Journal+of+the+American+Chemical+Society
-
 Simple Molecular Reagents to Treat Alzheimer’s Disease
- Researchers report minimalistic principles for designing small molecules with multiple reactivities against dementia. -
Sometimes the most complex problems actually have very simple solutions. A group of South Korean researchers reported an efficient and effective redox-based strategy for incorporating multiple functions into simple molecular reagents against neurodegenerative disorders. The team developed redox-active aromatic molecular reagents with a simple structural composition that can simultaneously target and modulate various pathogenic factors in complex neurodegenerative disorders such as Alzheimer’s disease.
Alzheimer’s disease is one of the most prevalent neurodegenerative disorders, affecting one in ten people over the age of 65. Early-onset dementia also increasingly affects younger people.
A number of pathogenic elements such as reactive oxygen species, amyloid-beta, and metal ions have been suggested as potential causes of Alzheimer’s disease. Each element itself can lead to Alzheimer’s disease, but interactions between them may also aggravate the patient’s condition or interfere with the appropriate clinical care.
For example, when interacting with amyloid-beta, metal ions foster the aggregation and accumulation of amyloid-beta peptides that can induce oxidative stress and toxicity in the brain and lead to neurodegeneration.
Because these pathogenic factors of Alzheimer’s disease are intertwined, developing therapeutic agents that are capable of simultaneously regulating metal ion dyshomeostasis, amyloid-beta agglutination, and oxidative stress responses remains a key to halting the progression of the disease.
A research team led by Professor Mi Hee Lim from the Department of Chemistry at KAIST demonstrated the feasibility of structure-mechanism-based molecular design for controlling a molecule’s chemical reactivity toward the various pathological factors of Alzheimer’s disease by tuning the redox properties of the molecule.
This study, featured as the ‘ACS Editors’ Choice’ in the May 6th issue of the Journal of the American Chemical Society (JACS), was conducted in conjunction with KAIST Professor Mu-Hyun Baik’s group and Professor Joo-Young Lee’s group at the Asan Medical Center.
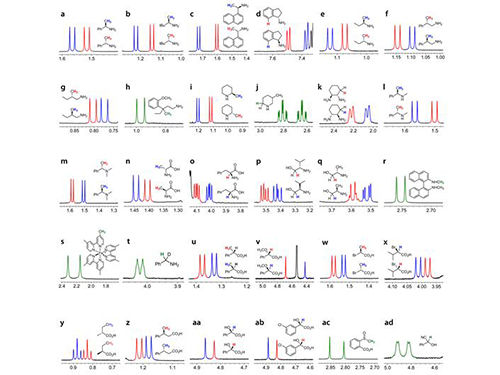
Professor Lim and her collaborators rationally designed and generated 10 compact aromatic molecules presenting a range of redox potentials by adjusting the electronic distribution of the phenyl, phenylene, or pyridyl moiety to impart redox-dependent reactivities against the multiple pathogenic factors in Alzheimer’s disease.
During the team’s biochemical and biophysical studies, these designed molecular reagents displayed redox-dependent reactivities against numerous desirable targets that are associated with Alzheimer’s disease such as free radicals, metal-free amyloid-beta, and metal-bound amyloid-beta.
Further mechanistic results revealed that the redox properties of these designed molecular reagents were essential for their function. The team demonstrated that these reagents engaged in oxidative reactions with metal-free and metal-bound amyloid-beta and led to chemical modifications. The products of such oxidative transformations were observed to form covalent adducts with amyloid-beta and alter its aggregation.
Moreover, the administration of the most promising candidate molecule significantly attenuated the amyloid pathology in the brains of Alzheimer’s disease transgenic mice and improved their cognitive defects.
Professor Lim said, “This strategy is straightforward, time-saving, and cost-effective, and its effect is significant. We are excited to help enable the advancement of new therapeutic agents for neurodegenerative disorders, which can improve the lives of so many patients.”
This work was supported by the National Research Foundation (NRF) of Korea, the Institute for Basic Science (IBS), and the Asan Institute for Life Sciences.
Image credit: Professor Mi Hee Lim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of the news coverage of this paper only.
Publication:
Kim, M. et al. (2020) ‘Minimalistic Principles for Designing Small Molecules with Multiple Reactivities against Pathological Factors in Dementia.’ Journal of the American Chemical Society (JACS), Volume 142, Issue 18, pp.8183-8193. Available online at https://doi.org/10.1021/jacs.9b13100
Profile:
Mi Hee Lim
Professor
miheelim@kaist.ac.kr
http://sites.google.com/site/miheelimlab
Lim Laboratory
Department of Chemistry
KAIST
Profile:
Mu-Hyun Baik
Professor
mbaik2805@kaist.ac.kr
https://baik-laboratory.com/
Baik Laboratory
Department of Chemistry
KAIST
Profile:
Joo-Yong Lee
Professor
jlee@amc.seoul.kr
Asan Institute for Life Sciences
Asan Medical Center
(END)
2020.05.11 View 17522
Simple Molecular Reagents to Treat Alzheimer’s Disease
- Researchers report minimalistic principles for designing small molecules with multiple reactivities against dementia. -
Sometimes the most complex problems actually have very simple solutions. A group of South Korean researchers reported an efficient and effective redox-based strategy for incorporating multiple functions into simple molecular reagents against neurodegenerative disorders. The team developed redox-active aromatic molecular reagents with a simple structural composition that can simultaneously target and modulate various pathogenic factors in complex neurodegenerative disorders such as Alzheimer’s disease.
Alzheimer’s disease is one of the most prevalent neurodegenerative disorders, affecting one in ten people over the age of 65. Early-onset dementia also increasingly affects younger people.
A number of pathogenic elements such as reactive oxygen species, amyloid-beta, and metal ions have been suggested as potential causes of Alzheimer’s disease. Each element itself can lead to Alzheimer’s disease, but interactions between them may also aggravate the patient’s condition or interfere with the appropriate clinical care.
For example, when interacting with amyloid-beta, metal ions foster the aggregation and accumulation of amyloid-beta peptides that can induce oxidative stress and toxicity in the brain and lead to neurodegeneration.
Because these pathogenic factors of Alzheimer’s disease are intertwined, developing therapeutic agents that are capable of simultaneously regulating metal ion dyshomeostasis, amyloid-beta agglutination, and oxidative stress responses remains a key to halting the progression of the disease.
A research team led by Professor Mi Hee Lim from the Department of Chemistry at KAIST demonstrated the feasibility of structure-mechanism-based molecular design for controlling a molecule’s chemical reactivity toward the various pathological factors of Alzheimer’s disease by tuning the redox properties of the molecule.
This study, featured as the ‘ACS Editors’ Choice’ in the May 6th issue of the Journal of the American Chemical Society (JACS), was conducted in conjunction with KAIST Professor Mu-Hyun Baik’s group and Professor Joo-Young Lee’s group at the Asan Medical Center.
Professor Lim and her collaborators rationally designed and generated 10 compact aromatic molecules presenting a range of redox potentials by adjusting the electronic distribution of the phenyl, phenylene, or pyridyl moiety to impart redox-dependent reactivities against the multiple pathogenic factors in Alzheimer’s disease.
During the team’s biochemical and biophysical studies, these designed molecular reagents displayed redox-dependent reactivities against numerous desirable targets that are associated with Alzheimer’s disease such as free radicals, metal-free amyloid-beta, and metal-bound amyloid-beta.
Further mechanistic results revealed that the redox properties of these designed molecular reagents were essential for their function. The team demonstrated that these reagents engaged in oxidative reactions with metal-free and metal-bound amyloid-beta and led to chemical modifications. The products of such oxidative transformations were observed to form covalent adducts with amyloid-beta and alter its aggregation.
Moreover, the administration of the most promising candidate molecule significantly attenuated the amyloid pathology in the brains of Alzheimer’s disease transgenic mice and improved their cognitive defects.
Professor Lim said, “This strategy is straightforward, time-saving, and cost-effective, and its effect is significant. We are excited to help enable the advancement of new therapeutic agents for neurodegenerative disorders, which can improve the lives of so many patients.”
This work was supported by the National Research Foundation (NRF) of Korea, the Institute for Basic Science (IBS), and the Asan Institute for Life Sciences.
Image credit: Professor Mi Hee Lim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of the news coverage of this paper only.
Publication:
Kim, M. et al. (2020) ‘Minimalistic Principles for Designing Small Molecules with Multiple Reactivities against Pathological Factors in Dementia.’ Journal of the American Chemical Society (JACS), Volume 142, Issue 18, pp.8183-8193. Available online at https://doi.org/10.1021/jacs.9b13100
Profile:
Mi Hee Lim
Professor
miheelim@kaist.ac.kr
http://sites.google.com/site/miheelimlab
Lim Laboratory
Department of Chemistry
KAIST
Profile:
Mu-Hyun Baik
Professor
mbaik2805@kaist.ac.kr
https://baik-laboratory.com/
Baik Laboratory
Department of Chemistry
KAIST
Profile:
Joo-Yong Lee
Professor
jlee@amc.seoul.kr
Asan Institute for Life Sciences
Asan Medical Center
(END)
2020.05.11 View 17522 -
 Quantum Dot Film Can Withstand High Temperatures and Humidity
The joint KAIST research team of Professor Byeong-Soo Bae of the Department of Materials Science and Engineering and Professor Doh Chang Lee of the Department of Chemical and Biomolecular Engineering was able to fabricate a siloxane-encapsulated quantum dot film, which exhibits stable emission intensity over one month even at high temperatures and humidity.
The results of this study were published in the Journal of the American Chemical Society (JACS) on November 29, 2016. The research article is entitled “Quantum Dot/Siloxane Composite Film Exceptionally Stable against Oxidation under Heat and Moisture.” (DOI: 10.1021/jacs.6b10681)
Quantum dots (QDs), light-emitting diodes (LEDs) for next-generation displays, are tiny particles or nanocrystals of semiconducting materials. Their emission wavelength can easily be adjusted by changing their sizes, which are just a few nanometers. A wide spectrum of their colors can also achieve ultra-high definition displays.
Due to these characteristics, QDs are coated on a film as a polymer resin in dispersed form, or they are spread on an LED light source. They are thus considered to be crucial for next generation displays.
Despite their exceptional optical properties, however, QDs are easily oxidized in a high temperature and high humidity environment, and, as a result, this greatly deteriorates their luminescence quality (quantum efficiency). Therefore, they are encapsulated in an extra thin layer to block oxygen and moisture.
QD displays in the current market have a film inserted to separate them from LEDs, which create heat. The high unit cost of this protective layer, however, increases the overall cost of displays, lowering their price competitiveness in the market.
For a solution, the research team applied the sol-gel condensation reaction of silane precursors with QDs. This technology uses the reactions of chemical substances to synthesize ceramics or glass at a low temperature.
The team applied QDs in a heat resistant siloxane polymer by employing this technology. The siloxane resin acted as a cup holding the QDs and also blocked heat and moisture. Thus, their performance can be maintained without an extra protective film.
QDs are evenly dispersed into the resin from a chemical process to fabricate a QD embedded film and retained the high quality luminescence not only at a high temperature of 85°C and in a high humidity of 85%, but also in a high acid and high base environment. Remarkably though, the luminescence actually increased in the high humidity environment.
If this technology is used, the overall price of displays will decrease by producing a stable QD film without an extra protective barrier. In the future, the QD film can be directly applied to a blue LED light source. As a result, it will be possible to develop a QD display that can reduce the amount of QDs needed and improve its performance.
Professor Bae said, “We have proposed a way to make quantum dots overcome their limitations and have wide applications as they are being developed for next-generation displays. Our technology will make significant contributions to the display industry in the country.”
He also added, “In the future, we plan to cooperate with companies both in and out of the country to improve the performance of quantum dots and concentrate on their commercialization.”
The research team is currently applying for related patents both in and out of the country. The team is also plan ning to transfer the patents to Sol Ip Technology Inc., a company founded at KAIST, to start the commercialization.
Picture 1:
Siloxane-encapsulated quantum dot (QD) films showing performance stability in boiling water
Picture 2 and 3:
So-gel condensation reaction in silane precursors between Methacryloxypropyltrimethoxysilane (MPTS) and diphenylsilanediol (DPSD). The inset shows photographs of a QD-oligosiloxane resin under room light (left) and a UV lamp (λ = 365 nm) (right).
Free radical addition reactions among carbon double bonds of methacryl functional groups and oleic acids. The inset shows photographs of a QD-silox film under room light (left) and a UV lamp (λ = 365 nm) (right).
2017.02.24 View 11077
Quantum Dot Film Can Withstand High Temperatures and Humidity
The joint KAIST research team of Professor Byeong-Soo Bae of the Department of Materials Science and Engineering and Professor Doh Chang Lee of the Department of Chemical and Biomolecular Engineering was able to fabricate a siloxane-encapsulated quantum dot film, which exhibits stable emission intensity over one month even at high temperatures and humidity.
The results of this study were published in the Journal of the American Chemical Society (JACS) on November 29, 2016. The research article is entitled “Quantum Dot/Siloxane Composite Film Exceptionally Stable against Oxidation under Heat and Moisture.” (DOI: 10.1021/jacs.6b10681)
Quantum dots (QDs), light-emitting diodes (LEDs) for next-generation displays, are tiny particles or nanocrystals of semiconducting materials. Their emission wavelength can easily be adjusted by changing their sizes, which are just a few nanometers. A wide spectrum of their colors can also achieve ultra-high definition displays.
Due to these characteristics, QDs are coated on a film as a polymer resin in dispersed form, or they are spread on an LED light source. They are thus considered to be crucial for next generation displays.
Despite their exceptional optical properties, however, QDs are easily oxidized in a high temperature and high humidity environment, and, as a result, this greatly deteriorates their luminescence quality (quantum efficiency). Therefore, they are encapsulated in an extra thin layer to block oxygen and moisture.
QD displays in the current market have a film inserted to separate them from LEDs, which create heat. The high unit cost of this protective layer, however, increases the overall cost of displays, lowering their price competitiveness in the market.
For a solution, the research team applied the sol-gel condensation reaction of silane precursors with QDs. This technology uses the reactions of chemical substances to synthesize ceramics or glass at a low temperature.
The team applied QDs in a heat resistant siloxane polymer by employing this technology. The siloxane resin acted as a cup holding the QDs and also blocked heat and moisture. Thus, their performance can be maintained without an extra protective film.
QDs are evenly dispersed into the resin from a chemical process to fabricate a QD embedded film and retained the high quality luminescence not only at a high temperature of 85°C and in a high humidity of 85%, but also in a high acid and high base environment. Remarkably though, the luminescence actually increased in the high humidity environment.
If this technology is used, the overall price of displays will decrease by producing a stable QD film without an extra protective barrier. In the future, the QD film can be directly applied to a blue LED light source. As a result, it will be possible to develop a QD display that can reduce the amount of QDs needed and improve its performance.
Professor Bae said, “We have proposed a way to make quantum dots overcome their limitations and have wide applications as they are being developed for next-generation displays. Our technology will make significant contributions to the display industry in the country.”
He also added, “In the future, we plan to cooperate with companies both in and out of the country to improve the performance of quantum dots and concentrate on their commercialization.”
The research team is currently applying for related patents both in and out of the country. The team is also plan ning to transfer the patents to Sol Ip Technology Inc., a company founded at KAIST, to start the commercialization.
Picture 1:
Siloxane-encapsulated quantum dot (QD) films showing performance stability in boiling water
Picture 2 and 3:
So-gel condensation reaction in silane precursors between Methacryloxypropyltrimethoxysilane (MPTS) and diphenylsilanediol (DPSD). The inset shows photographs of a QD-oligosiloxane resin under room light (left) and a UV lamp (λ = 365 nm) (right).
Free radical addition reactions among carbon double bonds of methacryl functional groups and oleic acids. The inset shows photographs of a QD-silox film under room light (left) and a UV lamp (λ = 365 nm) (right).
2017.02.24 View 11077 -
 KAIST Develops New Technique for Chiral Activity in Molecules
Professor Hyunwoo Kim of the Chemistry Department and his research team have developed a technique that can easily analyze the optical activity of charged compounds by using nuclear magnetic resonance (NMR) spectroscopy. The research finding entitled “H NMR Chiral Analysis of Charged Molecules via Ion Pairing with Aluminum Complexes” was published online in the October 19th issue of The Journal of the American Chemical Society.
The technique relies on observation of the behavior of optical isomers. Molecules with the same composition that are mirror images of each other are optical isomers. For example, the building blocks of all living organisms, amino acids, are a single optical isomer. In our bodies, optical isomers bring different physiological changes due to their distinct optical activities. Therefore, controlling and analyzing the optical activities are critical when developing a new drug.
High-performance liquid chromatography (HPLC) is the de facto standard of analyzing the optical activity of a compound. However, HPLC is very expensive that many laboratories can’t afford to have. In addition, with the machine, one analysis may take 30 minutes to one hour to complete. It lacks in signal sensitivity and chemical decomposition, and the application is limited to nonpolar compounds.
Usually adopted in analyzing the structure of a chemical compound, NMR spectroscopy requires only one to five minutes per single analysis. Since it is essential for analyzing the molecular structure, many chemistry labs have NMR equipment. However, until this technique was invented, no other research team had reported an effective way of using the NMR spectroscopy to decompose the signal of chiral activity of a compound.
The research team uses negatively-charged metal compounds in NMR spectroscopy. The technique employs negatively-charged metal compounds which bond ionically to positively- and negatively-charged optical compounds. As a result, the NMR spectroscopy can distinguish the signal from chiral activity. Not only can it analyze various chemicals without structural constraints, but it can also be used for both nonpolar and polar solvents.
As many compounds for new drugs have functional groups, which can be charged, this analysis method can be directly employed in the development process of drugs. Professor Kim said, “A revolutionary analysis method has been developed using simple chemical principles. I hope that our method will be applied to the development of new medicine.”
This research was sponsored by the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science and the Supercomputing Research Center of KAIST.
Picture 1: Separations of NMR Signals of Chemicals due to Interaction with Metal Compounds
Picture 2: Separations of NMR Signals in Different Chemicals
2015.11.20 View 12595
KAIST Develops New Technique for Chiral Activity in Molecules
Professor Hyunwoo Kim of the Chemistry Department and his research team have developed a technique that can easily analyze the optical activity of charged compounds by using nuclear magnetic resonance (NMR) spectroscopy. The research finding entitled “H NMR Chiral Analysis of Charged Molecules via Ion Pairing with Aluminum Complexes” was published online in the October 19th issue of The Journal of the American Chemical Society.
The technique relies on observation of the behavior of optical isomers. Molecules with the same composition that are mirror images of each other are optical isomers. For example, the building blocks of all living organisms, amino acids, are a single optical isomer. In our bodies, optical isomers bring different physiological changes due to their distinct optical activities. Therefore, controlling and analyzing the optical activities are critical when developing a new drug.
High-performance liquid chromatography (HPLC) is the de facto standard of analyzing the optical activity of a compound. However, HPLC is very expensive that many laboratories can’t afford to have. In addition, with the machine, one analysis may take 30 minutes to one hour to complete. It lacks in signal sensitivity and chemical decomposition, and the application is limited to nonpolar compounds.
Usually adopted in analyzing the structure of a chemical compound, NMR spectroscopy requires only one to five minutes per single analysis. Since it is essential for analyzing the molecular structure, many chemistry labs have NMR equipment. However, until this technique was invented, no other research team had reported an effective way of using the NMR spectroscopy to decompose the signal of chiral activity of a compound.
The research team uses negatively-charged metal compounds in NMR spectroscopy. The technique employs negatively-charged metal compounds which bond ionically to positively- and negatively-charged optical compounds. As a result, the NMR spectroscopy can distinguish the signal from chiral activity. Not only can it analyze various chemicals without structural constraints, but it can also be used for both nonpolar and polar solvents.
As many compounds for new drugs have functional groups, which can be charged, this analysis method can be directly employed in the development process of drugs. Professor Kim said, “A revolutionary analysis method has been developed using simple chemical principles. I hope that our method will be applied to the development of new medicine.”
This research was sponsored by the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science and the Supercomputing Research Center of KAIST.
Picture 1: Separations of NMR Signals of Chemicals due to Interaction with Metal Compounds
Picture 2: Separations of NMR Signals in Different Chemicals
2015.11.20 View 12595 -
 Polymers with Highly Improved Light-transformation Efficiency
A joint Korean research team, led by Professor Bum-Joon Kim of the Department of Chemical and Biomolecular Engineering at KAIST and Professor Young-Woo Han of the Department of Nanofusion Engineering at Pusan National University, has developed a new type of electrically-conductive polymer for solar batteries with an improved light-transformation efficiency of up to 5%. The team considers it a viable replacement for existing plastic batteries for solar power which is viewed as the energy source of the future.
Polymer solar cells have greater structural stability and heat resistance compared to fullerene organic solar cells. However, they have lower light-transformation efficiency—below 4%—compared to 10% of the latter. The low efficiency is due to the failure of blending among the polymers that compose the active layer of the cell. This phenomenon deters the formation and movement of electrons and thus lowers light-transformation efficiency.
By manipulating the structure and concentration of conductive polymers, the team was able to effectively increase the polymer blending and increase light-transformation efficiency. The team was able to maximize the efficiency up to 6% which is the highest reported ratio.
Professor Kim said, “This research demonstrates that conductive polymer plastics can be used widely for solar cells and batteries for mobile devices.”
The research findings were published in the February 18th issue of the Journal of the American Chemical Society (JACS).
Picture: Flexible Solar Cell Polymer Developed by the Research Team
2015.04.05 View 12168
Polymers with Highly Improved Light-transformation Efficiency
A joint Korean research team, led by Professor Bum-Joon Kim of the Department of Chemical and Biomolecular Engineering at KAIST and Professor Young-Woo Han of the Department of Nanofusion Engineering at Pusan National University, has developed a new type of electrically-conductive polymer for solar batteries with an improved light-transformation efficiency of up to 5%. The team considers it a viable replacement for existing plastic batteries for solar power which is viewed as the energy source of the future.
Polymer solar cells have greater structural stability and heat resistance compared to fullerene organic solar cells. However, they have lower light-transformation efficiency—below 4%—compared to 10% of the latter. The low efficiency is due to the failure of blending among the polymers that compose the active layer of the cell. This phenomenon deters the formation and movement of electrons and thus lowers light-transformation efficiency.
By manipulating the structure and concentration of conductive polymers, the team was able to effectively increase the polymer blending and increase light-transformation efficiency. The team was able to maximize the efficiency up to 6% which is the highest reported ratio.
Professor Kim said, “This research demonstrates that conductive polymer plastics can be used widely for solar cells and batteries for mobile devices.”
The research findings were published in the February 18th issue of the Journal of the American Chemical Society (JACS).
Picture: Flexible Solar Cell Polymer Developed by the Research Team
2015.04.05 View 12168 -
 Artificial Spore Production Technology Developed
The core technology needed in the development of ‘biosensors’ so crucial in diagnosing illnesses or pathogens was developed by Korean research team.
KAIST’s Professor Choi In Seung of the department of Chemistry developed the technology that allows for the production of Artificial Spore by selectively coating a live cell.
In the field of engineering the problem in developing the next generation bio sensor, the cell based sensor, was that it was difficult to keep a cell alive without division for a long time. Once a cell is taken out of the body, it will either divide or die easily.
Professor Choi’s research team mimicked the spore, which has the capability to survive harsh conditions without division, and chemically coated a live cell and artificially created a cell similar to that of a spore.
The physical and biological stabilities of the cell increased by coating an artificial shell over the yeast cell. The shell is composed with a protein similar to that of the protein that gives mussels its stickiness. In addition by controlling the thickness of the shell, the division rate of the yeast can be controlled.
Professor Choi commented that this technology will serve as the basis for the single cell based biosensor.
The research was conducted together with Professor Lee Hae Shin of KAIST department of Chemistry and Professor Jeong Taek Dong of Seoul National University’s department of Chemistry and was published as the cover paper of ‘Journal of the American Chemical Society’.
2011.04.01 View 14848
Artificial Spore Production Technology Developed
The core technology needed in the development of ‘biosensors’ so crucial in diagnosing illnesses or pathogens was developed by Korean research team.
KAIST’s Professor Choi In Seung of the department of Chemistry developed the technology that allows for the production of Artificial Spore by selectively coating a live cell.
In the field of engineering the problem in developing the next generation bio sensor, the cell based sensor, was that it was difficult to keep a cell alive without division for a long time. Once a cell is taken out of the body, it will either divide or die easily.
Professor Choi’s research team mimicked the spore, which has the capability to survive harsh conditions without division, and chemically coated a live cell and artificially created a cell similar to that of a spore.
The physical and biological stabilities of the cell increased by coating an artificial shell over the yeast cell. The shell is composed with a protein similar to that of the protein that gives mussels its stickiness. In addition by controlling the thickness of the shell, the division rate of the yeast can be controlled.
Professor Choi commented that this technology will serve as the basis for the single cell based biosensor.
The research was conducted together with Professor Lee Hae Shin of KAIST department of Chemistry and Professor Jeong Taek Dong of Seoul National University’s department of Chemistry and was published as the cover paper of ‘Journal of the American Chemical Society’.
2011.04.01 View 14848