluid leakage: when the fluid leak occur due to incorrect insertion, a sudden drop of temperature is detected.
This work was supported by grants from the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT.
2023.11.13 View 5733
An intravenous needle that irreversibly softens via body temperature on insertion
- A joint research team at KAIST developed an intravenous (IV) needle that softens upon insertion, minimizing risk of damage to blood vessels and tissues.
- Once used, it remains soft even at room temperature, preventing accidental needle stick injuries and unethical multiple use of needle.
- A thin-film temperature sensor can be embedded with this needle, enabling real-time monitoring of the patient's core body temperature, or detection of unintended fluid leakage, during IV medication.
Intravenous (IV) injection is a method commonly used in patient’s treatment worldwide as it induces rapid effects and allows treatment through continuous administration of medication by directly injecting drugs into the blood vessel. However, medical IV needles, made of hard materials such as stainless steel or plastic which do not mechanically match the soft biological tissues of the body, can cause critical problems in healthcare settings, starting from minor tissue damages in the injection sites to serious inflammations.
The structure and dexterity of rigid medical IV devices also enable unethical reuse of needles for reduction of injection costs, leading to transmission of deadly blood-borne disease infections such as human immunodeficiency virus (HIV) and hepatitis B/C viruses. Furthermore, unintended needlestick injuries are frequently occurring in medical settings worldwide, that are viable sources of such infections, with IV needles having the greatest susceptibility of being the medium of transmissible diseases. For these reasons, the World Health Organization (WHO) in 2015 launched a policy on safe injection practices to encourage the development and use of “smart” syringes that have features to prevent re-use, after a tremendous increase in the number of deadly infectious disease worldwide due to medical-sharps related issues.
KAIST announced on the 13th that Professor Jae-Woong Jeong and his research team of its School of Electrical Engineering succeeded in developing the Phase-Convertible, Adapting and non-REusable (P-CARE) needle with variable stiffness that can improve patient health and ensure the safety of medical staff through convergent joint research with another team led by Professor Won-Il Jeong of the Graduate School of Medical Sciences.
The new technology is expected to allow patients to move without worrying about pain at the injection site as it reduces the risk of damage to the wall of the blood vessel as patients receive IV medication. This is possible with the needle’s stiffness-tunable characteristics which will make it soft and flexible upon insertion into the body due to increased temperature, adapting to the movement of thin-walled vein. It is also expected to prevent blood-borne disease infections caused by accidental needlestick injuries or unethical re-using of syringes as the deformed needle remains perpetually soft even after it is retracted from the injection site.
The results of this research, in which Karen-Christian Agno, a doctoral researcher of the School of Electrical Engineering at and Dr. Keungmo Yang of the Graduate School of Medical Sciences participated as co-first authors, was published in Nature Biomedical Engineering on October 30. (Paper title: A temperature-responsive intravenous needle that irreversibly softens on insertion)
< Figure 1. Disposable variable stiffness intravenous needle. (a) Conceptual illustration of the key features of the P-CARE needle whose mechanical properties can be changed by body temperature, (b) Photograph of commonly used IV access devices and the P-CARE needle, (c) Performance of common IV access devices and the P-CARE needle >
“We’ve developed this special needle using advanced materials and micro/nano engineering techniques, and it can solve many global problems related to conventional medical needles used in healthcare worldwide”, said Jae-Woong Jeong, Ph.D., an associate professor of Electrical Engineering at KAIST and a lead senior author of the study.
The softening IV needle created by the research team is made up of liquid metal gallium that forms the hollow, mechanical needle frame encapsulated within an ultra-soft silicone material. In its solid state, gallium has sufficient hardness that enables puncturing of soft biological tissues. However, gallium melts when it is exposed to body temperature upon insertion, and changes it into a soft state like the surrounding tissue, enabling stable delivery of the drug without damaging blood vessels. Once used, a needle remains soft even at room temperature due to the supercooling phenomenon of gallium, fundamentally preventing needlestick accidents and reuse problems.
Biocompatibility of the softening IV needle was validated through in vivo studies in mice. The studies showed that implanted needles caused significantly less inflammation relative to the standard IV access devices of similar size made of metal needles or plastic catheters. The study also confirmed the new needle was able to deliver medications as reliably as commercial injection needles.
< Photo 1. Photo of the P-CARE needle that softens with body temperature. >
Researchers also showed possibility of integrating a customized ultra-thin temperature sensor with the softening IV needle to measure the on-site temperature which can further enhance patient’s well-being. The single assembly of sensor-needle device can be used to monitor the core body temperature, or even detect if there is a fluid leakage on-site during indwelling use, eliminating the need for additional medical tools or procedures to provide the patients with better health care services.
The researchers believe that this transformative IV needle can open new opportunities for wide range of applications particularly in clinical setups, in terms of redesigning other medical needles and sharp medical tools to reduce muscle tissue injury during indwelling use. The softening IV needle may become even more valuable in the present times as there is an estimated 16 billion medical injections administered annually in a global scale, yet not all needles are disposed of properly, based on a 2018 WHO report.
< Figure 2. Biocompatibility test for P-CARE needle: Images of H&E stained histology (the area inside the dashed box on the left is provided in an expanded view in the right), TUNEL staining (green), DAPI staining of nuclei (blue) and co-staining (TUNEL and DAPI) of muscle tissue from different organs. >
< Figure 3. Conceptual images of potential utilization for temperature monitoring function of P-CARE needle integrated with a temperature sensor. >
(a) Schematic diagram of injecting a drug through intravenous injection into the abdomen of a laboratory mouse (b) Change of body temperature upon injection of drug (c) Conceptual illustration of normal intravenous drug injection (top) and fluid leakage (bottom) (d) Comparison of body temperature during normal drug injection and fluid leakage: when the fluid leak occur due to incorrect insertion, a sudden drop of temperature is detected.
This work was supported by grants from the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT.
2023.11.13 View 5733