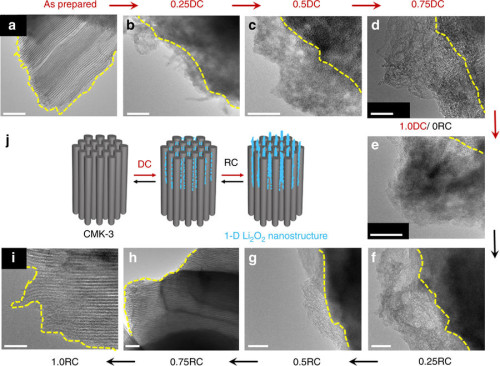
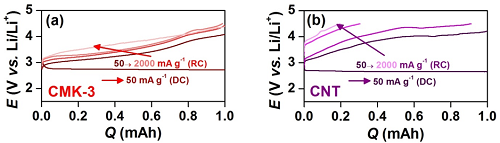
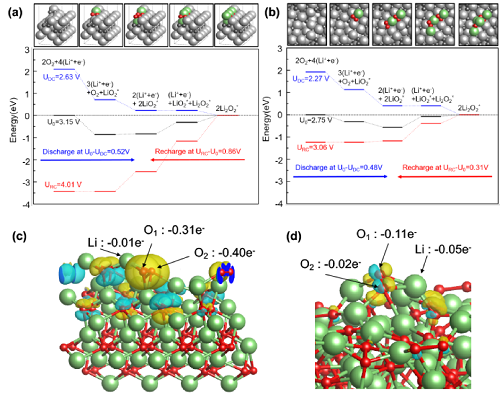
research

-
research KAIST Develops Stretchable Displays Featuring 25% Expansion Without Image Distortion
Stretchable displays, praised for their spatial efficiency, design flexibility, and human-like flexibility, are seen as the next generation of display technology. A team of Korean researchers has developed a stretchable display that can expand by 25% while maintaining clear image quality without distortion. It can also stretch and contract up to 5,000 times at 15% expansion without any performance degradation, making it the first deformation-free stretchable display with a negative Poisson's rat
2024-09-20 -
research KAIST Research Team Breaks Down Musical Instincts with AI
Music, often referred to as the universal language, is known to be a common component in all cultures. Then, could ‘musical instinct’ be something that is shared to some degree despite the extensive environmental differences amongst cultures? On January 16, a KAIST research team led by Professor Hawoong Jung from the Department of Physics announced to have identified the principle by which musical instincts emerge from the human brain without special learning using an artificial neu
2024-01-23 -
research North Korea and Beyond: AI-Powered Satellite Analysis Reveals the Unseen Economic Landscape of Underdeveloped Nations
- A joint research team in computer science, economics, and geography has developed an artificial intelligence (AI) technology to measure grid-level economic development within six-square-kilometer regions. - This AI technology is applicable in regions with limited statistical data (e.g., North Korea), supporting international efforts to propose policies for economic growth and poverty reduction in underdeveloped countries. - The research team plans to make this technology freely available f
2023-12-07 -
research KAIST builds a high-resolution 3D holographic sensor using a single mask
Holographic cameras can provide more realistic images than ordinary cameras thanks to their ability to acquire 3D information about objects. However, existing holographic cameras use interferometers that measure the wavelength and refraction of light through the interference of light waves, which makes them complex and sensitive to their surrounding environment. On August 23, a KAIST research team led by Professor YongKeun Park from the Department of Physics announced a new leap forward in 3D
2023-09-05 -
people Professor Shinhyun Choi’s team, selected for Nature Communications Editors’ highlight
[ From left, Ph.D. candidates See-On Park and Hakcheon Jeong, along with Master's student Jong-Yong Park and Professor Shinhyun Choi ] See-On Park, Hakcheon Jeong, Jong-Yong Park - a team of researchers under the leadership of Professor Shinhyun Choi of the School of Electrical Engineering, developed a highly reliable variable resistor (memristor) array that simulates the behavior of neurons using a metal oxide layer with an oxygen concentration gradient, and published their work in Nature
2022-11-01