Fuel+Cell
-
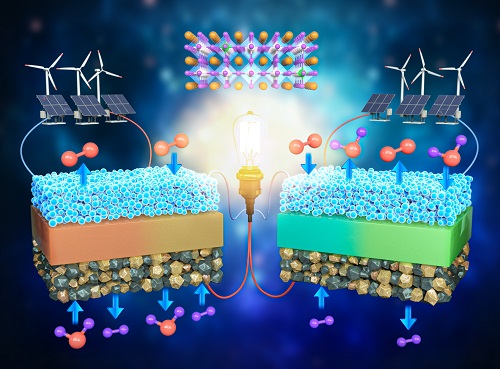 A KAIST Research Team Develops an Ultra-High Performing “Universal Electrode” for Next-Generation Fuel Cells
Fuel cells are devices that generate electricity with high efficiency using hydrogen, a clean energy source, and are expected to play an important part in the upcoming hydrogen society. The recent development of an excellent universal electrode material that is applicable to all next-generation fuel cells and can withstand 700 hours of operation has therefore garnered a great deal of attention.
On August 9, a joint research team led by Prof. WooChul Jung from the KAIST Department of Materials Science and Engineering, Prof. Kang Taek Lee from the KAIST Department of Mechanical Engineering, and Prof. Jun Hyuk Kim from the Department of Chemical Engineering at Hongik University announced the development of an electrode material that is applicable to both oxygen- and proton-conducting solid oxide cells.
Depending on the type of ion conducted by the electrolyte, ceramic fuel cells are categorized into either solid oxide fuel cells (SOFC) or protonic ceramic fuel cells (PCFC). As they can both convert between electricity and hydrogen production, fuel cells can be categorized into a total of four device types. These devices are applicable in hydrogen fuel cell vehicles, hydrogen charging stations, and power generation systems, and are henceforth emerging as core next-generation technologies for a carbon-neutral society.
However, these devices have a chronic problem where the speed of their slowest reaction would decrease with a drop of driving temperature, which greatly reduces device efficiency. Various studies have been conducted to solve this, but most reported that electrode materials have low catalytic activity and their applications are limited to specific devices, which limits them from being used as SOFCs that require reversible power conversion and hydrogen production.
< Figure 1. Schematic diagram of high-performance oxygen ion conductive solid oxide fuel cell (SOFC) and proton conductive ceramic fuel cell (PCFC) operates with the new universal electrodes >
To solve this issue, the research team doped a perovskite oxide material with Ta5+, a high valence ion that did not receive much attention in the field. Through this, the team successfully stabilized what is usually a highly unstable crystal structure, and confirmed that catalytic activity improved by 100 times.
The electrode material developed by the team was applied to all four of the mentioned device types. Furthermore, their efficiencies were greater than any of the devices reported thus far, and showed excellent performance by stably running for much longer (700 hours) compared to existing materials that deteriorated within the first 100 hours of operation.
< Figure 2. (a) Power conversion and hydrogen production performance chart for the protonic ceramic fuel cell (PCFC) with the new universal electrodes (b) and performance comparison with other reported devices >
This research, in which KAIST’s Ph.D. candidates Dongyeon Kim and Sejong Ahn, and Professor Jun Hyuk Kim from Hongik University contributed as co-first authors, was published in the internationally renowned Energy & Environmental Science under the title, "Oxygen-Electrode for Reversible Solid Oxide Electrochemical Cells at Reduced Temperatures".
Prof. WooChul Jung said, “We broke free from the idea that we must develop a completely new material to solve an existing problem, and instead suggested a way to control the crystal structure of a lesser-known material to develop a high-efficiency fuel cell, and that’s what makes these results more significant.”
Prof. Kang Taek Lee added, “Unlike previously reported materials that could only be applied to one device type at a time, our material has the flexibility of being applicable to all four. We therefore look forward to its contribution in the commercialization of eco-friendly energy technology including fuel cells and water-splitting equipment for hydrogen production.”
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Ministry of Science and ICT.
2023.08.22 View 8932
A KAIST Research Team Develops an Ultra-High Performing “Universal Electrode” for Next-Generation Fuel Cells
Fuel cells are devices that generate electricity with high efficiency using hydrogen, a clean energy source, and are expected to play an important part in the upcoming hydrogen society. The recent development of an excellent universal electrode material that is applicable to all next-generation fuel cells and can withstand 700 hours of operation has therefore garnered a great deal of attention.
On August 9, a joint research team led by Prof. WooChul Jung from the KAIST Department of Materials Science and Engineering, Prof. Kang Taek Lee from the KAIST Department of Mechanical Engineering, and Prof. Jun Hyuk Kim from the Department of Chemical Engineering at Hongik University announced the development of an electrode material that is applicable to both oxygen- and proton-conducting solid oxide cells.
Depending on the type of ion conducted by the electrolyte, ceramic fuel cells are categorized into either solid oxide fuel cells (SOFC) or protonic ceramic fuel cells (PCFC). As they can both convert between electricity and hydrogen production, fuel cells can be categorized into a total of four device types. These devices are applicable in hydrogen fuel cell vehicles, hydrogen charging stations, and power generation systems, and are henceforth emerging as core next-generation technologies for a carbon-neutral society.
However, these devices have a chronic problem where the speed of their slowest reaction would decrease with a drop of driving temperature, which greatly reduces device efficiency. Various studies have been conducted to solve this, but most reported that electrode materials have low catalytic activity and their applications are limited to specific devices, which limits them from being used as SOFCs that require reversible power conversion and hydrogen production.
< Figure 1. Schematic diagram of high-performance oxygen ion conductive solid oxide fuel cell (SOFC) and proton conductive ceramic fuel cell (PCFC) operates with the new universal electrodes >
To solve this issue, the research team doped a perovskite oxide material with Ta5+, a high valence ion that did not receive much attention in the field. Through this, the team successfully stabilized what is usually a highly unstable crystal structure, and confirmed that catalytic activity improved by 100 times.
The electrode material developed by the team was applied to all four of the mentioned device types. Furthermore, their efficiencies were greater than any of the devices reported thus far, and showed excellent performance by stably running for much longer (700 hours) compared to existing materials that deteriorated within the first 100 hours of operation.
< Figure 2. (a) Power conversion and hydrogen production performance chart for the protonic ceramic fuel cell (PCFC) with the new universal electrodes (b) and performance comparison with other reported devices >
This research, in which KAIST’s Ph.D. candidates Dongyeon Kim and Sejong Ahn, and Professor Jun Hyuk Kim from Hongik University contributed as co-first authors, was published in the internationally renowned Energy & Environmental Science under the title, "Oxygen-Electrode for Reversible Solid Oxide Electrochemical Cells at Reduced Temperatures".
Prof. WooChul Jung said, “We broke free from the idea that we must develop a completely new material to solve an existing problem, and instead suggested a way to control the crystal structure of a lesser-known material to develop a high-efficiency fuel cell, and that’s what makes these results more significant.”
Prof. Kang Taek Lee added, “Unlike previously reported materials that could only be applied to one device type at a time, our material has the flexibility of being applicable to all four. We therefore look forward to its contribution in the commercialization of eco-friendly energy technology including fuel cells and water-splitting equipment for hydrogen production.”
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Ministry of Science and ICT.
2023.08.22 View 8932 -
 A KAIST Research Team Develops Diesel Reforming Catalyst Enabling Hydrogen Production for Future Mobile Fuel Cells
This catalyst capability allowing stable hydrogen production from commercial diesel is expected to be applied in mobile fuel cell systems in the future hydrogen economy
On August 16, a joint research team led by Professors Joongmyeon Bae and Kang Taek Lee of KAIST’s Department of Mechanical Engineering and Dr. Chan-Woo Lee of Korea Institute of Energy Research (KIER) announced the successful development of a highly active and durable reforming catalyst allowing hydrogen production from commercial diesel.
Fuel reforming is a hydrogen production technique that extracts hydrogen from hydrocarbons through catalytic reactions. Diesel, being a liquid fuel, has a high storage density for hydrogen and is easy to transport and store. There have therefore been continuous research efforts to apply hydrogel supply systems using diesel reformation in mobile fuel cells, such as for auxiliary power in heavy trucks or air-independent propulsion (AIP) systems in submarines.
However, diesel is a mixture of high hydrocarbons including long-chained paraffin, double-bonded olefin, and aromatic hydrocarbons with benzene groups, and it requires a highly active catalyst to effectively break them down. In addition, the catalyst must be extremely durable against caulking and sintering, as they are often the main causes of catalyst degradation. Such challenges have limited the use of diesel reformation technologies to date.
The joint research team successfully developed a highly active and durable diesel reforming catalyst through elution (a heat treatment method used to uniformly grow active metals retained in an oxide support as ions in the form of metal nanoparticles), forming alloy nanoparticles. The design was based on the fact that eluted nanoparticles strongly interact with the support, allowing a high degree of dispersion at high temperatures, and that producing an alloy from dissimilar metals can increase the performance of catalysts through a synergistic effect.
The research team introduced a solution combustion synthesis method to produce a multi-component catalyst with a trace amount of platinum (Pt) and ruthenium (Ru) penetrated into a ceria (CeO2) lattice, which is a structure commonly used as a support for catalysts in redox reactions. When exposed to a diesel reforming reaction environment, the catalyst induces Pt-Ru alloy nanoparticle formation upon Pt and Ru elution onto the support surface.
In addition to the catalyst analysis, the research team also succeeded in characterizing the behaviour of active metal elution and alloy formation from an energetic perspective using a density functional theory-based calculation. In a performance comparison test between the Pt-Ru alloy catalyst against existing single-metal catalysts, the reforming activity was shown to have improved, as it showed a 100% fuel conversion rate even at a low temperature (600oC, compared to the original 800oC). In a long-term durability test (800oC, 200 hours), the catalyst showed commercial stability by successfully producing hydrogen from commercial diesel without performance degradation.
The study was conducted by Ph.D. candidate Jaemyung Lee of KAIST’s Department of Mechanical Engineering as the first author. Ph.D. candidate Changho Yeon of KIER, Dr. Jiwoo Oh of KAIST’s Department of Mechanical Engineering, Dr. Gwangwoo Han of KIER, Ph.D. candidate Jeong Do Yoo of KAIST’s Department of Mechanical Engineering, and Dr. Hyung Joong Yun of the Korea Basic Science Institute contributed as co-authors. Dr. Chan-Woo Lee of KIER and Professors Kang Taek Lee and Joongmyeon Bae of KAIST’s Department of Mechanical Engineering contributed as corresponding authors. The research was published in the online version of Applied Catalysis B: Environmental (IF 24.319, JCR 0.93%) on June 17, under the title “Highly Active and Stable Catalyst with Exsolved PtRu Alloy Nanoparticles for Hydrogen Production via Commercial Diesel Reforming”.
Professor Joongmyeon Bae said, “The fact that hydrogen can be stably produced from commercial diesel makes this a very meaningful achievement, and we look forward to this technology contributing to the active introduction of mobile fuel cell systems in the early hydrogen economy.” He added, “Our approach to catalyst design may be applied not only to reforming reactions, but also in various other fields.”
This research was supported by the National Research Foundation of Korea through funding from the Ministry of Science, ICT and Future Planning.
Figure. Schematic diagram of high-performance diesel reforming catalyst with eluted platinum-ruthenium alloy nanoparticles and long-term durability verification experiment results for commercial diesel reforming reaction
2022.09.07 View 15555
A KAIST Research Team Develops Diesel Reforming Catalyst Enabling Hydrogen Production for Future Mobile Fuel Cells
This catalyst capability allowing stable hydrogen production from commercial diesel is expected to be applied in mobile fuel cell systems in the future hydrogen economy
On August 16, a joint research team led by Professors Joongmyeon Bae and Kang Taek Lee of KAIST’s Department of Mechanical Engineering and Dr. Chan-Woo Lee of Korea Institute of Energy Research (KIER) announced the successful development of a highly active and durable reforming catalyst allowing hydrogen production from commercial diesel.
Fuel reforming is a hydrogen production technique that extracts hydrogen from hydrocarbons through catalytic reactions. Diesel, being a liquid fuel, has a high storage density for hydrogen and is easy to transport and store. There have therefore been continuous research efforts to apply hydrogel supply systems using diesel reformation in mobile fuel cells, such as for auxiliary power in heavy trucks or air-independent propulsion (AIP) systems in submarines.
However, diesel is a mixture of high hydrocarbons including long-chained paraffin, double-bonded olefin, and aromatic hydrocarbons with benzene groups, and it requires a highly active catalyst to effectively break them down. In addition, the catalyst must be extremely durable against caulking and sintering, as they are often the main causes of catalyst degradation. Such challenges have limited the use of diesel reformation technologies to date.
The joint research team successfully developed a highly active and durable diesel reforming catalyst through elution (a heat treatment method used to uniformly grow active metals retained in an oxide support as ions in the form of metal nanoparticles), forming alloy nanoparticles. The design was based on the fact that eluted nanoparticles strongly interact with the support, allowing a high degree of dispersion at high temperatures, and that producing an alloy from dissimilar metals can increase the performance of catalysts through a synergistic effect.
The research team introduced a solution combustion synthesis method to produce a multi-component catalyst with a trace amount of platinum (Pt) and ruthenium (Ru) penetrated into a ceria (CeO2) lattice, which is a structure commonly used as a support for catalysts in redox reactions. When exposed to a diesel reforming reaction environment, the catalyst induces Pt-Ru alloy nanoparticle formation upon Pt and Ru elution onto the support surface.
In addition to the catalyst analysis, the research team also succeeded in characterizing the behaviour of active metal elution and alloy formation from an energetic perspective using a density functional theory-based calculation. In a performance comparison test between the Pt-Ru alloy catalyst against existing single-metal catalysts, the reforming activity was shown to have improved, as it showed a 100% fuel conversion rate even at a low temperature (600oC, compared to the original 800oC). In a long-term durability test (800oC, 200 hours), the catalyst showed commercial stability by successfully producing hydrogen from commercial diesel without performance degradation.
The study was conducted by Ph.D. candidate Jaemyung Lee of KAIST’s Department of Mechanical Engineering as the first author. Ph.D. candidate Changho Yeon of KIER, Dr. Jiwoo Oh of KAIST’s Department of Mechanical Engineering, Dr. Gwangwoo Han of KIER, Ph.D. candidate Jeong Do Yoo of KAIST’s Department of Mechanical Engineering, and Dr. Hyung Joong Yun of the Korea Basic Science Institute contributed as co-authors. Dr. Chan-Woo Lee of KIER and Professors Kang Taek Lee and Joongmyeon Bae of KAIST’s Department of Mechanical Engineering contributed as corresponding authors. The research was published in the online version of Applied Catalysis B: Environmental (IF 24.319, JCR 0.93%) on June 17, under the title “Highly Active and Stable Catalyst with Exsolved PtRu Alloy Nanoparticles for Hydrogen Production via Commercial Diesel Reforming”.
Professor Joongmyeon Bae said, “The fact that hydrogen can be stably produced from commercial diesel makes this a very meaningful achievement, and we look forward to this technology contributing to the active introduction of mobile fuel cell systems in the early hydrogen economy.” He added, “Our approach to catalyst design may be applied not only to reforming reactions, but also in various other fields.”
This research was supported by the National Research Foundation of Korea through funding from the Ministry of Science, ICT and Future Planning.
Figure. Schematic diagram of high-performance diesel reforming catalyst with eluted platinum-ruthenium alloy nanoparticles and long-term durability verification experiment results for commercial diesel reforming reaction
2022.09.07 View 15555 -
 Professor Bumjoon Kim Named Scientist of the Month
Professor Bumjoon Kim from the Department of Chemical and Biomolecular Engineering won January’s Scientist of the Month Award presented by the Ministry of Science and ICT (MSIT) and the National Research Foundation of Korea (NRF) on January 6. Professor Kim also received 10 million won in prize money.
Professor Kim was recognized for his research in the field of fuel cells. Since the first paper on fuel cells was published in 1839 by the German chemist Friedrich Schonbein, there has been an increase in the number of fields in which fuel cells are used, including national defense, aerospace engineering, and autonomous vehicles.
Professor Kim developed carbonized block copolymer particles with high durability and a high-performance fuel cell. Block copolymers are two different polymers cross-linked into a chain structure. Various nanostructures can be made effectively by using the attractive and repulsive forces between the chains.
Professor Kim used the membrane emulsification technique, employing a high-performance separation membrane to develop a platform that makes the mass production of highly durable carbonized particles possible, which he then used to develop high-performance energy devices like fuel cells.
The carbonized particles designed by Professor Kim and his research team were used to create the world’s more durable fuel cells that boast outstanding performance while using only five percent of the costly platinum needed for existing commercialized products.
The team’s research results were published in the Journal of the American Chemical Society and Energy Environmental Science in May and July of last year.
“We have developed a fuel cell that ticks all the boxes including performance, durability, and cost,” said Professor Kim. “Related techniques will not be limited to fuel cells, but could also be applied to the development of various energy devices like solar cells and secondary cells,” he added.
(END)
2021.01.22 View 13814
Professor Bumjoon Kim Named Scientist of the Month
Professor Bumjoon Kim from the Department of Chemical and Biomolecular Engineering won January’s Scientist of the Month Award presented by the Ministry of Science and ICT (MSIT) and the National Research Foundation of Korea (NRF) on January 6. Professor Kim also received 10 million won in prize money.
Professor Kim was recognized for his research in the field of fuel cells. Since the first paper on fuel cells was published in 1839 by the German chemist Friedrich Schonbein, there has been an increase in the number of fields in which fuel cells are used, including national defense, aerospace engineering, and autonomous vehicles.
Professor Kim developed carbonized block copolymer particles with high durability and a high-performance fuel cell. Block copolymers are two different polymers cross-linked into a chain structure. Various nanostructures can be made effectively by using the attractive and repulsive forces between the chains.
Professor Kim used the membrane emulsification technique, employing a high-performance separation membrane to develop a platform that makes the mass production of highly durable carbonized particles possible, which he then used to develop high-performance energy devices like fuel cells.
The carbonized particles designed by Professor Kim and his research team were used to create the world’s more durable fuel cells that boast outstanding performance while using only five percent of the costly platinum needed for existing commercialized products.
The team’s research results were published in the Journal of the American Chemical Society and Energy Environmental Science in May and July of last year.
“We have developed a fuel cell that ticks all the boxes including performance, durability, and cost,” said Professor Kim. “Related techniques will not be limited to fuel cells, but could also be applied to the development of various energy devices like solar cells and secondary cells,” he added.
(END)
2021.01.22 View 13814 -
 International Workshop on EEWS 2010 was held.
On October 7 and 8th at Fusion Hall of KI Building, KAIST, the 2010 International Workshop on EEWS (Energy, Environment, Water, and Sustainability) was held.
The third to be held, forty national and international academic professionals including Mark Shannon, professor at University of Illinois at Urbana-Champaign, Domen Kazunari, Tokyo University professor, Dong Sub Kim, CTO of SK Energy and Doyoung Seung, Senior Vice President of GS Caltex, participated at this year’s workshop.
In twelve sessions, themes including Artificial Photosynthesis, Wireless Power Transfer, Green Aviation, Safe Nuclear Fuel Reuse, Fuel Cells in Action, LED 2.0, Foundation of Energy-Water Nexus, and Flexible Battery & Solar Cell were presented and discussed.
“Through this workshop, current EEWS policy and research progress from different countries and the future of related technologies will be foreseen,” said Jae Kyu Lee, Dean of KAIST EEWS Initiative. “I hope it became an opportunity to create cooperative relationships with leading researchers.”
EEWS is a research project conducted by KAIST to solve global issues that mankind faces today such as depletion of energy, environmental pollution, water shortage, and sustainability.
2010.10.15 View 19462
International Workshop on EEWS 2010 was held.
On October 7 and 8th at Fusion Hall of KI Building, KAIST, the 2010 International Workshop on EEWS (Energy, Environment, Water, and Sustainability) was held.
The third to be held, forty national and international academic professionals including Mark Shannon, professor at University of Illinois at Urbana-Champaign, Domen Kazunari, Tokyo University professor, Dong Sub Kim, CTO of SK Energy and Doyoung Seung, Senior Vice President of GS Caltex, participated at this year’s workshop.
In twelve sessions, themes including Artificial Photosynthesis, Wireless Power Transfer, Green Aviation, Safe Nuclear Fuel Reuse, Fuel Cells in Action, LED 2.0, Foundation of Energy-Water Nexus, and Flexible Battery & Solar Cell were presented and discussed.
“Through this workshop, current EEWS policy and research progress from different countries and the future of related technologies will be foreseen,” said Jae Kyu Lee, Dean of KAIST EEWS Initiative. “I hope it became an opportunity to create cooperative relationships with leading researchers.”
EEWS is a research project conducted by KAIST to solve global issues that mankind faces today such as depletion of energy, environmental pollution, water shortage, and sustainability.
2010.10.15 View 19462 -
 Prof. Woo's Team Discovers Eco-Friendly Solid-Oxide Fuel Cell System
A KAIST research team led by Prof. Seong-Ihl Woo of the Department of Chemical & Biomolecular Engineering has found a method to use glycerol, a byproduct from the production of biodiesel, as fuel for solid oxide fuel cells (SOFC), university authorities said on Tuesday (Oct. 27).
The research finding shows that glycerol can be an environmentally sustainable fuel when it is used for operating SOFCs with internal reforming, instead of hydrogen and methane. The finding was published in the Oct. 14, 2009 online edition of ChemSusChem, a sister journal of Angewandte Chemie, the world"s leading chemistry journal.
Biodiesel is an attractive alternative energy source because of its low sulfur content and demand is growing worldwide as oil price soars. Bio-derived glycerol will not contribute to the greenhouse effect and has the potential to contribute to reducing global warming.
Currently, glycereol is used as a raw material in the cosmetic, pharmacy, food, and tobacco industries. However, its supply exceeds its demand as the volume of biodiesel production increases. The production of 1 ton of biodiesel produces 0.1 ton of glycerol. Many researchers have investigated various routes for the consumption of surplus glycerol.
The research is expected to contribute to sustainable growth by reducing the emissions of carbon dioxide and reusing generated carbon dioxide for the production of biomass. The new method enables manufacturers to use glycerol as a fuel for operating SOFC.
2009.10.28 View 15332
Prof. Woo's Team Discovers Eco-Friendly Solid-Oxide Fuel Cell System
A KAIST research team led by Prof. Seong-Ihl Woo of the Department of Chemical & Biomolecular Engineering has found a method to use glycerol, a byproduct from the production of biodiesel, as fuel for solid oxide fuel cells (SOFC), university authorities said on Tuesday (Oct. 27).
The research finding shows that glycerol can be an environmentally sustainable fuel when it is used for operating SOFCs with internal reforming, instead of hydrogen and methane. The finding was published in the Oct. 14, 2009 online edition of ChemSusChem, a sister journal of Angewandte Chemie, the world"s leading chemistry journal.
Biodiesel is an attractive alternative energy source because of its low sulfur content and demand is growing worldwide as oil price soars. Bio-derived glycerol will not contribute to the greenhouse effect and has the potential to contribute to reducing global warming.
Currently, glycereol is used as a raw material in the cosmetic, pharmacy, food, and tobacco industries. However, its supply exceeds its demand as the volume of biodiesel production increases. The production of 1 ton of biodiesel produces 0.1 ton of glycerol. Many researchers have investigated various routes for the consumption of surplus glycerol.
The research is expected to contribute to sustainable growth by reducing the emissions of carbon dioxide and reusing generated carbon dioxide for the production of biomass. The new method enables manufacturers to use glycerol as a fuel for operating SOFC.
2009.10.28 View 15332