AT
-
 A 20-year-old puzzle solved: KAIST research team reveals the 'three-dimensional vortex' of zero-dimensional ferroelectrics
Materials that can maintain a magnetized state by themselves without an external magnetic field (i.e., permanent magnets) are called ferromagnets. Ferroelectrics can be thought of as the electric counterpart to ferromagnets, as they maintain a polarized state without an external electric field. It is well-known that ferromagnets lose their magnetic properties when reduced to nano sizes below a certain threshold. What happens when ferroelectrics are similarly made extremely small in all directions (i.e., into a zero-dimensional structure such as nanoparticles) has been a topic of controversy for a long time.
< (From left) Professor Yongsoo Yang, the corresponding author, and Chaehwa Jeong, the first author studying in the integrated master’s and doctoral program, of the KAIST Department of Physics >
The research team led by Dr. Yongsoo Yang from the Department of Physics at KAIST has, for the first time, experimentally clarified the three-dimensional, vortex-shaped polarization distribution inside ferroelectric nanoparticles through international collaborative research with POSTECH, SNU, KBSI, LBNL and University of Arkansas.
About 20 years ago, Prof. Laurent Bellaiche (currently at University of Arkansas) and his colleagues theoretically predicted that a unique form of polarization distribution, arranged in a toroidal vortex shape, could occur inside ferroelectric nanodots. They also suggested that if this vortex distribution could be properly controlled, it could be applied to ultra-high-density memory devices with capacities over 10,000 times greater than existing ones. However, experimental clarification had not been achieved due to the difficulty of measuring the three-dimensional polarization distribution within ferroelectric nanostructures.
The research team at KAIST successfully solved this 20-year-old challenge by implementing a technique called atomic electron tomography. This technique works by acquiring atomic-resolution transmission electron microscope images of the nanomaterials from multiple tilt angles, and then reconstructing them back into three-dimensional structures using advanced reconstruction algorithms. Electron tomography can be understood as essentially the same method with the CT scans used in hospitals to view internal organs in three dimensions; the KAIST team adapted it uniquely for nanomaterials, utilizing an electron microscope at the single-atom level.
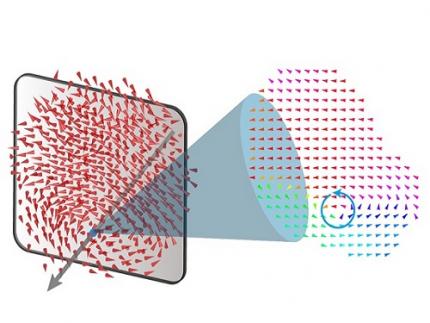
< Figure 1. Three-dimensional polarization distribution of BaTiO3 nanoparticles revealed by atomic electron tomography. >(Left) Schematic of the electron tomography technique, which involves acquiring transmission electron microscope images at multiple tilt angles and reconstructing them into 3D atomic structures.(Center) Experimentally determined three-dimensional polarization distribution inside a BaTiO3 nanoparticle via atomic electron tomography. A vortex-like structure is clearly visible near the bottom (blue dot).(Right) A two-dimensional cross-section of the polarization distribution, thinly sliced at the center of the vortex, with the color and arrows together indicating the direction of the polarization. A distinct vortex structure can be observed.
Using atomic electron tomography, the team completely measured the positions of cation atoms inside barium titanate (BaTiO3) nanoparticles, a well-known ferroelectric material, in three dimensions. From the precisely determined 3D atomic arrangements, they were able to further calculate the internal three-dimensional polarization distribution at the single-atom level. The analysis of the polarization distribution revealed, for the first time experimentally, that topological polarization orderings including vortices, anti-vortices, skyrmions, and a Bloch point occur inside the 0-dimensional ferroelectrics, as theoretically predicted 20 years ago. Furthermore, it was also found that the number of internal vortices can be controlled depending on their sizes.
Prof. Sergey Prosandeev and Prof. Bellaiche (who proposed with other co-workers the polar vortex ordering theoretically 20 years ago), joined this collaboration and further proved that the vortex distribution results obtained from experiments are consistent with theoretical calculations.
By controlling the number and orientation of these polarization distributions, it is expected that this can be utilized into next-generation high-density memory device that can store more than 10,000 times the amount of information in the same-sized device compared to existing ones.
Dr. Yang, who led the research, explained the significance of the results: “This result suggests that controlling the size and shape of ferroelectrics alone, without needing to tune the substrate or surrounding environmental effects such as epitaxial strain, can manipulate ferroelectric vortices or other topological orderings at the nano-scale. Further research could then be applied to the development of next-generation ultra-high-density memory.”
This research, with Chaehwa Jeong from the Department of Physics at KAIST as the first author, was published online in Nature Communications on May 8th (Title: Revealing the Three-Dimensional Arrangement of Polar Topology in Nanoparticles).
The study was mainly supported by the National Research Foundation of Korea (NRF) Grants funded by the Korean Government (MSIT).
2024.05.31 View 7646
A 20-year-old puzzle solved: KAIST research team reveals the 'three-dimensional vortex' of zero-dimensional ferroelectrics
Materials that can maintain a magnetized state by themselves without an external magnetic field (i.e., permanent magnets) are called ferromagnets. Ferroelectrics can be thought of as the electric counterpart to ferromagnets, as they maintain a polarized state without an external electric field. It is well-known that ferromagnets lose their magnetic properties when reduced to nano sizes below a certain threshold. What happens when ferroelectrics are similarly made extremely small in all directions (i.e., into a zero-dimensional structure such as nanoparticles) has been a topic of controversy for a long time.
< (From left) Professor Yongsoo Yang, the corresponding author, and Chaehwa Jeong, the first author studying in the integrated master’s and doctoral program, of the KAIST Department of Physics >
The research team led by Dr. Yongsoo Yang from the Department of Physics at KAIST has, for the first time, experimentally clarified the three-dimensional, vortex-shaped polarization distribution inside ferroelectric nanoparticles through international collaborative research with POSTECH, SNU, KBSI, LBNL and University of Arkansas.
About 20 years ago, Prof. Laurent Bellaiche (currently at University of Arkansas) and his colleagues theoretically predicted that a unique form of polarization distribution, arranged in a toroidal vortex shape, could occur inside ferroelectric nanodots. They also suggested that if this vortex distribution could be properly controlled, it could be applied to ultra-high-density memory devices with capacities over 10,000 times greater than existing ones. However, experimental clarification had not been achieved due to the difficulty of measuring the three-dimensional polarization distribution within ferroelectric nanostructures.
The research team at KAIST successfully solved this 20-year-old challenge by implementing a technique called atomic electron tomography. This technique works by acquiring atomic-resolution transmission electron microscope images of the nanomaterials from multiple tilt angles, and then reconstructing them back into three-dimensional structures using advanced reconstruction algorithms. Electron tomography can be understood as essentially the same method with the CT scans used in hospitals to view internal organs in three dimensions; the KAIST team adapted it uniquely for nanomaterials, utilizing an electron microscope at the single-atom level.
< Figure 1. Three-dimensional polarization distribution of BaTiO3 nanoparticles revealed by atomic electron tomography. >(Left) Schematic of the electron tomography technique, which involves acquiring transmission electron microscope images at multiple tilt angles and reconstructing them into 3D atomic structures.(Center) Experimentally determined three-dimensional polarization distribution inside a BaTiO3 nanoparticle via atomic electron tomography. A vortex-like structure is clearly visible near the bottom (blue dot).(Right) A two-dimensional cross-section of the polarization distribution, thinly sliced at the center of the vortex, with the color and arrows together indicating the direction of the polarization. A distinct vortex structure can be observed.
Using atomic electron tomography, the team completely measured the positions of cation atoms inside barium titanate (BaTiO3) nanoparticles, a well-known ferroelectric material, in three dimensions. From the precisely determined 3D atomic arrangements, they were able to further calculate the internal three-dimensional polarization distribution at the single-atom level. The analysis of the polarization distribution revealed, for the first time experimentally, that topological polarization orderings including vortices, anti-vortices, skyrmions, and a Bloch point occur inside the 0-dimensional ferroelectrics, as theoretically predicted 20 years ago. Furthermore, it was also found that the number of internal vortices can be controlled depending on their sizes.
Prof. Sergey Prosandeev and Prof. Bellaiche (who proposed with other co-workers the polar vortex ordering theoretically 20 years ago), joined this collaboration and further proved that the vortex distribution results obtained from experiments are consistent with theoretical calculations.
By controlling the number and orientation of these polarization distributions, it is expected that this can be utilized into next-generation high-density memory device that can store more than 10,000 times the amount of information in the same-sized device compared to existing ones.
Dr. Yang, who led the research, explained the significance of the results: “This result suggests that controlling the size and shape of ferroelectrics alone, without needing to tune the substrate or surrounding environmental effects such as epitaxial strain, can manipulate ferroelectric vortices or other topological orderings at the nano-scale. Further research could then be applied to the development of next-generation ultra-high-density memory.”
This research, with Chaehwa Jeong from the Department of Physics at KAIST as the first author, was published online in Nature Communications on May 8th (Title: Revealing the Three-Dimensional Arrangement of Polar Topology in Nanoparticles).
The study was mainly supported by the National Research Foundation of Korea (NRF) Grants funded by the Korean Government (MSIT).
2024.05.31 View 7646 -
 KAIST and Merck Sign MOU to Boost Biotech Innovation
< (From left) KAIST President Kwang-Hyung Lee and Merck CEO Matthias Heinzel >
KAIST (President Kwang-Hyung Lee) signed a Memorandum of Understanding (MOU) with Merck Life Science (CEO Matthias Heinzel) on May 29 to foster innovation and technology creation in advanced biotechnology.
Since May of last year, the two institutions have been discussing multidimensional innovation programs and will now focus on industry-academia cooperation to tackle bioindustry challenges with this MOU as a foundation.
KAIST will conduct joint research projects in various advanced biotechnology fields, such as synthetic biology, mRNA, cell line engineering, and organoids, using the chemical and biological portfolios provided by Merck.
Additionally, KAIST will establish an Experience Lab in collaboration with the Department of Materials Science and Engineering and the Graduate School of Medical Science and Engineering. This lab will support the discovery and analysis of candidate substances in materials science and biology.
Programs to enhance researchers' capabilities will also be offered. Scholarships for graduate students and awards for professors will be implemented. Researchers will have opportunities to participate in global academic events and educational programs hosted by Merck, such as the Curious 2024 Future Insight Conference and the Innovation Cup.
M Ventures, a venture capital subsidiary of Merck Group, will collaborate with KAIST's startup institute to support technology commercialization and continue to develop their startup ecosystem.
The signing ceremony at KAIST's main campus in Daejeon was attended by the CEO of Merck Life Science and the President of KAIST along with representatives from both institutions.
Matthias Heinzel, a member of the Executive Board of Merck and CEO Life Science, said, “This agreement with KAIST is a significant step toward accelerating the development of the life science industry both in Korea and globally. Advancing life science research and fostering the next generation of scientists is essential for discovering new medicines to meet global health needs.”
President Kwang-Hyung Lee responded, “We are pleased to share a vision for scientific advancement with Merck, a leading global technology company. We anticipate that this partnership will strengthen the connection between Merck’s life science business and the global scientific community.”
In March, Merck, a global science and technology company with over 350 years of history, announced a plan to invest 430 billion KRW (€300 million) to build a bioprocessing center in Daejeon, where KAIST is located. This is Merck's largest investment in the Asia-Pacific region.
2024.05.30 View 7782
KAIST and Merck Sign MOU to Boost Biotech Innovation
< (From left) KAIST President Kwang-Hyung Lee and Merck CEO Matthias Heinzel >
KAIST (President Kwang-Hyung Lee) signed a Memorandum of Understanding (MOU) with Merck Life Science (CEO Matthias Heinzel) on May 29 to foster innovation and technology creation in advanced biotechnology.
Since May of last year, the two institutions have been discussing multidimensional innovation programs and will now focus on industry-academia cooperation to tackle bioindustry challenges with this MOU as a foundation.
KAIST will conduct joint research projects in various advanced biotechnology fields, such as synthetic biology, mRNA, cell line engineering, and organoids, using the chemical and biological portfolios provided by Merck.
Additionally, KAIST will establish an Experience Lab in collaboration with the Department of Materials Science and Engineering and the Graduate School of Medical Science and Engineering. This lab will support the discovery and analysis of candidate substances in materials science and biology.
Programs to enhance researchers' capabilities will also be offered. Scholarships for graduate students and awards for professors will be implemented. Researchers will have opportunities to participate in global academic events and educational programs hosted by Merck, such as the Curious 2024 Future Insight Conference and the Innovation Cup.
M Ventures, a venture capital subsidiary of Merck Group, will collaborate with KAIST's startup institute to support technology commercialization and continue to develop their startup ecosystem.
The signing ceremony at KAIST's main campus in Daejeon was attended by the CEO of Merck Life Science and the President of KAIST along with representatives from both institutions.
Matthias Heinzel, a member of the Executive Board of Merck and CEO Life Science, said, “This agreement with KAIST is a significant step toward accelerating the development of the life science industry both in Korea and globally. Advancing life science research and fostering the next generation of scientists is essential for discovering new medicines to meet global health needs.”
President Kwang-Hyung Lee responded, “We are pleased to share a vision for scientific advancement with Merck, a leading global technology company. We anticipate that this partnership will strengthen the connection between Merck’s life science business and the global scientific community.”
In March, Merck, a global science and technology company with over 350 years of history, announced a plan to invest 430 billion KRW (€300 million) to build a bioprocessing center in Daejeon, where KAIST is located. This is Merck's largest investment in the Asia-Pacific region.
2024.05.30 View 7782 -
 Novel High-performance and Sustainable Paper Coating Material created by KAIST-Yonsei University Research Team to reduce microplastic pollution
What if there is a biodegradable packaging material with high performance without leaving microplastics?
Plastic pollution presents a global challenge that must be solved. In particular, packaging accounts for 30-50% of the total plastic consumption. While paper packaging is eco-friendly, it lacks crucial functionalities like moisture resistance and strength. Traditional coating materials exacerbate plastic pollution, prompting the need for sustainable alternatives.
Polyethylene (PE) and ethylene vinyl alcohol (EVOH) are typically used as coating materials to improve the low barrier properties of paper packaging, but these substances do not decompose and worsen microplastic pollution when disposed of in the natural environment. In response to this problem, packaging materials made from bio-based substances and biodegradable plastics have been developed, but in most cases, as the packaging performance improves, the biodegradability diminishes rapidly.
KAIST announced that a joint research team led by Professor Jaewook Myung of the Department of Civil and Environmental Engineering, Professor Hanseul Yang of the Department of Life Sciences, and Professor Jongcheol Seo of the Department of Packaging and Logistics <Figure 4. Back cover art of Green Chemistry journal of the latest volume, describing the boric acid cross-linked poly(vinyl alcohol) coated paper featuring marine biodegradability, biocompatibility, high barrier properties, and robustness developed through this study.>
at Yonsei University tackled the challenge of balancing packaging performance and sustainability. They successfully developed a sustainable, marine biodegradable, high-performance paper coating material.
* Biodegradable plastic: A plastic that can be decomposed by microorganisms in natural environments such as soil and ocean or artificial conditions such as industrial composting and anaerobic digestion by microorganisms.
*Microplastics: Tiny pieces of plastic less than 5 mm, produced during the decomposition of bulk plastic materials. Microplastics can persist in the sea for more than decades, causing severe marine pollution.
The team utilized boric acid-crosslinked poly(vinyl alcohol) (PVA), a biodegradable plastic, to coat the paper, thereby enhancing its biodegradability, barrier properties, and strength. The resulting coated paper exhibited superior performance compared to conventional plastics, with excellent barrier properties and physical strength, even in humid conditions.
<Figure 1. (a) Chemical structure of boric acid-crosslinked poly(vinyl alcohol) coating on paper, (b-c) Oxygen and water vapor barrier properties, (d-f) Tensile strength in dry and moist conditions. OTR: Oxygen transmission rate, WVTR: Water vapor transmission rate.>
The team also conducted an in-depth examination of biodegradation and biocompatibility to systematically evaluate the sustainability of the newly developed coated paper. Biodegradation was assessed by simulating the marine environment, known for its challenging biodegradability conditions. The team employed a respiratory system-based bioreactor to measure the degree of carbon mineralization into carbon dioxide. After 111 days of biodegradation, it was found that the coated papers achieved 59-82% biodegradation depending on the coating component. The phenomenon in which marine bacteria are decomposing the coating material was captured through a scanning electron microscope. In addition, in vitro biocompatibility was confirmed through human embryonic kidney and mouse embryonic fibroblast cells, as well as high in-vivo biocompatibility of the coated paper was verified through mouse experiments.
Through this study, the joint research team proposed a coating strategy that can improve packaging performance while upholding sustainability to address the drawbacks of paper packaging. The boric acid-crosslinked PVA-coated paper eliminates the need for artificial composting conditions or sewage treatment facilities. Being biodegradable in natural environments and characterized by low toxicity, this newly coated paper does not exacerbate environmental pollution when accidentally discarded. Thus, it presents a sustainable substitute for plastic packaging materials.
<Figure 2. (a) Normal paper and boric acid-crosslinked poly(vinyl alcohol) coated paper, (b) Biodegradation of the coated paper by marine bacteria, (c) Result of cytotoxicity test using human embryonic kidney and mouse embryonic fibroblast cells. (d) Vital organs after one-month exposure of the coated papers to mice.>
Professor Jaewook Myung at KAIST, who led the sustainability study of coated paper, said, "The development of a marine biodegradable high-performance paper coating is the result of combining the innovative technologies of three leading research teams in each field." He said, “We will continue to develop sustainable materials with excellent performance.” Meanwhile, Professor Jongchul Seo of Yonsei University, who led the research on the development of high-performance paper coating, mentioned, “Through this research, we have developed a sustainable paper packaging technology that can replace non-degradable plastic packaging, and we expect the research outcome will be applied in industry,”.
<Figure 3. End-of-life scenario of papers coated by BA-crosslinked PVA in the marine environment. The coated papers potentially be disintegrated by marine microorganisms and ocean waves and tides. The depolymerization of PVA coating and paper is then mediated by extracellular depolymerases such as oxidases and cellulases, after which the small subunits (oligomers and monomers) are assimilated by microbial cells. The carbon components in the coated papers are ultimately mineralized into CO2, posing no harm in the ocean.>
The work was published in Green Chemistry and Food Chemistry journals. This study was conducted with the support of the Korea Research Foundation and the Korea Institute for Agriculture, Food and Rural Affairs Technology Planning and Evaluation, etc.
*Title of paper published in Green Chemistry: Boric acid-crosslinked poly(vinyl alcohol): biodegradable, biocompatible, robust, and high-barrier paper coating
※ Selected as the article for the back cover of the journal .
- Authors: Shinhyeong Choe, Seulki You, Kitae Park, Youngju Kim, Jehee Park, Yongjun Cho, Jongchul Seo, Hanseul Yang, and Jaewook Myung)
- Date: April 17, 2024
- DOI: 10.1039/D4GC00618F
*Title of paper published in Food Chemistry: Effect of epichlorohydrin treatment on the coating process and performance of high-barrier paper packaging
- Authors: Kitae Park, Shinhyeong Choe, Kambiz Sadeghi, Pradeep Kumar Panda, Jaewook Myung, Dowan Kim, and Jongchul Seo
- Date: February 19, 2024
- DOI: 10.1016/j.foodchem.2024.138772
<Figure 4. Back cover art of Green Chemistry journal of the latest volume, describing the boric acid cross-linked poly(vinyl alcohol) coated paper featuring marine biodegradability, biocompatibility, high barrier properties, and robustness developed through this study.>
2024.05.22 View 8201
Novel High-performance and Sustainable Paper Coating Material created by KAIST-Yonsei University Research Team to reduce microplastic pollution
What if there is a biodegradable packaging material with high performance without leaving microplastics?
Plastic pollution presents a global challenge that must be solved. In particular, packaging accounts for 30-50% of the total plastic consumption. While paper packaging is eco-friendly, it lacks crucial functionalities like moisture resistance and strength. Traditional coating materials exacerbate plastic pollution, prompting the need for sustainable alternatives.
Polyethylene (PE) and ethylene vinyl alcohol (EVOH) are typically used as coating materials to improve the low barrier properties of paper packaging, but these substances do not decompose and worsen microplastic pollution when disposed of in the natural environment. In response to this problem, packaging materials made from bio-based substances and biodegradable plastics have been developed, but in most cases, as the packaging performance improves, the biodegradability diminishes rapidly.
KAIST announced that a joint research team led by Professor Jaewook Myung of the Department of Civil and Environmental Engineering, Professor Hanseul Yang of the Department of Life Sciences, and Professor Jongcheol Seo of the Department of Packaging and Logistics <Figure 4. Back cover art of Green Chemistry journal of the latest volume, describing the boric acid cross-linked poly(vinyl alcohol) coated paper featuring marine biodegradability, biocompatibility, high barrier properties, and robustness developed through this study.>
at Yonsei University tackled the challenge of balancing packaging performance and sustainability. They successfully developed a sustainable, marine biodegradable, high-performance paper coating material.
* Biodegradable plastic: A plastic that can be decomposed by microorganisms in natural environments such as soil and ocean or artificial conditions such as industrial composting and anaerobic digestion by microorganisms.
*Microplastics: Tiny pieces of plastic less than 5 mm, produced during the decomposition of bulk plastic materials. Microplastics can persist in the sea for more than decades, causing severe marine pollution.
The team utilized boric acid-crosslinked poly(vinyl alcohol) (PVA), a biodegradable plastic, to coat the paper, thereby enhancing its biodegradability, barrier properties, and strength. The resulting coated paper exhibited superior performance compared to conventional plastics, with excellent barrier properties and physical strength, even in humid conditions.
<Figure 1. (a) Chemical structure of boric acid-crosslinked poly(vinyl alcohol) coating on paper, (b-c) Oxygen and water vapor barrier properties, (d-f) Tensile strength in dry and moist conditions. OTR: Oxygen transmission rate, WVTR: Water vapor transmission rate.>
The team also conducted an in-depth examination of biodegradation and biocompatibility to systematically evaluate the sustainability of the newly developed coated paper. Biodegradation was assessed by simulating the marine environment, known for its challenging biodegradability conditions. The team employed a respiratory system-based bioreactor to measure the degree of carbon mineralization into carbon dioxide. After 111 days of biodegradation, it was found that the coated papers achieved 59-82% biodegradation depending on the coating component. The phenomenon in which marine bacteria are decomposing the coating material was captured through a scanning electron microscope. In addition, in vitro biocompatibility was confirmed through human embryonic kidney and mouse embryonic fibroblast cells, as well as high in-vivo biocompatibility of the coated paper was verified through mouse experiments.
Through this study, the joint research team proposed a coating strategy that can improve packaging performance while upholding sustainability to address the drawbacks of paper packaging. The boric acid-crosslinked PVA-coated paper eliminates the need for artificial composting conditions or sewage treatment facilities. Being biodegradable in natural environments and characterized by low toxicity, this newly coated paper does not exacerbate environmental pollution when accidentally discarded. Thus, it presents a sustainable substitute for plastic packaging materials.
<Figure 2. (a) Normal paper and boric acid-crosslinked poly(vinyl alcohol) coated paper, (b) Biodegradation of the coated paper by marine bacteria, (c) Result of cytotoxicity test using human embryonic kidney and mouse embryonic fibroblast cells. (d) Vital organs after one-month exposure of the coated papers to mice.>
Professor Jaewook Myung at KAIST, who led the sustainability study of coated paper, said, "The development of a marine biodegradable high-performance paper coating is the result of combining the innovative technologies of three leading research teams in each field." He said, “We will continue to develop sustainable materials with excellent performance.” Meanwhile, Professor Jongchul Seo of Yonsei University, who led the research on the development of high-performance paper coating, mentioned, “Through this research, we have developed a sustainable paper packaging technology that can replace non-degradable plastic packaging, and we expect the research outcome will be applied in industry,”.
<Figure 3. End-of-life scenario of papers coated by BA-crosslinked PVA in the marine environment. The coated papers potentially be disintegrated by marine microorganisms and ocean waves and tides. The depolymerization of PVA coating and paper is then mediated by extracellular depolymerases such as oxidases and cellulases, after which the small subunits (oligomers and monomers) are assimilated by microbial cells. The carbon components in the coated papers are ultimately mineralized into CO2, posing no harm in the ocean.>
The work was published in Green Chemistry and Food Chemistry journals. This study was conducted with the support of the Korea Research Foundation and the Korea Institute for Agriculture, Food and Rural Affairs Technology Planning and Evaluation, etc.
*Title of paper published in Green Chemistry: Boric acid-crosslinked poly(vinyl alcohol): biodegradable, biocompatible, robust, and high-barrier paper coating
※ Selected as the article for the back cover of the journal .
- Authors: Shinhyeong Choe, Seulki You, Kitae Park, Youngju Kim, Jehee Park, Yongjun Cho, Jongchul Seo, Hanseul Yang, and Jaewook Myung)
- Date: April 17, 2024
- DOI: 10.1039/D4GC00618F
*Title of paper published in Food Chemistry: Effect of epichlorohydrin treatment on the coating process and performance of high-barrier paper packaging
- Authors: Kitae Park, Shinhyeong Choe, Kambiz Sadeghi, Pradeep Kumar Panda, Jaewook Myung, Dowan Kim, and Jongchul Seo
- Date: February 19, 2024
- DOI: 10.1016/j.foodchem.2024.138772
<Figure 4. Back cover art of Green Chemistry journal of the latest volume, describing the boric acid cross-linked poly(vinyl alcohol) coated paper featuring marine biodegradability, biocompatibility, high barrier properties, and robustness developed through this study.>
2024.05.22 View 8201 -
 KAIST begins full-scale cooperation with Taiwan’s Formosa Group
< (From left) Senior Vice President for Planning and Budget Kyung-Soo Kim, and Professor Minee Choi of the Department of Brain and Cognitive Sciences of KAIST along with Chairman of Formosa Group Sandy Wang and KAIST President Kwang-Hyung Lee, and Dean Daesoo Kim of KAIST College of Life Science and Bioengineering >
KAIST is pursuing cooperation in the fields of advanced biotechnology and eco-friendly energy with Formosa Plastics Group, one of Taiwan's three largest companies.
To this end, Chairman Sandy Wang, a member of Formosa Group's standing committee and leader of the group's bio and eco-friendly energy sector, will visit KAIST on the 13th of this month. This is the first time that the owner of Formosa Group has made an official visit to KAIST.
Cooperation between the two institutions began last March when our university signed a memorandum of understanding on comprehensive exchange and cooperation with Ming Chi University of Science and Technology (明志科技大學), Chang Gung University(長庚大學), and Chang Gung Memorial Hospital(長庚記念醫院), three of many institutions established and supported by Formosa Group.
Based on this, Chairman Sandy Wang, who visits our university to promote more exchanges and cooperation, talked about ‘the education of children and corporate social return and practice of his father, Chairman Yung-Ching Wang,’ through a special lecture for the school leadership as a part of the Monthly Lecture on KAIST’s Leadership Innovation Day.
She then visited KAIST's research and engineering facilities related to Taiwan's future industries, such as advanced biotechnology and eco-friendly energy, and discussed global industry-academic cooperation plans. In the future, the two organizations plan to appoint adjunct professors and promote practical global cooperation, including joint student guidance and research cooperation. We plan to pursue effective mid- to long-term cooperation, such as conducting battery application research with the KAIST Next-Generation ESS Research Center and opening a graduate program specialized in stem cell and gene editing technology in connection with Chang Gung University and Chang Gung Memorial Hospital. The newly established cooperative relationship will also promote Formosa Group's investment and cooperation with KAIST's outstanding venture companies related to bio and eco-friendly energy to lay the foundation for innovative industrial cooperation between Taiwan and Korea.
President Kwang-Hyung Lee said, “The Formosa Group has a global network, so we regard it to be a key partner that will position KAIST’s bio and engineering technology in the global stages.” He also said, “With Chairman Sandy Wang’s visit, Taiwan is emerging as a global economic powerhouse,” and added, “We expect to continue our close cooperative relationship with the company.”
Formosa Group is a company founded by the late Chairman Yung-Ching Wang, the father of Chairman Sandy Wang. As the world's No. 1 plastic PVC producer, it is leading the core industries of Taiwan's economy, including semiconductors, steel, heavy industry, bio, and batteries. Chairman Yung-Ching Wang was respected by the Taiwanese people by setting an example of returning his wealth to society under the belief that the companies and assets he built ‘belonged to the people.’ Chang Gung University, Chang Gung Memorial Hospital, and Ming Chi University of Technology, which are pursuing cooperation with our university, were also established as part of the social contribution promoted by Chairman Yung-Ching Wang and are receiving financial support from Formosa Group.
2024.05.09 View 6700
KAIST begins full-scale cooperation with Taiwan’s Formosa Group
< (From left) Senior Vice President for Planning and Budget Kyung-Soo Kim, and Professor Minee Choi of the Department of Brain and Cognitive Sciences of KAIST along with Chairman of Formosa Group Sandy Wang and KAIST President Kwang-Hyung Lee, and Dean Daesoo Kim of KAIST College of Life Science and Bioengineering >
KAIST is pursuing cooperation in the fields of advanced biotechnology and eco-friendly energy with Formosa Plastics Group, one of Taiwan's three largest companies.
To this end, Chairman Sandy Wang, a member of Formosa Group's standing committee and leader of the group's bio and eco-friendly energy sector, will visit KAIST on the 13th of this month. This is the first time that the owner of Formosa Group has made an official visit to KAIST.
Cooperation between the two institutions began last March when our university signed a memorandum of understanding on comprehensive exchange and cooperation with Ming Chi University of Science and Technology (明志科技大學), Chang Gung University(長庚大學), and Chang Gung Memorial Hospital(長庚記念醫院), three of many institutions established and supported by Formosa Group.
Based on this, Chairman Sandy Wang, who visits our university to promote more exchanges and cooperation, talked about ‘the education of children and corporate social return and practice of his father, Chairman Yung-Ching Wang,’ through a special lecture for the school leadership as a part of the Monthly Lecture on KAIST’s Leadership Innovation Day.
She then visited KAIST's research and engineering facilities related to Taiwan's future industries, such as advanced biotechnology and eco-friendly energy, and discussed global industry-academic cooperation plans. In the future, the two organizations plan to appoint adjunct professors and promote practical global cooperation, including joint student guidance and research cooperation. We plan to pursue effective mid- to long-term cooperation, such as conducting battery application research with the KAIST Next-Generation ESS Research Center and opening a graduate program specialized in stem cell and gene editing technology in connection with Chang Gung University and Chang Gung Memorial Hospital. The newly established cooperative relationship will also promote Formosa Group's investment and cooperation with KAIST's outstanding venture companies related to bio and eco-friendly energy to lay the foundation for innovative industrial cooperation between Taiwan and Korea.
President Kwang-Hyung Lee said, “The Formosa Group has a global network, so we regard it to be a key partner that will position KAIST’s bio and engineering technology in the global stages.” He also said, “With Chairman Sandy Wang’s visit, Taiwan is emerging as a global economic powerhouse,” and added, “We expect to continue our close cooperative relationship with the company.”
Formosa Group is a company founded by the late Chairman Yung-Ching Wang, the father of Chairman Sandy Wang. As the world's No. 1 plastic PVC producer, it is leading the core industries of Taiwan's economy, including semiconductors, steel, heavy industry, bio, and batteries. Chairman Yung-Ching Wang was respected by the Taiwanese people by setting an example of returning his wealth to society under the belief that the companies and assets he built ‘belonged to the people.’ Chang Gung University, Chang Gung Memorial Hospital, and Ming Chi University of Technology, which are pursuing cooperation with our university, were also established as part of the social contribution promoted by Chairman Yung-Ching Wang and are receiving financial support from Formosa Group.
2024.05.09 View 6700 -
 Dr. Bong-Joon Jung Elected as Interim President of KAIST U.S. Alumni Association
On April 29th, 2024, the Office of Global Initiative at KAIST, led by Vice President Man-Sung Yim, successfully conducted the election for the interim president of the KAIST U.S. Alumni Association. The authority to organize and operate the election was delegated by the KAIST U.S. Alumni Interim President Selection Committee Foundation, consisting of Je Woo Kim, Director of KAIST U.S. Foundation; Terri Kim, Director of KAIST U.S. Foundation; and Myungjin Yim, President of the KAIST Silicon Valley Alumni Association.
The election was announced during the KAIST Online U.S. Alumni Event held on December 8th, 2023, with the aim of establishing interim executive principles for six months and ultimately forming the official executive body of the KAIST U.S. Alumni Association.
The election was conducted according to the requirements set by the KAIST U.S. Alumni Interim President Selection Committee Foundation, which stated that "if there is a single candidate, they will be elected if they receive more than half of the votes from participating voters." The voting period lasted from April 13th (Monday) to April 24th (Friday) and was specifically directed towards KAIST alumni living in the Americas.
All 50 eligible voters participated in the election, unanimously supporting Dr. Bong-Joon Jung (Ph.D., admitted in 1993, Department of Electrical Engineering) with a 100% approval rate as the interim president of the KAIST U.S. Alumni Association. Dr. Bong-Joon Jung currently serves as the Senior Technology Analyst and Partner at Ambex Venture Group and previously served as the President of the KAIST Silicon Valley Alumni Association. Dr. Jung expressed his intention to strengthen connections with KAIST alumni in the United States and contribute to the establishment of the U.S. alumni association as the interim president.
< Photo. Newly elected president of interim KAIST U.S. Alumni Association, Dr. Bong-Joon Jung. >
Man-Sung Yim, Vice President of the International Office, congratulated Dr. Bong-Joon Jung on his election and pledged continuous support for the activities of the interim KAIST U.S. Alumni Association, led by alumni. He stated, "The election of the interim president of the KAIST U.S. Alumni Association marks the beginning of active engagement not only from alumni associations in various regions but also from alumni across the Americas, reflecting sustained interest in their alma mater."
2024.04.29 View 5492
Dr. Bong-Joon Jung Elected as Interim President of KAIST U.S. Alumni Association
On April 29th, 2024, the Office of Global Initiative at KAIST, led by Vice President Man-Sung Yim, successfully conducted the election for the interim president of the KAIST U.S. Alumni Association. The authority to organize and operate the election was delegated by the KAIST U.S. Alumni Interim President Selection Committee Foundation, consisting of Je Woo Kim, Director of KAIST U.S. Foundation; Terri Kim, Director of KAIST U.S. Foundation; and Myungjin Yim, President of the KAIST Silicon Valley Alumni Association.
The election was announced during the KAIST Online U.S. Alumni Event held on December 8th, 2023, with the aim of establishing interim executive principles for six months and ultimately forming the official executive body of the KAIST U.S. Alumni Association.
The election was conducted according to the requirements set by the KAIST U.S. Alumni Interim President Selection Committee Foundation, which stated that "if there is a single candidate, they will be elected if they receive more than half of the votes from participating voters." The voting period lasted from April 13th (Monday) to April 24th (Friday) and was specifically directed towards KAIST alumni living in the Americas.
All 50 eligible voters participated in the election, unanimously supporting Dr. Bong-Joon Jung (Ph.D., admitted in 1993, Department of Electrical Engineering) with a 100% approval rate as the interim president of the KAIST U.S. Alumni Association. Dr. Bong-Joon Jung currently serves as the Senior Technology Analyst and Partner at Ambex Venture Group and previously served as the President of the KAIST Silicon Valley Alumni Association. Dr. Jung expressed his intention to strengthen connections with KAIST alumni in the United States and contribute to the establishment of the U.S. alumni association as the interim president.
< Photo. Newly elected president of interim KAIST U.S. Alumni Association, Dr. Bong-Joon Jung. >
Man-Sung Yim, Vice President of the International Office, congratulated Dr. Bong-Joon Jung on his election and pledged continuous support for the activities of the interim KAIST U.S. Alumni Association, led by alumni. He stated, "The election of the interim president of the KAIST U.S. Alumni Association marks the beginning of active engagement not only from alumni associations in various regions but also from alumni across the Americas, reflecting sustained interest in their alma mater."
2024.04.29 View 5492 -
 KAIST Develops Sodium Battery Capable of Rapid Charging in Just a Few Seconds
Sodium (Na), which is over 500 times more abundant than lithium (Li), has recently garnered significant attention for its potential in sodium-ion battery technologies. However, existing sodium-ion batteries face fundamental limitations, including lower power output, constrained storage properties, and longer charging times, necessitating the development of next-generation energy storage materials.
On the 11th of April, KAIST (represented by President Kwang Hyung Lee) announced that a research team led by Professor Jeung Ku Kang from the Department of Materials Science and Engineering had developed a high-energy, high-power hybrid sodium-ion battery capable of rapid charging.
The innovative hybrid energy storage system integrates anode materials typically used in batteries with cathodes suitable for supercapacitors. This combination allows the device to achieve both high storage capacities and rapid charge-discharge rates, positioning it as a viable next-generation alternative to lithium-ion batteries.
However, the development of a hybrid battery with high energy and high power density requires an improvement to the slow energy storage rate of battery-type anodes as well as the enhancement of the relatively low capacity of supercapacitor-type cathode materials.
< Figure 1. Schematic synthetic procedures of high-capacity/high-rate anode and cathode materials for a sodium-ion hybrid energy storages (SIHES) and their proposed energy storage mechanisms. Synthetic procedures for (a) ultrafine iron sulfide-embedded S-doped carbon/graphene (FS/C/G) anode and (b) zeolitic imidazolate framework-derived porous carbon (ZDPC) cathode materials. (c) Proposed energy storage mechanisms of Na+ ions in FS/C/G anode and ClO-4 ions in ZDPC cathode for an SIHES. >
To account for this, Professor Kang's team utilized two distinct metal-organic frameworks for the optimized synthesis of hybrid batteries. This approach led to the development of an anode material with improved kinetics through the inclusion of fine active materials in porous carbon derived from metal-organic frameworks. Additionally, a high-capacity cathode material was synthesized, and the combination of the cathode and anode materials allowed for the development of a sodium-ion storage system optimizing the balance and minimizing the disparities in energy storage rates between the electrodes.
The assembled full cell, comprising the newly developed anode and cathode, forms a high-performance hybrid sodium-ion energy storage device. This device surpasses the energy density of commercial lithium-ion batteries and exhibits the characteristics of supercapacitors' power density. It is expected to be suitable for rapid charging applications ranging from electric vehicles to smart electronic devices and aerospace technologies.
< Figure 2. Electrochemical characterizations of FS/C/G-20//ZDPC SIHES full cells (left). Ragone plots for FS/C/G-20//ZDPC (this work) and other previously reported sodium-ion electrochemical energy storage devices (right). >
Professor Kang noted that the hybrid sodium-ion energy storage device, capable of rapid charging and achieving an energy density of 247 Wh/kg and a power density of 34,748 W/kg, represents a breakthrough in overcoming the current limitations of energy storage systems. He anticipates broader applications across various electronic devices, including electric vehicles.
This research, co-authored by KAIST doctoral candidates Jong Hui Choi and Dong Won Kim, was published in the international journal Energy Storage Materials on March 29 with the title "Low-crystallinity conductive multivalence iron sulfide-embedded S-doped anode and high-surface-area O-doped cathode of 3D porous N-rich graphitic carbon frameworks for high-performance sodium-ion hybrid energy storages."
The study was conducted with support from the Ministry of Science and ICT and the National Research Foundation of Korea through the Nanomaterial Technology Development Project.
2024.04.18 View 18493
KAIST Develops Sodium Battery Capable of Rapid Charging in Just a Few Seconds
Sodium (Na), which is over 500 times more abundant than lithium (Li), has recently garnered significant attention for its potential in sodium-ion battery technologies. However, existing sodium-ion batteries face fundamental limitations, including lower power output, constrained storage properties, and longer charging times, necessitating the development of next-generation energy storage materials.
On the 11th of April, KAIST (represented by President Kwang Hyung Lee) announced that a research team led by Professor Jeung Ku Kang from the Department of Materials Science and Engineering had developed a high-energy, high-power hybrid sodium-ion battery capable of rapid charging.
The innovative hybrid energy storage system integrates anode materials typically used in batteries with cathodes suitable for supercapacitors. This combination allows the device to achieve both high storage capacities and rapid charge-discharge rates, positioning it as a viable next-generation alternative to lithium-ion batteries.
However, the development of a hybrid battery with high energy and high power density requires an improvement to the slow energy storage rate of battery-type anodes as well as the enhancement of the relatively low capacity of supercapacitor-type cathode materials.
< Figure 1. Schematic synthetic procedures of high-capacity/high-rate anode and cathode materials for a sodium-ion hybrid energy storages (SIHES) and their proposed energy storage mechanisms. Synthetic procedures for (a) ultrafine iron sulfide-embedded S-doped carbon/graphene (FS/C/G) anode and (b) zeolitic imidazolate framework-derived porous carbon (ZDPC) cathode materials. (c) Proposed energy storage mechanisms of Na+ ions in FS/C/G anode and ClO-4 ions in ZDPC cathode for an SIHES. >
To account for this, Professor Kang's team utilized two distinct metal-organic frameworks for the optimized synthesis of hybrid batteries. This approach led to the development of an anode material with improved kinetics through the inclusion of fine active materials in porous carbon derived from metal-organic frameworks. Additionally, a high-capacity cathode material was synthesized, and the combination of the cathode and anode materials allowed for the development of a sodium-ion storage system optimizing the balance and minimizing the disparities in energy storage rates between the electrodes.
The assembled full cell, comprising the newly developed anode and cathode, forms a high-performance hybrid sodium-ion energy storage device. This device surpasses the energy density of commercial lithium-ion batteries and exhibits the characteristics of supercapacitors' power density. It is expected to be suitable for rapid charging applications ranging from electric vehicles to smart electronic devices and aerospace technologies.
< Figure 2. Electrochemical characterizations of FS/C/G-20//ZDPC SIHES full cells (left). Ragone plots for FS/C/G-20//ZDPC (this work) and other previously reported sodium-ion electrochemical energy storage devices (right). >
Professor Kang noted that the hybrid sodium-ion energy storage device, capable of rapid charging and achieving an energy density of 247 Wh/kg and a power density of 34,748 W/kg, represents a breakthrough in overcoming the current limitations of energy storage systems. He anticipates broader applications across various electronic devices, including electric vehicles.
This research, co-authored by KAIST doctoral candidates Jong Hui Choi and Dong Won Kim, was published in the international journal Energy Storage Materials on March 29 with the title "Low-crystallinity conductive multivalence iron sulfide-embedded S-doped anode and high-surface-area O-doped cathode of 3D porous N-rich graphitic carbon frameworks for high-performance sodium-ion hybrid energy storages."
The study was conducted with support from the Ministry of Science and ICT and the National Research Foundation of Korea through the Nanomaterial Technology Development Project.
2024.04.18 View 18493 -
 KAIST introduces microbial food as a strategy food production of the future
The global food crisis is increasing due to rapid population growth and declining food productivity to climate change. Moreover, today's food production and supply system emit a huge amount of carbon dioxide, reaching 30% of the total amount emitted by humanity, aggravating climate change. Sustainable and nutritious microbial food is attracting attention as a key to overcoming this impasse.
KAIST (President Kwang Hyung Lee) announced on April 12th that Research Professor Kyeong Rok Choi of the BioProcess Engineering Research Center and Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering published a paper that proposes a direction of research on ‘microbial food production from sustainable raw materials.’
Microbial food refers to various foods and food ingredients produced using microorganisms. Microbial biomass contains a large amount of protein per unit in dry mass, comparable to that of meat, and emits the smallest amount of carbon dioxide and is required to produce a unit mass compared to various livestock, fish, shellfish, and crops. Since the amount of water and space requirement is small, it can be an eco-friendly, sustainable and highly nutritious food resource.
Fermented foods are the most readily available microbial foods around us. Although the proportion of microbial biomass in fermented foods is small, compounds with relatively low nutritional value, such as carbohydrates, are consumed during the fermentation process, and as microorganisms proliferate, the content of nutrients with higher nutritional value, such as proteins and vitamins, increases.
Various food compounds isolated and purified from biomass or culture media obtained through microbial culture are also a branch of microbial food. Examples that can be found around us include various amino acids, including monosodium glutamate, food proteins, enzymes, flavoring compounds, food colorings, and bioactive substances.
< Figure 1. Schematic diagram portraying various microbial biomass production strategies utlizing sustainable feedstocks >
Lastly, the most ultimate and fundamental form of microbial food can be said to be microbial biomass or extracts produced through microbial culture and foods cooked using them. A representative example is single-cell protein, which collectively refers to microbial biomass or microbial proteins extracted from it.
In this paper, the researchers comprehensively covered various non-edible raw materials and strategies for using them that can be used to produce microbial food in a more sustainable way. Furthermore, it covers various microbial foods that are actually produced in the industry using the relevant raw materials and their characteristics, as well as prospects for the production and generalization of sustainable microbial foods.
Research Professor Kyeong Rok Choi, the first author of this paper, said, “Microbial foods produced from various sustainable raw materials will soon be commonly encountered at our tables.” Second author Seok Yeong Jung, a doctoral student, also said, “Microbial foods of the future will not be limited foods consumed only out of a sense of obligation to the environment, but will be complete foods that are consumed by choice because of their nutritional value and taste.” In addition, Distinguished Professor Sang Yup Lee said, “It is time for the industry and academia, as well as the public and private sectors, to cooperate more closely so that more diverse microbial foods can be developed and supplied in order to create a sustainable society for ourselves and our descendants.”
< Figure 2. Compositions and environmental footprints of animal, plant and microbial biomass. >
This paper was published online on April 9 in ‘Nature Microbiology’ published by Nature.
※ Paper title: From sustainable feedstocks to microbial foods
※ Author information: Kyeong Rok Choi (first author), Seok Yeong Jung (second author) and Sang Yup Lee (corresponding author)
This research was conducted under the development of platform technologies of microbial cell factories for the next-generation biorefineries project (project leader KAIST Distinguished Professor Sang Yup Lee) supported by the Ministry of Science and ICT and the Cooperative Research Program for Agriculture Science and Technology Development (Project leader KAIST Research Professor Kyeong Rok Choi) of the Agricultural Microbiology Project Group (Director, Professor Pahn-Shick Chang) supported by the Rural Development Administration.
2024.04.12 View 7918
KAIST introduces microbial food as a strategy food production of the future
The global food crisis is increasing due to rapid population growth and declining food productivity to climate change. Moreover, today's food production and supply system emit a huge amount of carbon dioxide, reaching 30% of the total amount emitted by humanity, aggravating climate change. Sustainable and nutritious microbial food is attracting attention as a key to overcoming this impasse.
KAIST (President Kwang Hyung Lee) announced on April 12th that Research Professor Kyeong Rok Choi of the BioProcess Engineering Research Center and Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering published a paper that proposes a direction of research on ‘microbial food production from sustainable raw materials.’
Microbial food refers to various foods and food ingredients produced using microorganisms. Microbial biomass contains a large amount of protein per unit in dry mass, comparable to that of meat, and emits the smallest amount of carbon dioxide and is required to produce a unit mass compared to various livestock, fish, shellfish, and crops. Since the amount of water and space requirement is small, it can be an eco-friendly, sustainable and highly nutritious food resource.
Fermented foods are the most readily available microbial foods around us. Although the proportion of microbial biomass in fermented foods is small, compounds with relatively low nutritional value, such as carbohydrates, are consumed during the fermentation process, and as microorganisms proliferate, the content of nutrients with higher nutritional value, such as proteins and vitamins, increases.
Various food compounds isolated and purified from biomass or culture media obtained through microbial culture are also a branch of microbial food. Examples that can be found around us include various amino acids, including monosodium glutamate, food proteins, enzymes, flavoring compounds, food colorings, and bioactive substances.
< Figure 1. Schematic diagram portraying various microbial biomass production strategies utlizing sustainable feedstocks >
Lastly, the most ultimate and fundamental form of microbial food can be said to be microbial biomass or extracts produced through microbial culture and foods cooked using them. A representative example is single-cell protein, which collectively refers to microbial biomass or microbial proteins extracted from it.
In this paper, the researchers comprehensively covered various non-edible raw materials and strategies for using them that can be used to produce microbial food in a more sustainable way. Furthermore, it covers various microbial foods that are actually produced in the industry using the relevant raw materials and their characteristics, as well as prospects for the production and generalization of sustainable microbial foods.
Research Professor Kyeong Rok Choi, the first author of this paper, said, “Microbial foods produced from various sustainable raw materials will soon be commonly encountered at our tables.” Second author Seok Yeong Jung, a doctoral student, also said, “Microbial foods of the future will not be limited foods consumed only out of a sense of obligation to the environment, but will be complete foods that are consumed by choice because of their nutritional value and taste.” In addition, Distinguished Professor Sang Yup Lee said, “It is time for the industry and academia, as well as the public and private sectors, to cooperate more closely so that more diverse microbial foods can be developed and supplied in order to create a sustainable society for ourselves and our descendants.”
< Figure 2. Compositions and environmental footprints of animal, plant and microbial biomass. >
This paper was published online on April 9 in ‘Nature Microbiology’ published by Nature.
※ Paper title: From sustainable feedstocks to microbial foods
※ Author information: Kyeong Rok Choi (first author), Seok Yeong Jung (second author) and Sang Yup Lee (corresponding author)
This research was conducted under the development of platform technologies of microbial cell factories for the next-generation biorefineries project (project leader KAIST Distinguished Professor Sang Yup Lee) supported by the Ministry of Science and ICT and the Cooperative Research Program for Agriculture Science and Technology Development (Project leader KAIST Research Professor Kyeong Rok Choi) of the Agricultural Microbiology Project Group (Director, Professor Pahn-Shick Chang) supported by the Rural Development Administration.
2024.04.12 View 7918 -
 KAIST researchers developed a novel ultra-low power memory for neuromorphic computing
A team of Korean researchers is making headlines by developing a new memory device that can be used to replace existing memory or used in implementing neuromorphic computing for next-generation artificial intelligence hardware for its low processing costs and its ultra-low power consumption.
KAIST (President Kwang-Hyung Lee) announced on April 4th that Professor Shinhyun Choi's research team in the School of Electrical Engineering has developed a next-generation phase change memory* device featuring ultra-low-power consumption that can replace DRAM and NAND flash memory.
☞ Phase change memory: A memory device that stores and/or processes information by changing the crystalline states of materials to be amorphous or crystalline using heat, thereby changing its resistance state.
Existing phase change memory has the problems such as expensive fabrication process for making highly scaled device and requiring substantial amount of power for operation. To solve these problems, Professor Choi’s research team developed an ultra-low power phase change memory device by electrically forming a very small nanometer (nm) scale phase changeable filament without expensive fabrication processes. This new development has the groundbreaking advantage of not only having a very low processing cost but also of enabling operating with ultra-low power consumption.
DRAM, one of the most popularly used memory, is very fast, but has volatile characteristics in which data disappears when the power is turned off. NAND flash memory, a storage device, has relatively slow read/write speeds, but it has non-volatile characteristic that enables it to preserve the data even when the power is cut off.
Phase change memory, on the other hand, combines the advantages of both DRAM and NAND flash memory, offering high speed and non-volatile characteristics. For this reason, phase change memory is being highlighted as the next-generation memory that can replace existing memory, and is being actively researched as a memory technology or neuromorphic computing technology that mimics the human brain.
However, conventional phase change memory devices require a substantial amount of power to operate, making it difficult to make practical large-capacity memory products or realize a neuromorphic computing system. In order to maximize the thermal efficiency for memory device operation, previous research efforts focused on reducing the power consumption by shrinking the physical size of the device through the use of the state-of-the-art lithography technologies, but they were met with limitations in terms of practicality as the degree of improvement in power consumption was minimal whereas the cost and the difficulty of fabrication increased with each improvement.
In order to solve the power consumption problem of phase change memory, Professor Shinhyun Choi’s research team created a method to electrically form phase change materials in extremely small area, successfully implementing an ultra-low-power phase change memory device that consumes 15 times less power than a conventional phase change memory device fabricated with the expensive lithography tool.
< Figure 1. Illustrations of the ultra-low power phase change memory device developed through this study and the comparison of power consumption by the newly developed phase change memory device compared to conventional phase change memory devices. >
Professor Shinhyun Choi expressed strong confidence in how this research will span out in the future in the new field of research saying, "The phase change memory device we have developed is significant as it offers a novel approach to solve the lingering problems in producing a memory device at a greatly improved manufacturing cost and energy efficiency. We expect the results of our study to become the foundation of future electronic engineering, enabling various applications including high-density three-dimensional vertical memory and neuromorphic computing systems as it opened up the possibilities to choose from a variety of materials.” He went on to add, “I would like to thank the National Research Foundation of Korea and the National NanoFab Center for supporting this research.”
This study, in which See-On Park, a student of MS-PhD Integrated Program, and Seokman Hong, a doctoral student of the School of Electrical Engineering at KAIST, participated as first authors, was published on April 4 in the April issue of the renowned international academic journal Nature. (Paper title: Phase-Change Memory via a Phase-Changeable Self-Confined Nano-Filament)
This research was conducted with support from the Next-Generation Intelligent Semiconductor Technology Development Project, PIM AI Semiconductor Core Technology Development (Device) Project, Excellent Emerging Research Program of the National Research Foundation of Korea, and the Semiconductor Process-based Nanomedical Devices Development Project of the National NanoFab Center.
2024.04.04 View 8530
KAIST researchers developed a novel ultra-low power memory for neuromorphic computing
A team of Korean researchers is making headlines by developing a new memory device that can be used to replace existing memory or used in implementing neuromorphic computing for next-generation artificial intelligence hardware for its low processing costs and its ultra-low power consumption.
KAIST (President Kwang-Hyung Lee) announced on April 4th that Professor Shinhyun Choi's research team in the School of Electrical Engineering has developed a next-generation phase change memory* device featuring ultra-low-power consumption that can replace DRAM and NAND flash memory.
☞ Phase change memory: A memory device that stores and/or processes information by changing the crystalline states of materials to be amorphous or crystalline using heat, thereby changing its resistance state.
Existing phase change memory has the problems such as expensive fabrication process for making highly scaled device and requiring substantial amount of power for operation. To solve these problems, Professor Choi’s research team developed an ultra-low power phase change memory device by electrically forming a very small nanometer (nm) scale phase changeable filament without expensive fabrication processes. This new development has the groundbreaking advantage of not only having a very low processing cost but also of enabling operating with ultra-low power consumption.
DRAM, one of the most popularly used memory, is very fast, but has volatile characteristics in which data disappears when the power is turned off. NAND flash memory, a storage device, has relatively slow read/write speeds, but it has non-volatile characteristic that enables it to preserve the data even when the power is cut off.
Phase change memory, on the other hand, combines the advantages of both DRAM and NAND flash memory, offering high speed and non-volatile characteristics. For this reason, phase change memory is being highlighted as the next-generation memory that can replace existing memory, and is being actively researched as a memory technology or neuromorphic computing technology that mimics the human brain.
However, conventional phase change memory devices require a substantial amount of power to operate, making it difficult to make practical large-capacity memory products or realize a neuromorphic computing system. In order to maximize the thermal efficiency for memory device operation, previous research efforts focused on reducing the power consumption by shrinking the physical size of the device through the use of the state-of-the-art lithography technologies, but they were met with limitations in terms of practicality as the degree of improvement in power consumption was minimal whereas the cost and the difficulty of fabrication increased with each improvement.
In order to solve the power consumption problem of phase change memory, Professor Shinhyun Choi’s research team created a method to electrically form phase change materials in extremely small area, successfully implementing an ultra-low-power phase change memory device that consumes 15 times less power than a conventional phase change memory device fabricated with the expensive lithography tool.
< Figure 1. Illustrations of the ultra-low power phase change memory device developed through this study and the comparison of power consumption by the newly developed phase change memory device compared to conventional phase change memory devices. >
Professor Shinhyun Choi expressed strong confidence in how this research will span out in the future in the new field of research saying, "The phase change memory device we have developed is significant as it offers a novel approach to solve the lingering problems in producing a memory device at a greatly improved manufacturing cost and energy efficiency. We expect the results of our study to become the foundation of future electronic engineering, enabling various applications including high-density three-dimensional vertical memory and neuromorphic computing systems as it opened up the possibilities to choose from a variety of materials.” He went on to add, “I would like to thank the National Research Foundation of Korea and the National NanoFab Center for supporting this research.”
This study, in which See-On Park, a student of MS-PhD Integrated Program, and Seokman Hong, a doctoral student of the School of Electrical Engineering at KAIST, participated as first authors, was published on April 4 in the April issue of the renowned international academic journal Nature. (Paper title: Phase-Change Memory via a Phase-Changeable Self-Confined Nano-Filament)
This research was conducted with support from the Next-Generation Intelligent Semiconductor Technology Development Project, PIM AI Semiconductor Core Technology Development (Device) Project, Excellent Emerging Research Program of the National Research Foundation of Korea, and the Semiconductor Process-based Nanomedical Devices Development Project of the National NanoFab Center.
2024.04.04 View 8530 -
 A KAIST-SNUH Team Devises a Way to Make Mathematical Predictions to find Metabolites Related to Somatic Mutations in Cancers
Cancer is characterized by abnormal metabolic processes different from those of normal cells. Therefore, cancer metabolism has been extensively studied to develop effective diagnosis and treatment strategies. Notable achievements of cancer metabolism studies include the discovery of oncometabolites* and the approval of anticancer drugs by the U.S. Food and Drug Administration (FDA) that target enzymes associated with oncometabolites. Approved anticancer drugs such as ‘Tibsovo (active ingredient: ivosidenib)’ and ‘Idhifa (active ingredient: enasidenib)’ are both used for the treatment of acute myeloid leukemia. Despite such achievements, studying cancer metabolism, especially oncometabolites, remains challenging due to time-consuming and expensive methodologies such as metabolomics. Thus, the number of confirmed oncometabolites is very small although a relatively large number of cancer-associated gene mutations have been well studied.
*Oncometabolite: A metabolite that shows pro-oncogenic function when abnormally accumulated in cancer cells. An oncometabolite is often generated as a result of gene mutations, and this accumulation promotes the growth and survival of cancer cells. Representative oncometabolites include 2-hydroxyglutarate, succinate, and fumarate.
On March 18th, a KAIST research team led by Professor Hyun Uk Kim from the Department of Chemical and Biomolecular Engineering developed a computational workflow that systematically predicts metabolites and metabolic pathways associated with somatic mutations in cancer through collaboration with research teams under Prof Youngil Koh, Prof. Hongseok Yun, and Prof. Chang Wook Jeong from Seoul National University Hospital.
The research teams have successfully reconstructed patient-specific genome-scale metabolic models (GEMs)* for 1,043 cancer patients across 24 cancer types by integrating publicly available cancer patients’ transcriptome data (i.e., from international cancer genome consortiums such as PCAWG and TCGA) into a generic human GEM. The resulting patient-specific GEMs make it possible to predict each patient’s metabolic phenotypes.
*Genome-scale metabolic model (GEM): A computational model that mathematically describes all of the biochemical reactions that take place inside a cell. It allows for the prediction of the cell’s metabolic phenotypes under various genetic and/or environmental conditions.
< Figure 1. Schematic diagram of a computational methodology for predicting metabolites and metabolic pathways associated with cancer somatic mutations. of a computational methodology for predicting metabolites and metabolic pathways associated with cancer somatic mutations. >
The team developed a four-step computational workflow using the patient-specific GEMs from 1,043 cancer patients and somatic mutation data obtained from the corresponding cancer patients. This workflow begins with the calculation of the flux-sum value of each metabolite by simulating the patient-specific GEMs. The flux-sum value quantifies the intracellular importance of a metabolite. Next, the workflow identifies metabolites that appear to be significantly associated with specific gene mutations through a statistical analysis of the predicted flux-sum data and the mutation data. Finally, the workflow selects altered metabolic pathways that significantly contribute to the biosynthesis of the predicted oncometabolite candidates, ultimately generating metabolite-gene-pathway sets as an output.
The two co-first authors, Dr. GaRyoung Lee (currently a postdoctoral fellow at the Dana-Farber Cancer Institute and Harvard Medical School) and Dr. Sang Mi Lee (currently a postdoctoral fellow at Harvard Medical School) said, “The computational workflow developed can systematically predict how genetic mutations affect cellular metabolism through metabolic pathways. Importantly, it can easily be applied to different types of cancer based on the mutation and transcriptome data of cancer patient cohorts.”
Prof. Kim said, “The computational workflow and its resulting prediction outcomes will serve as the groundwork for identifying novel oncometabolites and for facilitating the development of various treatment and diagnosis strategies”.
This study, which was supported by the National Research Foundation of Korea, has been published online in Genome Biology, a representative journal in the field of biotechnology and genetics, under the title "Prediction of metabolites associated with somatic mutations in cancers by using genome‑scale metabolic models and mutation data".
2024.03.18 View 7029
A KAIST-SNUH Team Devises a Way to Make Mathematical Predictions to find Metabolites Related to Somatic Mutations in Cancers
Cancer is characterized by abnormal metabolic processes different from those of normal cells. Therefore, cancer metabolism has been extensively studied to develop effective diagnosis and treatment strategies. Notable achievements of cancer metabolism studies include the discovery of oncometabolites* and the approval of anticancer drugs by the U.S. Food and Drug Administration (FDA) that target enzymes associated with oncometabolites. Approved anticancer drugs such as ‘Tibsovo (active ingredient: ivosidenib)’ and ‘Idhifa (active ingredient: enasidenib)’ are both used for the treatment of acute myeloid leukemia. Despite such achievements, studying cancer metabolism, especially oncometabolites, remains challenging due to time-consuming and expensive methodologies such as metabolomics. Thus, the number of confirmed oncometabolites is very small although a relatively large number of cancer-associated gene mutations have been well studied.
*Oncometabolite: A metabolite that shows pro-oncogenic function when abnormally accumulated in cancer cells. An oncometabolite is often generated as a result of gene mutations, and this accumulation promotes the growth and survival of cancer cells. Representative oncometabolites include 2-hydroxyglutarate, succinate, and fumarate.
On March 18th, a KAIST research team led by Professor Hyun Uk Kim from the Department of Chemical and Biomolecular Engineering developed a computational workflow that systematically predicts metabolites and metabolic pathways associated with somatic mutations in cancer through collaboration with research teams under Prof Youngil Koh, Prof. Hongseok Yun, and Prof. Chang Wook Jeong from Seoul National University Hospital.
The research teams have successfully reconstructed patient-specific genome-scale metabolic models (GEMs)* for 1,043 cancer patients across 24 cancer types by integrating publicly available cancer patients’ transcriptome data (i.e., from international cancer genome consortiums such as PCAWG and TCGA) into a generic human GEM. The resulting patient-specific GEMs make it possible to predict each patient’s metabolic phenotypes.
*Genome-scale metabolic model (GEM): A computational model that mathematically describes all of the biochemical reactions that take place inside a cell. It allows for the prediction of the cell’s metabolic phenotypes under various genetic and/or environmental conditions.
< Figure 1. Schematic diagram of a computational methodology for predicting metabolites and metabolic pathways associated with cancer somatic mutations. of a computational methodology for predicting metabolites and metabolic pathways associated with cancer somatic mutations. >
The team developed a four-step computational workflow using the patient-specific GEMs from 1,043 cancer patients and somatic mutation data obtained from the corresponding cancer patients. This workflow begins with the calculation of the flux-sum value of each metabolite by simulating the patient-specific GEMs. The flux-sum value quantifies the intracellular importance of a metabolite. Next, the workflow identifies metabolites that appear to be significantly associated with specific gene mutations through a statistical analysis of the predicted flux-sum data and the mutation data. Finally, the workflow selects altered metabolic pathways that significantly contribute to the biosynthesis of the predicted oncometabolite candidates, ultimately generating metabolite-gene-pathway sets as an output.
The two co-first authors, Dr. GaRyoung Lee (currently a postdoctoral fellow at the Dana-Farber Cancer Institute and Harvard Medical School) and Dr. Sang Mi Lee (currently a postdoctoral fellow at Harvard Medical School) said, “The computational workflow developed can systematically predict how genetic mutations affect cellular metabolism through metabolic pathways. Importantly, it can easily be applied to different types of cancer based on the mutation and transcriptome data of cancer patient cohorts.”
Prof. Kim said, “The computational workflow and its resulting prediction outcomes will serve as the groundwork for identifying novel oncometabolites and for facilitating the development of various treatment and diagnosis strategies”.
This study, which was supported by the National Research Foundation of Korea, has been published online in Genome Biology, a representative journal in the field of biotechnology and genetics, under the title "Prediction of metabolites associated with somatic mutations in cancers by using genome‑scale metabolic models and mutation data".
2024.03.18 View 7029 -
 KAIST Develops Healthcare Device Tracking Chronic Diabetic Wounds
A KAIST research team has developed an effective wireless system that monitors the wound healing process by tracking the spatiotemporal temperature changes and heat transfer characteristics of damaged areas such as diabetic wounds.
On the 5th of March, KAIST (represented by President Kwang Hyung Lee) announced that the research team led by Professor Kyeongha Kwon from KAIST’s School of Electrical Engineering, in association with Chung-Ang University professor Hanjun Ryu, developed digital healthcare technology that tracks the wound healing process in real time, which allows appropriate treatments to be administered.
< Figure 1. Schematic illustrations and diagrams of real-time wound monitoring systems. >
The skin serves as a barrier protecting the body from harmful substances, therefore damage to the skin may cause severe health risks to patients in need of intensive care. Especially in the case of diabetic patients, chronic wounds are easily formed due to complications in normal blood circulation and the wound healing process. In the United States alone, hundreds of billions of dollars of medical costs stem from regenerating the skin from such wounds. While various methods exist to promote wound healing, personalized management is essential depending on the condition of each patient's wounds.
Accordingly, the research team tracked the heating response within the wound by utilizing the differences in temperature between the damaged area and the surrounding healthy skin. They then measured heat transfer characteristics to observe moisture changes near the skin surface, ultimately establishing a basis for understanding the formation process of scar tissue. The team conducted experiments using diabetic mice models regarding the delay in wound healing under pathological conditions, and it was demonstrated that the collected data accurately tracks the wound healing process and the formation of scar tissue.
To minimize the tissue damage that may occur in the process of removing the tracking device after healing, the system integrates biodegradable sensor modules capable of natural decomposition within the body. These biodegradable modules disintegrate within the body after use, thus reducing the risk of additional discomfort or tissue damage upon device removal. Furthermore, the device could one day be used for monitoring inside the wound area as there is no need for removal.
Professor Kyeongha Kwon, who led the research, anticipates that continuous monitoring of wound temperature and heat transfer characteristics will enable medical professionals to more accurately assess the status of diabetic patients' wounds and provide appropriate treatment. He further predicted that the implementation of biodegradable sensors allows for the safe decomposition of the device after wound healing without the need for removal, making live monitoring possible not only in hospitals but also at home.
The research team plans to integrate antimicrobial materials into this device, aiming to expand its technological capabilities to enable the observation and prevention of inflammatory responses, bacterial infections, and other complications. The goal is to provide a multi-purpose wound monitoring platform capable of real-time antimicrobial monitoring in hospitals or homes by detecting changes in temperature and heat transfer characteristics indicative of infection levels.
< Image 1. Image of the bioresorbable temperature sensor >
The results of this study were published on February 19th in the international journal Advanced Healthcare Materials and selected as the inside back cover article, titled "Materials and Device Designs for Wireless Monitoring of Temperature and Thermal Transport Properties of Wound Beds during Healing."
This research was conducted with support from the Basic Research Program, the Regional Innovation Center Program, and the BK21 Program.
2024.03.11 View 7810
KAIST Develops Healthcare Device Tracking Chronic Diabetic Wounds
A KAIST research team has developed an effective wireless system that monitors the wound healing process by tracking the spatiotemporal temperature changes and heat transfer characteristics of damaged areas such as diabetic wounds.
On the 5th of March, KAIST (represented by President Kwang Hyung Lee) announced that the research team led by Professor Kyeongha Kwon from KAIST’s School of Electrical Engineering, in association with Chung-Ang University professor Hanjun Ryu, developed digital healthcare technology that tracks the wound healing process in real time, which allows appropriate treatments to be administered.
< Figure 1. Schematic illustrations and diagrams of real-time wound monitoring systems. >
The skin serves as a barrier protecting the body from harmful substances, therefore damage to the skin may cause severe health risks to patients in need of intensive care. Especially in the case of diabetic patients, chronic wounds are easily formed due to complications in normal blood circulation and the wound healing process. In the United States alone, hundreds of billions of dollars of medical costs stem from regenerating the skin from such wounds. While various methods exist to promote wound healing, personalized management is essential depending on the condition of each patient's wounds.
Accordingly, the research team tracked the heating response within the wound by utilizing the differences in temperature between the damaged area and the surrounding healthy skin. They then measured heat transfer characteristics to observe moisture changes near the skin surface, ultimately establishing a basis for understanding the formation process of scar tissue. The team conducted experiments using diabetic mice models regarding the delay in wound healing under pathological conditions, and it was demonstrated that the collected data accurately tracks the wound healing process and the formation of scar tissue.
To minimize the tissue damage that may occur in the process of removing the tracking device after healing, the system integrates biodegradable sensor modules capable of natural decomposition within the body. These biodegradable modules disintegrate within the body after use, thus reducing the risk of additional discomfort or tissue damage upon device removal. Furthermore, the device could one day be used for monitoring inside the wound area as there is no need for removal.
Professor Kyeongha Kwon, who led the research, anticipates that continuous monitoring of wound temperature and heat transfer characteristics will enable medical professionals to more accurately assess the status of diabetic patients' wounds and provide appropriate treatment. He further predicted that the implementation of biodegradable sensors allows for the safe decomposition of the device after wound healing without the need for removal, making live monitoring possible not only in hospitals but also at home.
The research team plans to integrate antimicrobial materials into this device, aiming to expand its technological capabilities to enable the observation and prevention of inflammatory responses, bacterial infections, and other complications. The goal is to provide a multi-purpose wound monitoring platform capable of real-time antimicrobial monitoring in hospitals or homes by detecting changes in temperature and heat transfer characteristics indicative of infection levels.
< Image 1. Image of the bioresorbable temperature sensor >
The results of this study were published on February 19th in the international journal Advanced Healthcare Materials and selected as the inside back cover article, titled "Materials and Device Designs for Wireless Monitoring of Temperature and Thermal Transport Properties of Wound Beds during Healing."
This research was conducted with support from the Basic Research Program, the Regional Innovation Center Program, and the BK21 Program.
2024.03.11 View 7810 -
 The World’s First Hacking-preventing Cryptographic Semiconductor Chip
With the dramatic increase in the amount of information exchanged between components or devices in the 5G/6G era, such as for the Internet of Things (IoT) and autonomous driving, hacking attacks are becoming more sophisticated. Consequently, enhancing security functions is essential for safely transmitting data between and among devices.
On February 29th, a KAIST research team led by Professors Yang-gyu Choi and Seung-tak Ryu from the School of Electrical Engineering announced the successful development of the world's first security cryptographic semiconductor.
The team has developed the Cryptoristor, a cryptographic transistor based on FinFET technology, produced through a 100% silicon-compatible process, for the first time in the world. Cryptoristor is a random number generator (RNG) with unparalleled characteristics, featuring a unique structure comprising a single transistor and a distinctive mechanism.
In all security environments, including artificial intelligence, the most crucial element is the RNG. In the most commonly used security chip, the Advanced Encryption Standard (AES), the RNG is a core component, occupying approximately 75% of the total chip area and more than 85% of its energy consumption. Hence, there is an urgent need for the development of low-power/ultra-small RNGs suitable for mobile or IoT devices.
Existing RNGs come with limitations as they lack compatibility with silicon CMOS processes and circuit-based RNGs occupy a large surface area.
In contrast, the team’s newly developed Cryptoristor, a cryptographic semiconductor based on a single-component structure, consumes and occupies less than .001 of the power and area compared to the current chips being used. Utilizing the inherent randomness of FinFETs, fabricated on a Silicon-on-Insulator (SOI) substrate with an insulating layer formed beneath the silicon, the team developed an RNG that unpredictably produces zeroes and ones.
< Figure 1. Conceptual diagram of the security cryptographic transistor device. >
Generally speaking, preventing hackers from predicting the encrypted algorithms during data exchanges through mobile devices is pivotal. Therefore, this method ensures unpredictability by generating random sequences of zeroes and ones that change every time.
Moreover, while the Cryptoristor-based RNG research is the world's first of its kind without any international implementation cases, it shares the same transistor structure as existing logic or memory components. This enables 100% production through rapid mass production processes using existing semiconductor facilities at a low cost.
Seung-il Kim, a PhD student who led the research, explained the significance of the study, stating, "As a cryptographic semiconductor, the ultra-small/low-power random number generator enhances security through its distinctive unpredictability, supporting safe hyperconnectivity with secure transmissions between chips or devices. Particularly, compared to previous research, it offers excellent advantages in terms of energy consumption, integration density, and cost, making it suitable for IoT device environments."
This research, with master’s student Hyung-jin Yoo as the co-author, was officially published in the online edition of Science Advances, a sister journal of Science, in February 2024 (research paper title: Cryptographic transistor for true random number generator with low power consumption).
This research received support from the Next-Generation Intelligent Semiconductor Technology Development Project and the Core Technology Development Project for the National Semiconductor Research Laboratory.
2024.03.07 View 8793
The World’s First Hacking-preventing Cryptographic Semiconductor Chip
With the dramatic increase in the amount of information exchanged between components or devices in the 5G/6G era, such as for the Internet of Things (IoT) and autonomous driving, hacking attacks are becoming more sophisticated. Consequently, enhancing security functions is essential for safely transmitting data between and among devices.
On February 29th, a KAIST research team led by Professors Yang-gyu Choi and Seung-tak Ryu from the School of Electrical Engineering announced the successful development of the world's first security cryptographic semiconductor.
The team has developed the Cryptoristor, a cryptographic transistor based on FinFET technology, produced through a 100% silicon-compatible process, for the first time in the world. Cryptoristor is a random number generator (RNG) with unparalleled characteristics, featuring a unique structure comprising a single transistor and a distinctive mechanism.
In all security environments, including artificial intelligence, the most crucial element is the RNG. In the most commonly used security chip, the Advanced Encryption Standard (AES), the RNG is a core component, occupying approximately 75% of the total chip area and more than 85% of its energy consumption. Hence, there is an urgent need for the development of low-power/ultra-small RNGs suitable for mobile or IoT devices.
Existing RNGs come with limitations as they lack compatibility with silicon CMOS processes and circuit-based RNGs occupy a large surface area.
In contrast, the team’s newly developed Cryptoristor, a cryptographic semiconductor based on a single-component structure, consumes and occupies less than .001 of the power and area compared to the current chips being used. Utilizing the inherent randomness of FinFETs, fabricated on a Silicon-on-Insulator (SOI) substrate with an insulating layer formed beneath the silicon, the team developed an RNG that unpredictably produces zeroes and ones.
< Figure 1. Conceptual diagram of the security cryptographic transistor device. >
Generally speaking, preventing hackers from predicting the encrypted algorithms during data exchanges through mobile devices is pivotal. Therefore, this method ensures unpredictability by generating random sequences of zeroes and ones that change every time.
Moreover, while the Cryptoristor-based RNG research is the world's first of its kind without any international implementation cases, it shares the same transistor structure as existing logic or memory components. This enables 100% production through rapid mass production processes using existing semiconductor facilities at a low cost.
Seung-il Kim, a PhD student who led the research, explained the significance of the study, stating, "As a cryptographic semiconductor, the ultra-small/low-power random number generator enhances security through its distinctive unpredictability, supporting safe hyperconnectivity with secure transmissions between chips or devices. Particularly, compared to previous research, it offers excellent advantages in terms of energy consumption, integration density, and cost, making it suitable for IoT device environments."
This research, with master’s student Hyung-jin Yoo as the co-author, was officially published in the online edition of Science Advances, a sister journal of Science, in February 2024 (research paper title: Cryptographic transistor for true random number generator with low power consumption).
This research received support from the Next-Generation Intelligent Semiconductor Technology Development Project and the Core Technology Development Project for the National Semiconductor Research Laboratory.
2024.03.07 View 8793 -
 KAIST Research Team Creates the Scent of Jasmine from Microorganisms
The fragrance of jasmine and ylang-ylang, used widely in the manufacturing of cosmetics, foods, and beverages, can be produced by direct extraction from their respective flowers. In reality, this makes it difficult for production to meet demand, so companies use benzyl acetate, a major aromatic component of the two fragrances that is chemically synthesized from raw materials derived from petroleum.
On February 26, a KAIST research team led by Research Professor Kyeong Rok Choi from the BioProcess Engineering Research Center and Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering revealed the development of the first microbial process to effectively produce benzyl acetate, an industrially useful compound, from renewable carbon sources such as glucose. The results were published in their paper titled “A microbial process for the production of benzyl acetate”.
< Figure 1. Production of benzyl acetate through co-culture of upstream and downstream strains harboring the benzoic acid-dependent pathway. >
The team, led by Distinguished Professor Lee, aimed to produce benzyl acetate through an environmentally friendly and sustainable method, and developed an Escherichia coli strand to convert glucose into benzyl acetate through system metabolic engineering*.
*System metabolic engineering: a field of research founded by Distinguished Professor Lee to effectively develop microbial cell plants, a core component of the bio-industry that will replace the existing chemical industry, which is highly dependent on petroleum.
The research team developed a metabolic pathway that biosynthesizes benzyl acetate from benzoic acid derived from glucose, and successfully produced benzyl acetate by co-culturing** the strain.
**co-culture: simultaneously synthesizing two or more types of microorganisms in a mixture
However, it has been confirmed that the enzyme used to convert benzoic acid into benzyl acetate in this co-culturing technique acts non-specifically on an intermediate product during benzoic acid biosynthesis, producing a by-product called cinnamyl acetate. This process consumes the intermediate product needed for benzoic acid biosynthesis, thereby reducing the production efficiency of the target compound, benzyl acetate.
To overcome this problem, Distinguished Professor Lee and his team devised a delayed co-culture method, where they first produced benzoic acid in the earlier stages of fermentation by only culturing the top strain that produces benzoic acid from glucose, and later inoculated the bottom strain to convert the accumulated benzoic acid in the culture medium into benzyl acetate.
By applying this co-culture technique, the team suppressed the formation of by-products without further strain improvement or applying additional enzymes, and multiplied the concentration of the target compound by 10 times, producing 2.2 g/L of benzyl acetate. In addition, the team confirmed its potential for the commercial production of benzyl acetate through a technical economic analysis on this microbial process.
< Figure 2. Delayed co-culture of the Bn1 and Bn-BnAc3 strains for improved production of benzyl acetate through the benzoic acid-independent pathway.>
Research Professor Keyong Rok Choi, who was the first author of this paper, said, “This work is significant in that we have developed an effective microbial process to produce the industrially useful compound benzyl acetate, and also in that we have suggested a new approach to overcome the target chemical efficiency diminution and by-product formation issues caused commonly through non-specific enzyme activities during metabolic engineering.”
Distinguished Professor Lee said, “If we can increase the variety and number of microbial processes that produce useful chemicals through sustainable methods and at the same time develop effective strategies to solve chronic and inevitable problems that arise during microbial strain development, we will be able to accelerate the transition from the petrochemical industry into the eco-friendly and sustainable bio-industry.
This work was published online in Nature Chemical Engineering, issued by Nature.
This research was supported by the ‘Implementation of Intelligent Cell Factory Technology (PI: Distinguished Professor Sang Yup Lee) Project by the Ministry of Science and ICT, and the ‘Development of Protein Production Technology from Inorganic Substances through Microbiological Metabolic System Control’ (PI: Research Professor Kyeong Rok Choi) by the Agricultural Microbiological Project Group at the Rural Development Administration.
2024.03.05 View 7227
KAIST Research Team Creates the Scent of Jasmine from Microorganisms
The fragrance of jasmine and ylang-ylang, used widely in the manufacturing of cosmetics, foods, and beverages, can be produced by direct extraction from their respective flowers. In reality, this makes it difficult for production to meet demand, so companies use benzyl acetate, a major aromatic component of the two fragrances that is chemically synthesized from raw materials derived from petroleum.
On February 26, a KAIST research team led by Research Professor Kyeong Rok Choi from the BioProcess Engineering Research Center and Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering revealed the development of the first microbial process to effectively produce benzyl acetate, an industrially useful compound, from renewable carbon sources such as glucose. The results were published in their paper titled “A microbial process for the production of benzyl acetate”.
< Figure 1. Production of benzyl acetate through co-culture of upstream and downstream strains harboring the benzoic acid-dependent pathway. >
The team, led by Distinguished Professor Lee, aimed to produce benzyl acetate through an environmentally friendly and sustainable method, and developed an Escherichia coli strand to convert glucose into benzyl acetate through system metabolic engineering*.
*System metabolic engineering: a field of research founded by Distinguished Professor Lee to effectively develop microbial cell plants, a core component of the bio-industry that will replace the existing chemical industry, which is highly dependent on petroleum.
The research team developed a metabolic pathway that biosynthesizes benzyl acetate from benzoic acid derived from glucose, and successfully produced benzyl acetate by co-culturing** the strain.
**co-culture: simultaneously synthesizing two or more types of microorganisms in a mixture
However, it has been confirmed that the enzyme used to convert benzoic acid into benzyl acetate in this co-culturing technique acts non-specifically on an intermediate product during benzoic acid biosynthesis, producing a by-product called cinnamyl acetate. This process consumes the intermediate product needed for benzoic acid biosynthesis, thereby reducing the production efficiency of the target compound, benzyl acetate.
To overcome this problem, Distinguished Professor Lee and his team devised a delayed co-culture method, where they first produced benzoic acid in the earlier stages of fermentation by only culturing the top strain that produces benzoic acid from glucose, and later inoculated the bottom strain to convert the accumulated benzoic acid in the culture medium into benzyl acetate.
By applying this co-culture technique, the team suppressed the formation of by-products without further strain improvement or applying additional enzymes, and multiplied the concentration of the target compound by 10 times, producing 2.2 g/L of benzyl acetate. In addition, the team confirmed its potential for the commercial production of benzyl acetate through a technical economic analysis on this microbial process.
< Figure 2. Delayed co-culture of the Bn1 and Bn-BnAc3 strains for improved production of benzyl acetate through the benzoic acid-independent pathway.>
Research Professor Keyong Rok Choi, who was the first author of this paper, said, “This work is significant in that we have developed an effective microbial process to produce the industrially useful compound benzyl acetate, and also in that we have suggested a new approach to overcome the target chemical efficiency diminution and by-product formation issues caused commonly through non-specific enzyme activities during metabolic engineering.”
Distinguished Professor Lee said, “If we can increase the variety and number of microbial processes that produce useful chemicals through sustainable methods and at the same time develop effective strategies to solve chronic and inevitable problems that arise during microbial strain development, we will be able to accelerate the transition from the petrochemical industry into the eco-friendly and sustainable bio-industry.
This work was published online in Nature Chemical Engineering, issued by Nature.
This research was supported by the ‘Implementation of Intelligent Cell Factory Technology (PI: Distinguished Professor Sang Yup Lee) Project by the Ministry of Science and ICT, and the ‘Development of Protein Production Technology from Inorganic Substances through Microbiological Metabolic System Control’ (PI: Research Professor Kyeong Rok Choi) by the Agricultural Microbiological Project Group at the Rural Development Administration.
2024.03.05 View 7227