Electrochemical+Energy+Device+Lab
-
 New LSB with Theoretical Capacity over 90%
(Professor Hee-Tak Kim and Hyunwon Chu)
A KAIST research team has developed a lithium sulfur battery (LSB) that realizes 92% of the theoretical capacity and an areal capacity of 4mAh/cm2.
LSBs are gaining a great deal of attention as an alternative for lithium ion batteries (LIBs) because they have a theoretical energy density up to six to seven times higher than that of LIBs, and can be manufactured in a more cost-effective way.
However, LSBs face the obstacle of having a capacity reaching its theoretical maximum because they are prone to uncontrolled growth of lithium sulfide on the electrodes, which leads to blocking electron transfer.
To address the problem of electrode passivation, researchers introduced additional conductive agent into the electrode; however, it drastically lowered the energy density of LSBs, making it difficult to exceed 70% of the theoretical capacity.
Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering and his team replaced the lithium salt anions used in conventional LSB electrolytes with anions with a high donor number. The team successfully induced the three-dimensional growth of lithium sulfide on electrode surfaces and efficiently delayed the electrode passivation.
Based on this electrolyte design, the research team achieved 92% of the theoretical capacity with their high-capacity sulfur electrode (4mAh/cm2), which is equivalent to that of conventional LIB cathode. Furthermore, they were able to form a stable passivation film on the surface of the lithium anode and exhibited stable operation over 100 cycles.
This technology, which can be flexibly used with various types of sulfur electrodes, can mark a new milestone in the battery industry.
Professor Kim said, “We proposed a new physiochemical principle to overcome the limitations of conventional LSBs. I believe our achievement of obtaining 90% of the LBSs’ theoretical capacity without any capacity loss after 100 cycles will become a new milestone.”
This research, first-authored by Hyunwon Chu and Hyungjun Noh, was published in Nature Communications on January 14, 2019. It was also selected in the editor’s highlight for its outstanding achievements.
Figure 1. Lithium sulfur growth and its deposition mechanism for different sulfide growth behaviors
Figure 2. Capacity and cycle life characteristics of the LSBs
2019.02.11 View 8698
New LSB with Theoretical Capacity over 90%
(Professor Hee-Tak Kim and Hyunwon Chu)
A KAIST research team has developed a lithium sulfur battery (LSB) that realizes 92% of the theoretical capacity and an areal capacity of 4mAh/cm2.
LSBs are gaining a great deal of attention as an alternative for lithium ion batteries (LIBs) because they have a theoretical energy density up to six to seven times higher than that of LIBs, and can be manufactured in a more cost-effective way.
However, LSBs face the obstacle of having a capacity reaching its theoretical maximum because they are prone to uncontrolled growth of lithium sulfide on the electrodes, which leads to blocking electron transfer.
To address the problem of electrode passivation, researchers introduced additional conductive agent into the electrode; however, it drastically lowered the energy density of LSBs, making it difficult to exceed 70% of the theoretical capacity.
Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering and his team replaced the lithium salt anions used in conventional LSB electrolytes with anions with a high donor number. The team successfully induced the three-dimensional growth of lithium sulfide on electrode surfaces and efficiently delayed the electrode passivation.
Based on this electrolyte design, the research team achieved 92% of the theoretical capacity with their high-capacity sulfur electrode (4mAh/cm2), which is equivalent to that of conventional LIB cathode. Furthermore, they were able to form a stable passivation film on the surface of the lithium anode and exhibited stable operation over 100 cycles.
This technology, which can be flexibly used with various types of sulfur electrodes, can mark a new milestone in the battery industry.
Professor Kim said, “We proposed a new physiochemical principle to overcome the limitations of conventional LSBs. I believe our achievement of obtaining 90% of the LBSs’ theoretical capacity without any capacity loss after 100 cycles will become a new milestone.”
This research, first-authored by Hyunwon Chu and Hyungjun Noh, was published in Nature Communications on January 14, 2019. It was also selected in the editor’s highlight for its outstanding achievements.
Figure 1. Lithium sulfur growth and its deposition mechanism for different sulfide growth behaviors
Figure 2. Capacity and cycle life characteristics of the LSBs
2019.02.11 View 8698 -
 KAIST Develops VRFB with Longer Durability
(from left: PhD candidate Soohyun Kim, Professor Hee-Tak Kim and PhD candidate Junghoon Choi)
There has been growing demand for large-scale storage for energy produced from renewable energy sources in an efficient and stable way. To meet this demand, a KAIST research team developed a new vanadium redox-flow battery (VRFB) with 15 times greater capacity retention and five times longer durability. This VRFB battery can be an excellent candidate for a large-scale rechargeable battery with no risk of explosion.
The VRFB has received much attention for its high efficiency and reliability with the absence of cross-contamination. However, it has the limitation of having insufficient charge and discharge efficiency and a low capacity retention rate because its perfluorinated membrane is very permeable to any active materials. To minimize energy loss, it needs a membrane that has low vanadium ion permeability and high ion conductivity. Hence, there was an attempt to incorporate a hydrocarbon membrane that has low cost and high ion selectivity but it turned out that the VO₂+ caused chemical degradation, which led to shortening the battery life drastically.
To develop a membrane with pore sizes smaller than the hydrated size of vanadium ions yet larger than that of the protons, a research team co-led by Professor Hee-Tae Jung and Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering implemented a graphene-oxide framework (GOF) membrane by cross-linking graphene oxide nanosheets. They believed that GOF, having strong ion selectivity, would be a good candidate for the membrane component for the VRFB. The interlayer spacing between the GO sheets limited moisture expansion and provided selective ion permeation.
The GOF membrane increased the capacity retention of the VRFB, which showed a 15 times higher rate than that of perfluorinated membranes. Its cycling stability was also enhanced up to five times, compared to conventional hydrocarbon membranes.
These pore-sized-tuned graphene oxide frameworks will allow pore-sized tuning of membranes and will be applicable to electrochemical systems that utilize ions of various sizes, such as rechargeable batteries and sensors.
Professor Kim said, “Developing a membrane that prevents the mixing of positive and negative active materials has been a chronic issue in the field of redox-flow batteries. Through this research, we showed that nanotechnology can prevent this crossover issue and membrane degradation. I believe that this technology can be applied to various rechargeable batteries requiring large-scale storage.”
This research was published in Nano Letters on May 3.
Figure 1. Electrochemical performances of the VRFBs with Nafion 115, SPAES (sulfonated poly), and GOF/SPAES: discharge capacity
Figure 2. Schematic of the selective ion transfer of hydrated vanadium ions and protons in the GOF membrane and the molecular structure of the GOF membrane, showing that the GO nanosheets are cross-linked with EDA (ethylenediamine)
2018.09.20 View 8052
KAIST Develops VRFB with Longer Durability
(from left: PhD candidate Soohyun Kim, Professor Hee-Tak Kim and PhD candidate Junghoon Choi)
There has been growing demand for large-scale storage for energy produced from renewable energy sources in an efficient and stable way. To meet this demand, a KAIST research team developed a new vanadium redox-flow battery (VRFB) with 15 times greater capacity retention and five times longer durability. This VRFB battery can be an excellent candidate for a large-scale rechargeable battery with no risk of explosion.
The VRFB has received much attention for its high efficiency and reliability with the absence of cross-contamination. However, it has the limitation of having insufficient charge and discharge efficiency and a low capacity retention rate because its perfluorinated membrane is very permeable to any active materials. To minimize energy loss, it needs a membrane that has low vanadium ion permeability and high ion conductivity. Hence, there was an attempt to incorporate a hydrocarbon membrane that has low cost and high ion selectivity but it turned out that the VO₂+ caused chemical degradation, which led to shortening the battery life drastically.
To develop a membrane with pore sizes smaller than the hydrated size of vanadium ions yet larger than that of the protons, a research team co-led by Professor Hee-Tae Jung and Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering implemented a graphene-oxide framework (GOF) membrane by cross-linking graphene oxide nanosheets. They believed that GOF, having strong ion selectivity, would be a good candidate for the membrane component for the VRFB. The interlayer spacing between the GO sheets limited moisture expansion and provided selective ion permeation.
The GOF membrane increased the capacity retention of the VRFB, which showed a 15 times higher rate than that of perfluorinated membranes. Its cycling stability was also enhanced up to five times, compared to conventional hydrocarbon membranes.
These pore-sized-tuned graphene oxide frameworks will allow pore-sized tuning of membranes and will be applicable to electrochemical systems that utilize ions of various sizes, such as rechargeable batteries and sensors.
Professor Kim said, “Developing a membrane that prevents the mixing of positive and negative active materials has been a chronic issue in the field of redox-flow batteries. Through this research, we showed that nanotechnology can prevent this crossover issue and membrane degradation. I believe that this technology can be applied to various rechargeable batteries requiring large-scale storage.”
This research was published in Nano Letters on May 3.
Figure 1. Electrochemical performances of the VRFBs with Nafion 115, SPAES (sulfonated poly), and GOF/SPAES: discharge capacity
Figure 2. Schematic of the selective ion transfer of hydrated vanadium ions and protons in the GOF membrane and the molecular structure of the GOF membrane, showing that the GO nanosheets are cross-linked with EDA (ethylenediamine)
2018.09.20 View 8052 -
 Using Donut-shaped Lithium Sulfide for Higher Performing Batteries
(from left: Research Professor Fangmin Ye and Professor Hee-Tak Kim)
A KAIST research team developed a lithium-sulfur battery with a doughnut-shaped active material structure showing a record lifecycle of over 600 cycles. Having higher energy density and lower production cost than a lithium-ion battery (LIB), it can be used in electric vehicles that require a longer battery life.
There has been an intense research conducted for developing lithium-sulfur batteries with high energy density because LIBs only allow for a very short travel distance per charge. However, Li-S batteries are still unable to provide a longer lifecycle due to the poor reversibility of the lithium metal cathode.
To tackle this issue, Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering and his team used lithium sulfide (Li₂S) cathodes and combine them with graphite anodes to enhance energy density and lifecycles for the batteries.
Yet, lithium sulfide is costly and, so far, there has not been an electrode architecture and electrolyte design that enables a longer lifecycle between the graphite anodes and lithium sulfide cathodes.
Hence, the team produced a doughnut-shaped lithium sulfide cathode active material from low-cost lithium sulfide developed from raw materials. They have also developed a lithium sulfide ion battery with a graphite anode and lithium sulfide cathode using a high concentration salt electrolyte.
This doughnut-shaped lithium sulfide showed outstanding charge and discharge reversibility through improving the transfer of lithium ions. Its highly concentrated salt electrolyte formed a stable film on the surface of the graphite electrode, which showed strong durability.
Through this technology, the team achieved 30% higher energy density than that of conventional LIBs and secured a lifecycle of more than 600 cycles. This doughnut-shaped lithium sulfide-based electrode can be manufactured using low-cost raw materials and a single heat treatment process. The electrode can also be applied to existing LIBs.
Professor Kim said, “We have demonstrated that applying low-cost sulfur compounds to LIBs can improve both energy density and the lifecycle simultaneously.”
This research, led by Research Professor Fangmin Ye, was published in Advanced Science on May 7.
Figure 1. Structural characterization of Li₂SO₄/CNT and Li₂S/CNT electrodes and suggested mechanism for the formation of the holey-Li₂S nanoarchitecture
2018.09.19 View 6225
Using Donut-shaped Lithium Sulfide for Higher Performing Batteries
(from left: Research Professor Fangmin Ye and Professor Hee-Tak Kim)
A KAIST research team developed a lithium-sulfur battery with a doughnut-shaped active material structure showing a record lifecycle of over 600 cycles. Having higher energy density and lower production cost than a lithium-ion battery (LIB), it can be used in electric vehicles that require a longer battery life.
There has been an intense research conducted for developing lithium-sulfur batteries with high energy density because LIBs only allow for a very short travel distance per charge. However, Li-S batteries are still unable to provide a longer lifecycle due to the poor reversibility of the lithium metal cathode.
To tackle this issue, Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering and his team used lithium sulfide (Li₂S) cathodes and combine them with graphite anodes to enhance energy density and lifecycles for the batteries.
Yet, lithium sulfide is costly and, so far, there has not been an electrode architecture and electrolyte design that enables a longer lifecycle between the graphite anodes and lithium sulfide cathodes.
Hence, the team produced a doughnut-shaped lithium sulfide cathode active material from low-cost lithium sulfide developed from raw materials. They have also developed a lithium sulfide ion battery with a graphite anode and lithium sulfide cathode using a high concentration salt electrolyte.
This doughnut-shaped lithium sulfide showed outstanding charge and discharge reversibility through improving the transfer of lithium ions. Its highly concentrated salt electrolyte formed a stable film on the surface of the graphite electrode, which showed strong durability.
Through this technology, the team achieved 30% higher energy density than that of conventional LIBs and secured a lifecycle of more than 600 cycles. This doughnut-shaped lithium sulfide-based electrode can be manufactured using low-cost raw materials and a single heat treatment process. The electrode can also be applied to existing LIBs.
Professor Kim said, “We have demonstrated that applying low-cost sulfur compounds to LIBs can improve both energy density and the lifecycle simultaneously.”
This research, led by Research Professor Fangmin Ye, was published in Advanced Science on May 7.
Figure 1. Structural characterization of Li₂SO₄/CNT and Li₂S/CNT electrodes and suggested mechanism for the formation of the holey-Li₂S nanoarchitecture
2018.09.19 View 6225 -
 Membrane
Scientists at KAIST have developed a new way of making fuel cell membranes using nanoscale fasteners, paving the way for lower-cost, higher-efficiency and more easily manufactured fuel cells.
The internal workings of fuel cells vary, but basically all types mix hydrogen and oxygen to produce a chemical reaction that delivers usable electricity and exhausts ordinary water as a by-product. One of the most efficient types is the proton exchange membrane (PEM) fuel cell, which operates at low enough temperatures to be used in homes and vehicles.
To generate electricity, PEM fuel cells rely on two chemical compartments separated by a permeable catalyst membrane. This membrane acts as an electrolyte; a negative electrode is bonded to one side of the membrane and a positive electrode is bonded to the other. The electrolyte membrane is often based on a polymer of perfluorosulfonic acid. Due to its high cost, however, a less expensive hydrocarbon-based electrolyte membrane has attracted interest in this technology sector.
Until now, the challenge in adopting such a hydrocarbon membrane has been that the interface between the electrode and hydrocarbon membrane is weak. This causes the membrane to delaminate relatively easily, falling apart and losing efficiency with use.
Professor Hee-Tak Kim of the Department of Chemical and Biomolecular Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and his research team have developed a new fastening system that bonds the two materials mechanically rather than chemically. This opens the way to the development of fuel cell membranes that are less expensive, easier to manufacture, stronger and more efficient.
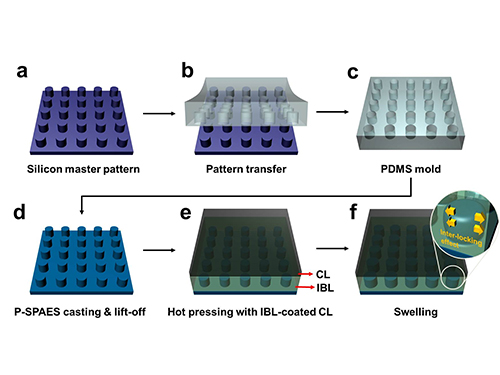
The researchers achieved this by moulding a pattern of tiny cylindrical pillars on the face of the hydrocarbon membrane. The pillars protrude into a softened skin of the electrode with heat. The mechanical bond sets and strengthens as the material cools and absorbs water. The pillar-patterned hydrocarbon membrane is cast using silicone moulds.
Professor Kim said, “This physically fastened bond is almost five times stronger and harder to separate than current bonds between the same layers.”
The new interlocking method also appears to offer a way to bond many types of hydrocarbon membranes that, until now, have been rejected because they couldn’t be fastened robustly. This would make hydrocarbon membranes practical for a number of applications beyond fuel cells such as rechargeable “redox flow” batteries.
The research team is now developing a stronger and more scalable interlocking interface for their nanoscale fasteners.
Picture: Schematic Diagram of the Fabrication of the Pillar P-SPAES Membrane and Its Working Principle of Interlocking Effects
2015.11.06 View 10683
Membrane
Scientists at KAIST have developed a new way of making fuel cell membranes using nanoscale fasteners, paving the way for lower-cost, higher-efficiency and more easily manufactured fuel cells.
The internal workings of fuel cells vary, but basically all types mix hydrogen and oxygen to produce a chemical reaction that delivers usable electricity and exhausts ordinary water as a by-product. One of the most efficient types is the proton exchange membrane (PEM) fuel cell, which operates at low enough temperatures to be used in homes and vehicles.
To generate electricity, PEM fuel cells rely on two chemical compartments separated by a permeable catalyst membrane. This membrane acts as an electrolyte; a negative electrode is bonded to one side of the membrane and a positive electrode is bonded to the other. The electrolyte membrane is often based on a polymer of perfluorosulfonic acid. Due to its high cost, however, a less expensive hydrocarbon-based electrolyte membrane has attracted interest in this technology sector.
Until now, the challenge in adopting such a hydrocarbon membrane has been that the interface between the electrode and hydrocarbon membrane is weak. This causes the membrane to delaminate relatively easily, falling apart and losing efficiency with use.
Professor Hee-Tak Kim of the Department of Chemical and Biomolecular Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and his research team have developed a new fastening system that bonds the two materials mechanically rather than chemically. This opens the way to the development of fuel cell membranes that are less expensive, easier to manufacture, stronger and more efficient.
The researchers achieved this by moulding a pattern of tiny cylindrical pillars on the face of the hydrocarbon membrane. The pillars protrude into a softened skin of the electrode with heat. The mechanical bond sets and strengthens as the material cools and absorbs water. The pillar-patterned hydrocarbon membrane is cast using silicone moulds.
Professor Kim said, “This physically fastened bond is almost five times stronger and harder to separate than current bonds between the same layers.”
The new interlocking method also appears to offer a way to bond many types of hydrocarbon membranes that, until now, have been rejected because they couldn’t be fastened robustly. This would make hydrocarbon membranes practical for a number of applications beyond fuel cells such as rechargeable “redox flow” batteries.
The research team is now developing a stronger and more scalable interlocking interface for their nanoscale fasteners.
Picture: Schematic Diagram of the Fabrication of the Pillar P-SPAES Membrane and Its Working Principle of Interlocking Effects
2015.11.06 View 10683