NDA
-
 Professor Hee-Sung Park Named Scientist of May
(Professor Hee-Sung Park)
Professor Hee-Sung Park from the Department of Chemistry was named ‘Scientist of May’ sponsored by the Ministry of Science and ICT and the National Research Foundation of Korea. Professor Park was honored in recognition of his developing a tool to engineer designer proteins via diverse chemical modifications. This approach provides a novel platform for investigating numerous diseases such as cancer and dementia.
His research focuses on the production of synthetic proteins and the generation of diverse protein functions as well as the designing and engineering of new translation machinery for genetic code expansion, and the application of synthetic biology techniques for basic cell biology and applied medical science.
Post-translational modifications (PTMs) are constantly taking place during or after protein biosynthesis. PTMs play a vital role in expanding protein functional diversity and, as a result, critically affect numerous biological processes. Abnormal PTMs have been known to trigger various diseases including cancer and dementia. Therefore, this technology enables proteins to reproduce with specific modifications at selected residues and will significantly help establish experimental strategies to investigate fundamental biological mechanisms including the development of targeted cancer therapies.
Professor Park also received 10 million KRW in prize money.
2018.05.04 View 12071
Professor Hee-Sung Park Named Scientist of May
(Professor Hee-Sung Park)
Professor Hee-Sung Park from the Department of Chemistry was named ‘Scientist of May’ sponsored by the Ministry of Science and ICT and the National Research Foundation of Korea. Professor Park was honored in recognition of his developing a tool to engineer designer proteins via diverse chemical modifications. This approach provides a novel platform for investigating numerous diseases such as cancer and dementia.
His research focuses on the production of synthetic proteins and the generation of diverse protein functions as well as the designing and engineering of new translation machinery for genetic code expansion, and the application of synthetic biology techniques for basic cell biology and applied medical science.
Post-translational modifications (PTMs) are constantly taking place during or after protein biosynthesis. PTMs play a vital role in expanding protein functional diversity and, as a result, critically affect numerous biological processes. Abnormal PTMs have been known to trigger various diseases including cancer and dementia. Therefore, this technology enables proteins to reproduce with specific modifications at selected residues and will significantly help establish experimental strategies to investigate fundamental biological mechanisms including the development of targeted cancer therapies.
Professor Park also received 10 million KRW in prize money.
2018.05.04 View 12071 -
 Professor Gou Young Koh, 2018 Laureate of Ho-Am Prize
Distinguished Professor Gou Young Koh from the Graduate School of Medical Science and Engineering was appointed a 2018 laureate in medicine of the Ho-Am Prize by the Ho-Am Foundation. Professor Koh is a renowned expert in the field of tumor angiogenesis by exploring the hidden nature of capillary and lymphatic vessels in human organs.
He was recognized for demonstrating the effective reduction of tumor progression and metastasis via tumor vessel normalization. This counterintuitive study result is regarded as a stepping stone for a drug discovery to prevent microvascular diseases.
Besides Professor Koh, Professor Hee Oh from Yale University (Science), Professor Nam-Gyu Park from Sungkyunkwan University (Engineering), Opera Singer Kwangchul Youn (The Arts) and Sister Carla Kang (Community Service) received awards.
The Ho-Am Prize is presented to individuals who have contributed to academics, the arts, and social development, or furthered the welfare of humanity, and commemorates the noble spirit of public service espoused by the late Chairman Byung-chull Lee, who used the pen name Ho-Am.
It was established in 1990 by Kun-Hee Lee, the chairman of Samsung. Awards have been presented to 143 individuals worth a total of 24.4 billion KRW.
2018.04.11 View 9557
Professor Gou Young Koh, 2018 Laureate of Ho-Am Prize
Distinguished Professor Gou Young Koh from the Graduate School of Medical Science and Engineering was appointed a 2018 laureate in medicine of the Ho-Am Prize by the Ho-Am Foundation. Professor Koh is a renowned expert in the field of tumor angiogenesis by exploring the hidden nature of capillary and lymphatic vessels in human organs.
He was recognized for demonstrating the effective reduction of tumor progression and metastasis via tumor vessel normalization. This counterintuitive study result is regarded as a stepping stone for a drug discovery to prevent microvascular diseases.
Besides Professor Koh, Professor Hee Oh from Yale University (Science), Professor Nam-Gyu Park from Sungkyunkwan University (Engineering), Opera Singer Kwangchul Youn (The Arts) and Sister Carla Kang (Community Service) received awards.
The Ho-Am Prize is presented to individuals who have contributed to academics, the arts, and social development, or furthered the welfare of humanity, and commemorates the noble spirit of public service espoused by the late Chairman Byung-chull Lee, who used the pen name Ho-Am.
It was established in 1990 by Kun-Hee Lee, the chairman of Samsung. Awards have been presented to 143 individuals worth a total of 24.4 billion KRW.
2018.04.11 View 9557 -
 KAIST Develops IoT Platform for Food Safety
A research team led by the KAIST Auto-ID Labs developed a GS1 international standard-based IoTs infrastructure platform dubbed Oliot (Open Language of Internet of Things). This platform will be applied to Wanju Local Food, the nation’s largest cooperative, and will be in operation from April 5.
A total of eleven organizations participated in the development of Oliot, with KAIST as the center. This consortium is based on the GS1 international standard-based Oliot platform, which allows collecting and sharing data along the entire process of agrifood from production to processing, distribution, and consumption. It aims at increasing farm incomes and establishing a global ecosystem of domestic agriculture and stockbreeding that provides safe food.
Wanju Local Food is now the world’s first local food co-op with a traceability system from the initial stage of production planning to end sales based on GS1 international standards, which will ensure food safety.
KAIST has been sharing Oliot data in order to apply it to industries around the world. As of April 2018, approximately 900 enterprises and developers from more than 100 countries have downloaded it.
Professor Daeyoung Kim from the School of Computing, who is also Research Director of Auto-ID Labs said, “We are planning to disseminate Oliot to local food cooperatives throughout the nation. We will also cooperate with other countries, like China, Holland, and Hong Kong to create a better ecosystem for the global food industry.
“We are currently collaborating with related business to converge Oliot with AI or blockchain technology that can be applied to various services, such as healthcare and smart factories. Its tangible outcome will be revealed soon,” he added.
Auto-ID Labs are a global research consortium of six academic institutions that research and develop new technologies for advancing global commerce, partnering with GS1 (Global Standard 1), a non-profit organization that established standards for global commerce such as introducing barcodes to the retail industry. The Auto-ID Labs include MIT, University of Cambridge, Keio University, Fudan University, ETH Zurich/University of St. Gallen, and KAIST.
The consortium was supported by the Ministry of Science and ICT as well as the Institute for Information and Communications Technology Promotion for three years from 2015.
The launching of Oliot at Wanju Local Food will be held on April 5.
2018.04.03 View 10836
KAIST Develops IoT Platform for Food Safety
A research team led by the KAIST Auto-ID Labs developed a GS1 international standard-based IoTs infrastructure platform dubbed Oliot (Open Language of Internet of Things). This platform will be applied to Wanju Local Food, the nation’s largest cooperative, and will be in operation from April 5.
A total of eleven organizations participated in the development of Oliot, with KAIST as the center. This consortium is based on the GS1 international standard-based Oliot platform, which allows collecting and sharing data along the entire process of agrifood from production to processing, distribution, and consumption. It aims at increasing farm incomes and establishing a global ecosystem of domestic agriculture and stockbreeding that provides safe food.
Wanju Local Food is now the world’s first local food co-op with a traceability system from the initial stage of production planning to end sales based on GS1 international standards, which will ensure food safety.
KAIST has been sharing Oliot data in order to apply it to industries around the world. As of April 2018, approximately 900 enterprises and developers from more than 100 countries have downloaded it.
Professor Daeyoung Kim from the School of Computing, who is also Research Director of Auto-ID Labs said, “We are planning to disseminate Oliot to local food cooperatives throughout the nation. We will also cooperate with other countries, like China, Holland, and Hong Kong to create a better ecosystem for the global food industry.
“We are currently collaborating with related business to converge Oliot with AI or blockchain technology that can be applied to various services, such as healthcare and smart factories. Its tangible outcome will be revealed soon,” he added.
Auto-ID Labs are a global research consortium of six academic institutions that research and develop new technologies for advancing global commerce, partnering with GS1 (Global Standard 1), a non-profit organization that established standards for global commerce such as introducing barcodes to the retail industry. The Auto-ID Labs include MIT, University of Cambridge, Keio University, Fudan University, ETH Zurich/University of St. Gallen, and KAIST.
The consortium was supported by the Ministry of Science and ICT as well as the Institute for Information and Communications Technology Promotion for three years from 2015.
The launching of Oliot at Wanju Local Food will be held on April 5.
2018.04.03 View 10836 -
 Professor Emeritus Jung Ki Park Won the IBA Technology Award 2018
(Professor Emeritus Jung Ki Park)
Professor Emeritus Jung Ki Park from the Department of Chemical and Biomolecular Engineering received the IBA Technology Award from the International Battery Association (IBA).
IBA 2018 was held from March 11 to 16 on Jeju Island, which was the first time it was hosted in Korea. The conference was an excellent opportunity to let the world know the level of the Korean rechargeable battery industry and its technology.
Professor Park delivered his keynote speech titled Advances in Lithium Batteries in Korea at the conference and received the IBA Technology Award as the first Korean recipient.
Professor Park is a world-renowned scholar who was a groundbreaker in the rechargeable battery industry. He was recognized by the IBA Award Committee for his contributions carrying out research and development, fostering competent people, and enhancing the lithium rechargeable battery industry in Korea over the last 30 years.
Professor Park said, “It is my great honor to receive this award, which is the best international award in the field of rechargeable batteries. I would like to share this with my colleagues and students. As competition in the rechargeable industry intensifies, systemic cooperation among industries, academia, and government is needed for the continued development of the battery industry in Korea.
2018.03.19 View 8630
Professor Emeritus Jung Ki Park Won the IBA Technology Award 2018
(Professor Emeritus Jung Ki Park)
Professor Emeritus Jung Ki Park from the Department of Chemical and Biomolecular Engineering received the IBA Technology Award from the International Battery Association (IBA).
IBA 2018 was held from March 11 to 16 on Jeju Island, which was the first time it was hosted in Korea. The conference was an excellent opportunity to let the world know the level of the Korean rechargeable battery industry and its technology.
Professor Park delivered his keynote speech titled Advances in Lithium Batteries in Korea at the conference and received the IBA Technology Award as the first Korean recipient.
Professor Park is a world-renowned scholar who was a groundbreaker in the rechargeable battery industry. He was recognized by the IBA Award Committee for his contributions carrying out research and development, fostering competent people, and enhancing the lithium rechargeable battery industry in Korea over the last 30 years.
Professor Park said, “It is my great honor to receive this award, which is the best international award in the field of rechargeable batteries. I would like to share this with my colleagues and students. As competition in the rechargeable industry intensifies, systemic cooperation among industries, academia, and government is needed for the continued development of the battery industry in Korea.
2018.03.19 View 8630 -
 Scientist of March, Professor Hee-Seung Lee
(Professor Hee-Seung Lee)
Professor Hee-Seung Lee from the Department of Chemistry at KAIST received the ‘Science and Technology Award of the Month’ awarded by the Ministry of ICT and Science, and the National Research Foundation of Korea for March 2018.
Professor Lee has been recognized for successfully producing peptide-based molecular machines, which used to be made of metals.
The methodology can be translated into magnetotactic behavior at the macroscopic scale, which is reminiscent of magnetosomes in magnetotactic bacteria.
The team employed foldectures, self-assembled molecular architectures of β-peptide foldamers, to develop the peptide-based molecular machines that uniformly align with respect to an applied static magnetic field.
Professor Lee said, “Molecular machines are widely used in the field of medical engineering or material science; however, there were limitations for developing the machines using magnetic fields. By developing peptide-based molecular machines, we were able to develop body-friendly molecular machines.”
Every month, the Ministry of ICT and Science and the National Research Foundation of Korea award a cash prize worth 10,000,000 KRW to a scientist who has contributed to science and technology with outstanding research and development performance.
2018.03.15 View 10878
Scientist of March, Professor Hee-Seung Lee
(Professor Hee-Seung Lee)
Professor Hee-Seung Lee from the Department of Chemistry at KAIST received the ‘Science and Technology Award of the Month’ awarded by the Ministry of ICT and Science, and the National Research Foundation of Korea for March 2018.
Professor Lee has been recognized for successfully producing peptide-based molecular machines, which used to be made of metals.
The methodology can be translated into magnetotactic behavior at the macroscopic scale, which is reminiscent of magnetosomes in magnetotactic bacteria.
The team employed foldectures, self-assembled molecular architectures of β-peptide foldamers, to develop the peptide-based molecular machines that uniformly align with respect to an applied static magnetic field.
Professor Lee said, “Molecular machines are widely used in the field of medical engineering or material science; however, there were limitations for developing the machines using magnetic fields. By developing peptide-based molecular machines, we were able to develop body-friendly molecular machines.”
Every month, the Ministry of ICT and Science and the National Research Foundation of Korea award a cash prize worth 10,000,000 KRW to a scientist who has contributed to science and technology with outstanding research and development performance.
2018.03.15 View 10878 -
 The 2018 Commencement of KAIST at a Glance
KAIST awarded a total of 2, 736 degrees at the 2018 commencement ceremony on February 23. Among the honorees, Chairman and CEO of Samsung Electronics and Samsung Advanced Institute of Technology (SAIT) Oh-Hyun Kwon was recognized as the first alumnus honorary doctorate recipient of KAIST.
More than 5,000 family, friends, and graduates including distinguished guests of Minister of Science and ICT Young-Min Yu, the Member of National Assembly Kyung-Jin Kim, Chairman of the KAIST Board of Trustees Jang-Moo Lee, and the Chairperson of the KAIST Development Foundation Soo-Young Lee attended to celebrate the graduates. During the commencement, a total of 2,736 students earned degrees: 644 PhD degrees, 1,352 master’s degrees, and 740 bachelor’s degrees.
(Minister of Science and ICT Young-Min Yu)
(The Member of National Assembly Kyung-Jin Kim)
This year, Chairman and CEO of Samsung Electronics and SAIT Kwon shared the spotlight with many other graduates. Kwon received his Master’s degree in Electrical Engineering from KAIST in 1977 and completed his Ph.D. in Electrical Engineering from Stanford University in 1985. During his more than 33-year career at Samsung, he has made significant contribution to the development of 4M DRAM and the world’s first 64M DRAM. The success of 4M DRAM and 64 DRAM led Samsung to clinch the top position in the DRAM and NAND flash business around the world. This helped Samsung emerge as a global leader in the semiconductor industry.
(From left: Chairman and CEO of Samsung Electronics and SAIT Oh-Hyun Kwon and KAIST President Sung-Chul Shin)
During the commencement speech, Kwon and President Shin both highlighted the importance of collaboration instead of competition.
Kwon encouraged the graduates to understand others to make wonderful synergy. “When you first notice the true value of another person and interact with them, the value of the individual will be doubled and will bring about a greater impact,” he said.
Also, he stressed having a collaborative mindset by saying, “All of you here, including myself, are people who have benefited from society. We must cooperate with each other and give back to society for the vest results.”
While highlighting the core values of KAIST, creativity, challenge and caring, President Shin also emphasized collaboration with others. He said, “In the future, expertise in a single discipline will not lead to new inventions or discoveries. This highlights the importance of multidisciplinary, convergence research. The key to success lies in the acknowledgement of your peers as partners for mutual growth. Your partners will make up your weak areas and become your most important asset. May you expand your personal network by finding valuable partners not only within your laboratory and workplace, but beyond Korea.”
“Go out into the world and change it as a global shaper, global innovator, and global mover. I hope that each and every one of you will add benefits the world and your legacy will be remembered for generations to come. This is your obligation as a graduate of KAIST,” he said.
Click here to view the full text of President Sung-Chul Shin’s address to the graduates
+ List of academically outstanding undergraduate degree recipients who received honors during the Commencement 2018 of KAIST
Award
Department
Winner
Minister of Science and ICT Award
Dept. of Mathematical Sciences
Seong-Hyeok Park
KAIST Board Chairperson Award
School of Computing
Hyeong-Seok Kim
KAIST President Award
Dept. of Chemistry
Hoi-Min Cheong
KAIST Development Foundation Chairperson Award
Dept. of Biological Sciences
Gi-Song Kim
Dept. of Industrial & Systems Engineering
Seung-Hun Lee
2018.02.23 View 13009
The 2018 Commencement of KAIST at a Glance
KAIST awarded a total of 2, 736 degrees at the 2018 commencement ceremony on February 23. Among the honorees, Chairman and CEO of Samsung Electronics and Samsung Advanced Institute of Technology (SAIT) Oh-Hyun Kwon was recognized as the first alumnus honorary doctorate recipient of KAIST.
More than 5,000 family, friends, and graduates including distinguished guests of Minister of Science and ICT Young-Min Yu, the Member of National Assembly Kyung-Jin Kim, Chairman of the KAIST Board of Trustees Jang-Moo Lee, and the Chairperson of the KAIST Development Foundation Soo-Young Lee attended to celebrate the graduates. During the commencement, a total of 2,736 students earned degrees: 644 PhD degrees, 1,352 master’s degrees, and 740 bachelor’s degrees.
(Minister of Science and ICT Young-Min Yu)
(The Member of National Assembly Kyung-Jin Kim)
This year, Chairman and CEO of Samsung Electronics and SAIT Kwon shared the spotlight with many other graduates. Kwon received his Master’s degree in Electrical Engineering from KAIST in 1977 and completed his Ph.D. in Electrical Engineering from Stanford University in 1985. During his more than 33-year career at Samsung, he has made significant contribution to the development of 4M DRAM and the world’s first 64M DRAM. The success of 4M DRAM and 64 DRAM led Samsung to clinch the top position in the DRAM and NAND flash business around the world. This helped Samsung emerge as a global leader in the semiconductor industry.
(From left: Chairman and CEO of Samsung Electronics and SAIT Oh-Hyun Kwon and KAIST President Sung-Chul Shin)
During the commencement speech, Kwon and President Shin both highlighted the importance of collaboration instead of competition.
Kwon encouraged the graduates to understand others to make wonderful synergy. “When you first notice the true value of another person and interact with them, the value of the individual will be doubled and will bring about a greater impact,” he said.
Also, he stressed having a collaborative mindset by saying, “All of you here, including myself, are people who have benefited from society. We must cooperate with each other and give back to society for the vest results.”
While highlighting the core values of KAIST, creativity, challenge and caring, President Shin also emphasized collaboration with others. He said, “In the future, expertise in a single discipline will not lead to new inventions or discoveries. This highlights the importance of multidisciplinary, convergence research. The key to success lies in the acknowledgement of your peers as partners for mutual growth. Your partners will make up your weak areas and become your most important asset. May you expand your personal network by finding valuable partners not only within your laboratory and workplace, but beyond Korea.”
“Go out into the world and change it as a global shaper, global innovator, and global mover. I hope that each and every one of you will add benefits the world and your legacy will be remembered for generations to come. This is your obligation as a graduate of KAIST,” he said.
Click here to view the full text of President Sung-Chul Shin’s address to the graduates
+ List of academically outstanding undergraduate degree recipients who received honors during the Commencement 2018 of KAIST
Award
Department
Winner
Minister of Science and ICT Award
Dept. of Mathematical Sciences
Seong-Hyeok Park
KAIST Board Chairperson Award
School of Computing
Hyeong-Seok Kim
KAIST President Award
Dept. of Chemistry
Hoi-Min Cheong
KAIST Development Foundation Chairperson Award
Dept. of Biological Sciences
Gi-Song Kim
Dept. of Industrial & Systems Engineering
Seung-Hun Lee
2018.02.23 View 13009 -
 Soul-Searching & Odds-Defying Determination: A Commencement Story of Dr. Tae-Hyun Oh
(Dr. Tae-Hyun Oh, one of the 2736 graduates of the 2018)
Each and every one of the 2,736 graduates has come a long way to the 2018 Commencement. Tae-Hyun Oh, who just started his new research career at MIT after completing his Ph.D. at KAIST, is no exception.
Unlike the most KAIST freshmen straight out of the ingenious science academies of Korea, he is among the many who endured very challenging and turbulent adolescent years. Buffeted by family instability and struggling during his time at school, he saw himself trapped by seemingly impenetrable barriers. His mother, who hated to see his struggling, advised him to take a break to reflect on who he is and what he wanted to do.
After dropping out of high school in his first year, ways to make money and support his family occupied his thoughts. He took on odd jobs from a car body shop to a gas station, but the real world was very tough and sometimes even cruel to the high school dropout.
Bias and prejudice stigmatizing dropouts hurt him so much. He often overheard a parent who dropped by the body shop that he worked in saying, “If you do not study hard, you will end up like this guy.” Hearing such things terrified him and awoke his sense of purpose. So he decided to do something meaningful and be a better man than he was.
“I didn’t like the person I was growing up to become. I needed to find myself and get away from the place I was growing up. It was my adventure and it was the best decision I ever made,” says Oh.
After completing his high school diploma national certificate, he planned to apply to an engineering college. On his second try, he gained admission into the Department of Electrical Engineering at Kwang Woon University with a full scholarship. He was so thrilled for this opportunity and hoped he could do well at college. Signal processing and image processing became the interest of his research and he finished his undergraduate degree summa cum laude.
Gaining confidence in his studies, he searched around graduate school department websites in Korea to select the path he was interested in. Among others, the Robotics and Computer Vision Lab of Professor In-So Kweon at the Department of Electrical Engineering at KAIST was attractive to him.
Professor Kweon’s lab is globally renowned for robot vision technology. Their technologies were applied into HUBO, the KAIST-developed bimodal humanoid robot that won the 2015 DARPA Challenges.
“I am so appreciate of Professor Kweon, who accepted and guided me,” he said. Under Professor Kweon’s advising, he could finish his Master’s and Ph.D. courses in seven years. The mathematical modeling on fundamental computer algorithms became his main research topic.
While at KAIST, his academic research has blossomed. He won a total of 13 research prizes sponsored by corporations at home and abroad such as Kolon, Samsung, Hyundai Motors, and Qualcomm.
In 2015, he won the Microsoft Research Asia Fellowship as the sole Korean among 13 Ph.D. candidates in the Asian region. With the MSRA fellowship, he could intern at the MS Research Beijing Office for half a year and then in Redmond, Washington in the US.
“Professor Kweon’s lab filled me up with knowledge. Whenever I presented our team’s paper at an international conference, I was amazed by the strong interest shown by foreign experts, researchers, and professors. Their strong support and interest encouraged me a lot. I was fully charged with the belief that I could go abroad and explore more opportunities,” he said.
Dr. Oh, who completed his dissertation last fall, now works at the Department of Electrical Engineering and Computer Science at MIT under Professor Wojciech Matusik.
“I think the research environment at KAIST is on par with MIT. I have very rich resources for my studies and research at both schools, but at MIT the working culture is a little different and it remains a big challenge for me. I am still not familiar with collaborative work with colleagues from very diverse backgrounds and countries, and to persuade them and communicate with them is very tough. But I think I am getting better and better,” he said.
Oh, who is an avid computer game player as well, said life seems to be a game. The level of the game will be upgraded to the next level after something is accomplished. He feels great joy when he is moving up and he believes such diverse experiences have helped him become a better person day by day. Once he identified what gave him a strong sense of purpose, he wasn’t stressed out by his studies any more. He was so excited to be able to follow his passion and is ready for the next challenge.
2018.02.23 View 12673
Soul-Searching & Odds-Defying Determination: A Commencement Story of Dr. Tae-Hyun Oh
(Dr. Tae-Hyun Oh, one of the 2736 graduates of the 2018)
Each and every one of the 2,736 graduates has come a long way to the 2018 Commencement. Tae-Hyun Oh, who just started his new research career at MIT after completing his Ph.D. at KAIST, is no exception.
Unlike the most KAIST freshmen straight out of the ingenious science academies of Korea, he is among the many who endured very challenging and turbulent adolescent years. Buffeted by family instability and struggling during his time at school, he saw himself trapped by seemingly impenetrable barriers. His mother, who hated to see his struggling, advised him to take a break to reflect on who he is and what he wanted to do.
After dropping out of high school in his first year, ways to make money and support his family occupied his thoughts. He took on odd jobs from a car body shop to a gas station, but the real world was very tough and sometimes even cruel to the high school dropout.
Bias and prejudice stigmatizing dropouts hurt him so much. He often overheard a parent who dropped by the body shop that he worked in saying, “If you do not study hard, you will end up like this guy.” Hearing such things terrified him and awoke his sense of purpose. So he decided to do something meaningful and be a better man than he was.
“I didn’t like the person I was growing up to become. I needed to find myself and get away from the place I was growing up. It was my adventure and it was the best decision I ever made,” says Oh.
After completing his high school diploma national certificate, he planned to apply to an engineering college. On his second try, he gained admission into the Department of Electrical Engineering at Kwang Woon University with a full scholarship. He was so thrilled for this opportunity and hoped he could do well at college. Signal processing and image processing became the interest of his research and he finished his undergraduate degree summa cum laude.
Gaining confidence in his studies, he searched around graduate school department websites in Korea to select the path he was interested in. Among others, the Robotics and Computer Vision Lab of Professor In-So Kweon at the Department of Electrical Engineering at KAIST was attractive to him.
Professor Kweon’s lab is globally renowned for robot vision technology. Their technologies were applied into HUBO, the KAIST-developed bimodal humanoid robot that won the 2015 DARPA Challenges.
“I am so appreciate of Professor Kweon, who accepted and guided me,” he said. Under Professor Kweon’s advising, he could finish his Master’s and Ph.D. courses in seven years. The mathematical modeling on fundamental computer algorithms became his main research topic.
While at KAIST, his academic research has blossomed. He won a total of 13 research prizes sponsored by corporations at home and abroad such as Kolon, Samsung, Hyundai Motors, and Qualcomm.
In 2015, he won the Microsoft Research Asia Fellowship as the sole Korean among 13 Ph.D. candidates in the Asian region. With the MSRA fellowship, he could intern at the MS Research Beijing Office for half a year and then in Redmond, Washington in the US.
“Professor Kweon’s lab filled me up with knowledge. Whenever I presented our team’s paper at an international conference, I was amazed by the strong interest shown by foreign experts, researchers, and professors. Their strong support and interest encouraged me a lot. I was fully charged with the belief that I could go abroad and explore more opportunities,” he said.
Dr. Oh, who completed his dissertation last fall, now works at the Department of Electrical Engineering and Computer Science at MIT under Professor Wojciech Matusik.
“I think the research environment at KAIST is on par with MIT. I have very rich resources for my studies and research at both schools, but at MIT the working culture is a little different and it remains a big challenge for me. I am still not familiar with collaborative work with colleagues from very diverse backgrounds and countries, and to persuade them and communicate with them is very tough. But I think I am getting better and better,” he said.
Oh, who is an avid computer game player as well, said life seems to be a game. The level of the game will be upgraded to the next level after something is accomplished. He feels great joy when he is moving up and he believes such diverse experiences have helped him become a better person day by day. Once he identified what gave him a strong sense of purpose, he wasn’t stressed out by his studies any more. He was so excited to be able to follow his passion and is ready for the next challenge.
2018.02.23 View 12673 -
 Scientist of November, Professor Hyung Jin Sung
Professor Hyung Jin Sung from the Department of Mechanical Engineering at KAIST received a ‘Science and Technology Award of the Month’ given by the Ministry of ICT and Science and the National Research Foundation of Korea for November 2017. He developed technology that can exquisitely control a micrometer-scaled liquid drop on a dime-sized lab-on-a-chip. With his work, he was recognized for reinforcing research capability on microfluidics.
Lab-on-a-chip is an emerging experiment and diagnostic technology in the form of a bio-microchip that facilitates complex and various experiments with only a minimal sample size required. This technology draws a lot of attention not only from medical and pharmaceutical areas, but also the health and environmental field. The biggest problem was that technology for the temperature control of a fluid sample, which is one of the core technologies in microfluidics, has low accuracy. This limit had to be overcome in order to use the lab-on-a-chip more widely.
Professor Sung developed an acoustic and thermal method which controls the temperature of a droplet quickly and meticulously by using sound and energy. This is a thermal method that uses heat generated during the absorption of an acoustic wave into viscoelastic substances. It facilitates a rapid heating rate and spatial-temporal temperature control, allowing heating in desired areas. In addition, Professor Sung applied his technology to polymerase chain reactions, which are used to amplify DNA.
Through this experiment, he successfully shortened the reaction time from 1-2 hours to only three minutes, making this a groundbreaking achievement.
Professor Sung said, “My research is significant for enhancing the applicability of microfluidics. I expect that it will lead to technological innovations in healthcare fields including biochemistry, medical checkups, and new medicine development.”
2017.11.03 View 11661
Scientist of November, Professor Hyung Jin Sung
Professor Hyung Jin Sung from the Department of Mechanical Engineering at KAIST received a ‘Science and Technology Award of the Month’ given by the Ministry of ICT and Science and the National Research Foundation of Korea for November 2017. He developed technology that can exquisitely control a micrometer-scaled liquid drop on a dime-sized lab-on-a-chip. With his work, he was recognized for reinforcing research capability on microfluidics.
Lab-on-a-chip is an emerging experiment and diagnostic technology in the form of a bio-microchip that facilitates complex and various experiments with only a minimal sample size required. This technology draws a lot of attention not only from medical and pharmaceutical areas, but also the health and environmental field. The biggest problem was that technology for the temperature control of a fluid sample, which is one of the core technologies in microfluidics, has low accuracy. This limit had to be overcome in order to use the lab-on-a-chip more widely.
Professor Sung developed an acoustic and thermal method which controls the temperature of a droplet quickly and meticulously by using sound and energy. This is a thermal method that uses heat generated during the absorption of an acoustic wave into viscoelastic substances. It facilitates a rapid heating rate and spatial-temporal temperature control, allowing heating in desired areas. In addition, Professor Sung applied his technology to polymerase chain reactions, which are used to amplify DNA.
Through this experiment, he successfully shortened the reaction time from 1-2 hours to only three minutes, making this a groundbreaking achievement.
Professor Sung said, “My research is significant for enhancing the applicability of microfluidics. I expect that it will lead to technological innovations in healthcare fields including biochemistry, medical checkups, and new medicine development.”
2017.11.03 View 11661 -
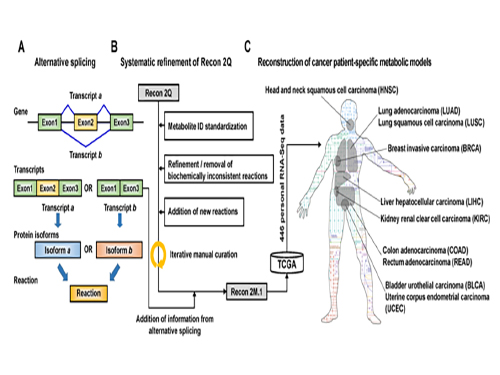 Development of a Highly-Accurate Computational Model of Human Metabolism
A research team from KAIST developed a computational framework that enables the reconstruction of a comprehensive computational model of human metabolism, which allows for an accurate prediction of personal metabolic features (or phenotypes).
Understanding personal metabolic phenotypes allows us to design effective therapeutic strategies for various chronic and infectious diseases. A human computational model called the genome-scale metabolic model (GEM) contains information on thousands of metabolic genes and their corresponding reactions and metabolites, and has played an important role in predicting metabolic phenotypes. Although several versions of human GEMs have been released, they had room for further development, especially as to incorporating biological information coming from a human genetics mechanism called “alternative splicing.” Alternative splicing is a genetic mechanism that allows a gene to give rise to multiple reactions, and is strongly associated with pathology.
To tackle this problem, Jae Yong Ryu (a Ph.D. student), Dr. Hyun Uk Kim (Research Fellow), and Distinguished Professor Sang Yup Lee, all from the Department of Chemical and Biomolecular Engineering at KAIST, developed a computational framework that systematically generates metabolic reactions, and adds them to the human GEM. The resulting human GEM was demonstrated to accurately predict metabolic phenotypes under varied environmental conditions. The research results were published online in Proceedings of the National Academy of Sciences (PNAS) on October 24, 2017, under the title “Framework and resource for more than 11,000 gene-transcript-protein-reaction associations in human metabolism.”
The research team first updated the biological contents of a previous version of the human GEM. The updated biological contents include metabolic genes and their corresponding metabolites and reactions. In particular, metabolic reactions catalyzed by already-known protein isoforms were additionally incorporated into the human GEM; protein isoforms are multiple variants of proteins generated from individual genes through the alternative splicing process. Each protein isoform is often responsible for the operation of a metabolic reaction. Although multiple protein isoforms generated from one gene can play different functions by having different sets of protein domains and/or subcellular localizations, such information was not properly considered in previous versions of human GEMs.
Upon the initial update of the human GEM, named Recon 2M.1, the research team subsequently implemented a computational framework that systematically generates information on Gene-Transcript-Protein-Reaction Associations (GeTPRA) in order to identify protein isoforms that were previously not identified. This framework was developed in this study. As a result of the implementation of the framework for GeTPRA, more than 11,000 GeTPRA were automatically predicted, and thoroughly validated. Additional metabolic reactions were then added to Recon 2M.1 based on the predicted GeTPRA for the previously uncharacterized protein isoforms; Recon 2M.1 was renamed Recon 2M.2 from this upgrade.
Finally, Recon 2M.2 was integrated with 446 sets of personal biological data (RNA-Seq data) in order to build patient-specific cancer models. These patient-specific cancer models were used to predict cancer metabolism activities and anticancer targets.
The development of a new version of human GEMs along with the computational framework for GeTPRA is expected to boost studies in fundamental human genetics and medicine. Model files of the human GEMs Recon 2M.1 and 2M.2, a full list of the GeTPRA and the source code for the computational framework to predict the GeTPRA are all available as part of the publication of this study.
Distinguished Professor Lee said, “The predicted GeTPRA from the computational framework is expected to serve as a guideline for future experiments on human genetics and biochemistry, whereas the resulting Recon 2M.2 can be used to predict drug targets for various human diseases.”
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012M1A2A2026556 and NRF-2012M1A2A2026557) from the Ministry of Science and ICT through the National Research Foundation (NRF) of Korea.
(Figure 1:A scheme of Recon 2M.1 development and its use in reconstructing personal genome-scale metabolic models (GEMs). (A) A concept of alternative splicing of human genes and its use in Gene-Transcript-Protein-Reaction Associations (GeTPRA) of Recon 2M.1. (B) A procedure of systematic refinement of the Recon 2Q. Recon 2Q is one of the previously released human GEMs. Biochemically inconsistent reactions include unbalanced, artificial, blocked, and/or redundant reactions. Iterative manual curation was conducted while validating the Recon 2M.1. (C) Reconstruction of cancer patient-specific GEMs using Recon 2M.1 for further simulation studies. In this study, personal biological data (RNA-Seq data) were obtained from The Cancer Genome Atlas (TCGA; https://cancergenome.nih.gov/ ) across the ten cancer types.
(Figure 2: Computational framework for the systematic generation of Gene-Transcript-Protein-Reaction Associations (GeTPRA; red box in the flowchart). Peptide sequences of metabolic genes defined in Recon 2M.1 were retrieved from a database called Ensembl. EC numbers and subcellular localizations of all the protein isoforms of metabolic genes in Recon 2M.1 were predicted using software programs EFICAz2.5 and Wolf PSort, respectively. Information on the newly predicted GeTPRA was systematically incorporated into the Recon 2M.1, thereby resulting in Recon 2M.2.)
2017.10.25 View 10965
Development of a Highly-Accurate Computational Model of Human Metabolism
A research team from KAIST developed a computational framework that enables the reconstruction of a comprehensive computational model of human metabolism, which allows for an accurate prediction of personal metabolic features (or phenotypes).
Understanding personal metabolic phenotypes allows us to design effective therapeutic strategies for various chronic and infectious diseases. A human computational model called the genome-scale metabolic model (GEM) contains information on thousands of metabolic genes and their corresponding reactions and metabolites, and has played an important role in predicting metabolic phenotypes. Although several versions of human GEMs have been released, they had room for further development, especially as to incorporating biological information coming from a human genetics mechanism called “alternative splicing.” Alternative splicing is a genetic mechanism that allows a gene to give rise to multiple reactions, and is strongly associated with pathology.
To tackle this problem, Jae Yong Ryu (a Ph.D. student), Dr. Hyun Uk Kim (Research Fellow), and Distinguished Professor Sang Yup Lee, all from the Department of Chemical and Biomolecular Engineering at KAIST, developed a computational framework that systematically generates metabolic reactions, and adds them to the human GEM. The resulting human GEM was demonstrated to accurately predict metabolic phenotypes under varied environmental conditions. The research results were published online in Proceedings of the National Academy of Sciences (PNAS) on October 24, 2017, under the title “Framework and resource for more than 11,000 gene-transcript-protein-reaction associations in human metabolism.”
The research team first updated the biological contents of a previous version of the human GEM. The updated biological contents include metabolic genes and their corresponding metabolites and reactions. In particular, metabolic reactions catalyzed by already-known protein isoforms were additionally incorporated into the human GEM; protein isoforms are multiple variants of proteins generated from individual genes through the alternative splicing process. Each protein isoform is often responsible for the operation of a metabolic reaction. Although multiple protein isoforms generated from one gene can play different functions by having different sets of protein domains and/or subcellular localizations, such information was not properly considered in previous versions of human GEMs.
Upon the initial update of the human GEM, named Recon 2M.1, the research team subsequently implemented a computational framework that systematically generates information on Gene-Transcript-Protein-Reaction Associations (GeTPRA) in order to identify protein isoforms that were previously not identified. This framework was developed in this study. As a result of the implementation of the framework for GeTPRA, more than 11,000 GeTPRA were automatically predicted, and thoroughly validated. Additional metabolic reactions were then added to Recon 2M.1 based on the predicted GeTPRA for the previously uncharacterized protein isoforms; Recon 2M.1 was renamed Recon 2M.2 from this upgrade.
Finally, Recon 2M.2 was integrated with 446 sets of personal biological data (RNA-Seq data) in order to build patient-specific cancer models. These patient-specific cancer models were used to predict cancer metabolism activities and anticancer targets.
The development of a new version of human GEMs along with the computational framework for GeTPRA is expected to boost studies in fundamental human genetics and medicine. Model files of the human GEMs Recon 2M.1 and 2M.2, a full list of the GeTPRA and the source code for the computational framework to predict the GeTPRA are all available as part of the publication of this study.
Distinguished Professor Lee said, “The predicted GeTPRA from the computational framework is expected to serve as a guideline for future experiments on human genetics and biochemistry, whereas the resulting Recon 2M.2 can be used to predict drug targets for various human diseases.”
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012M1A2A2026556 and NRF-2012M1A2A2026557) from the Ministry of Science and ICT through the National Research Foundation (NRF) of Korea.
(Figure 1:A scheme of Recon 2M.1 development and its use in reconstructing personal genome-scale metabolic models (GEMs). (A) A concept of alternative splicing of human genes and its use in Gene-Transcript-Protein-Reaction Associations (GeTPRA) of Recon 2M.1. (B) A procedure of systematic refinement of the Recon 2Q. Recon 2Q is one of the previously released human GEMs. Biochemically inconsistent reactions include unbalanced, artificial, blocked, and/or redundant reactions. Iterative manual curation was conducted while validating the Recon 2M.1. (C) Reconstruction of cancer patient-specific GEMs using Recon 2M.1 for further simulation studies. In this study, personal biological data (RNA-Seq data) were obtained from The Cancer Genome Atlas (TCGA; https://cancergenome.nih.gov/ ) across the ten cancer types.
(Figure 2: Computational framework for the systematic generation of Gene-Transcript-Protein-Reaction Associations (GeTPRA; red box in the flowchart). Peptide sequences of metabolic genes defined in Recon 2M.1 were retrieved from a database called Ensembl. EC numbers and subcellular localizations of all the protein isoforms of metabolic genes in Recon 2M.1 were predicted using software programs EFICAz2.5 and Wolf PSort, respectively. Information on the newly predicted GeTPRA was systematically incorporated into the Recon 2M.1, thereby resulting in Recon 2M.2.)
2017.10.25 View 10965 -
 Photoacoustic Imaging and Photothermal Cancer Therapy Using BR Nanoparticles
(Professor Sangyong Jon and PhD Candidate Dong Yun Lee)
Sangyong Jon, a professor in the Department of Biological Sciences at KAIST, and his team developed combined photoacoustic imaging and photothermal therapy for cancer by using Bilirubin (BR) nanoparticles.
The research team applied the properties of a bile pigment called BR, which exerts potent antioxidant and anti-inflammatory effects, to this research.
The team expects this research, which shows high biocompatibility as well as outstanding photoacoustic imaging and photothermal therapy, to be an appropriate system in the field of treatment for cancer.
In the past, the research team developed a PEGylated bilirubin-based nanoparticle system by combining water-insoluble BR with water-soluble Polyethylene Glycol (PEG).
This technology facilitated BR exerting antioxidants yet prevented them from being accumulated in the body. Its efficiency and safety was identified in an animal disease model, for conditions such as inflammatory bowel disease, islet cell transportation, and asthma.
Differing from previous research methods, this research applied the different physicochemical properties of BR to cancer treatment.
When the causative agent of jaundice, yellow BR, is exposed to a certain wavelength of blue light, the agent becomes a photonic nanomaterial as it responses to the light. This light-responsive nanomaterial can be used to cure jaundice because it allows for active excretion in infants.
Secondly, the team identified that BR is a major component of black pigment gallstones which can be often found in gall bladders or bile ducts under certain pathological conditions. The findings show that BR forms black pigment gallstones without the role of an intermediate or cation, such as calcium and copper. The research team combined cisplatin, a platinum metal-based anticancer drug, with BR so that BR nanoparticles changed the solution color from yellow to purple.
The team also examined the possibility of cisplatin-chelated BR nanoparticles as a probe for photoacoustic images. They found that considerable photoacoustic activity was shown when it was exposed to near infrared light. In fact, the photoacoustic signal was increased significantly in tumors of animals with colorectal cancer when the nanoparticles were administered to it intravenously. The team expects a more accurate diagnosis of tumors through this technology.
Moreover, the team assessed the photothermal effects of cisplatin-chelated BR nanoparticles. The research showed that the temperature of tumors increased by 25 degrees Celsius within five minutes when they were exposed to near infrared light, due to the photothermal effect. After two weeks, their size was reduced compared to that of other groups, and sometimes the tumors were even necrotized.
Professor Jon said, “Existing substances have a low biocompatibility and limitation for clinical therapy because they are artificially oriented; therefore, they might have toxicity. I am hoping that these cisplatin-chelated BR-based nanoparticles will provide a new platform for preclinical, translational research and clinical adaptation of the photoacoustic imaging and photothermal therapy.”
The paper (Dong Yun Lee as a first author) was published online in the renowned journal in the field of applied chemistry, Angewandte Chemi International Edition, on September 4. This research was sponsored by the National Research Foundation of Korea.
(Schematic diagram of the research)
(From left: Bilirubin nanoparticles, cisplatin-chelated Bilirubin nanoparticles)
2017.09.26 View 9743
Photoacoustic Imaging and Photothermal Cancer Therapy Using BR Nanoparticles
(Professor Sangyong Jon and PhD Candidate Dong Yun Lee)
Sangyong Jon, a professor in the Department of Biological Sciences at KAIST, and his team developed combined photoacoustic imaging and photothermal therapy for cancer by using Bilirubin (BR) nanoparticles.
The research team applied the properties of a bile pigment called BR, which exerts potent antioxidant and anti-inflammatory effects, to this research.
The team expects this research, which shows high biocompatibility as well as outstanding photoacoustic imaging and photothermal therapy, to be an appropriate system in the field of treatment for cancer.
In the past, the research team developed a PEGylated bilirubin-based nanoparticle system by combining water-insoluble BR with water-soluble Polyethylene Glycol (PEG).
This technology facilitated BR exerting antioxidants yet prevented them from being accumulated in the body. Its efficiency and safety was identified in an animal disease model, for conditions such as inflammatory bowel disease, islet cell transportation, and asthma.
Differing from previous research methods, this research applied the different physicochemical properties of BR to cancer treatment.
When the causative agent of jaundice, yellow BR, is exposed to a certain wavelength of blue light, the agent becomes a photonic nanomaterial as it responses to the light. This light-responsive nanomaterial can be used to cure jaundice because it allows for active excretion in infants.
Secondly, the team identified that BR is a major component of black pigment gallstones which can be often found in gall bladders or bile ducts under certain pathological conditions. The findings show that BR forms black pigment gallstones without the role of an intermediate or cation, such as calcium and copper. The research team combined cisplatin, a platinum metal-based anticancer drug, with BR so that BR nanoparticles changed the solution color from yellow to purple.
The team also examined the possibility of cisplatin-chelated BR nanoparticles as a probe for photoacoustic images. They found that considerable photoacoustic activity was shown when it was exposed to near infrared light. In fact, the photoacoustic signal was increased significantly in tumors of animals with colorectal cancer when the nanoparticles were administered to it intravenously. The team expects a more accurate diagnosis of tumors through this technology.
Moreover, the team assessed the photothermal effects of cisplatin-chelated BR nanoparticles. The research showed that the temperature of tumors increased by 25 degrees Celsius within five minutes when they were exposed to near infrared light, due to the photothermal effect. After two weeks, their size was reduced compared to that of other groups, and sometimes the tumors were even necrotized.
Professor Jon said, “Existing substances have a low biocompatibility and limitation for clinical therapy because they are artificially oriented; therefore, they might have toxicity. I am hoping that these cisplatin-chelated BR-based nanoparticles will provide a new platform for preclinical, translational research and clinical adaptation of the photoacoustic imaging and photothermal therapy.”
The paper (Dong Yun Lee as a first author) was published online in the renowned journal in the field of applied chemistry, Angewandte Chemi International Edition, on September 4. This research was sponsored by the National Research Foundation of Korea.
(Schematic diagram of the research)
(From left: Bilirubin nanoparticles, cisplatin-chelated Bilirubin nanoparticles)
2017.09.26 View 9743 -
 Research Center for Smart Submerged Floating Tunnel Systems Opens
(Distinguished guests including President Shin (fourth from the right) and Director Lee (third from left) at the opening ceremony)
The Research Center for a Smart Submerged Floating Tunnel Systems was recently established at KAIST with the purpose of taking the lead in developing fundamental and applicable technology for submerged floating tunnels as well as fostering creative and talented people. Haeng-Ki Lee, a professor in the Department of Civil & Environmental Engineering at KAIST is heading the center.
KAIST held its opening ceremony on September 7, 2017 in the Applied Engineering Building located on the main campus.
Distinguished guests, including KAIST president Sung-Chul Shin, the President of the Korea Institute of Ocean Science and Technology Gi-Hoon Hong, the President of the Korean Society of Civil Engineering Young-Seok Park, and the Director in the Division of Engineering at the National Research Foundation of Korea Joong-Kon Park attended the ceremony.
The National Research Foundation of Korea provides Engineering Research Center (ERC) projects which find and foster groups with outstanding research performance in a field of engineering. The projects support these groups so that they can strengthen their global competitiveness while enhancing national competence in basic research.
The ‘Research Center for Smart Submerged Floating Tunnel Systems’ was selected as one of the ERC projects in 2017. For the next seven years, the research center will work to develop a submerged floating tunnel system resistant depths greater than 100 meters.
To achieve its goal, the center has defined crucial research topics including: i) a structural analysis program and integrated design technology specific for submerged floating tunnel systems, ii) high-durability marine construction materials and submerged construction integrated systems, and iii) safety and maintenance integrated technology for smart submerged floating tunnel systems.
The ‘Research Center for Smart Submerged Floating Tunnel Systems’ will devote itself to developing a variety of fundamental and applicable technology that will be leading global maritime construction. Moreover, it will concentrate on fostering professional research manpower in related areas.
The Director of the Center Lee said, “The center will cooperate with KAIST researchers who are experts in various fields, including structures, materials, construction, and maritime research. Based on this collaboration, the center will contribute to achieving autonomous technologies by developing fundamental and applicable technology related with submerged floating tunnel systems. It will also take the role of a leading global research hub in the field of submerged floating tunnels as well as construction technologies.”
2017.09.07 View 11095
Research Center for Smart Submerged Floating Tunnel Systems Opens
(Distinguished guests including President Shin (fourth from the right) and Director Lee (third from left) at the opening ceremony)
The Research Center for a Smart Submerged Floating Tunnel Systems was recently established at KAIST with the purpose of taking the lead in developing fundamental and applicable technology for submerged floating tunnels as well as fostering creative and talented people. Haeng-Ki Lee, a professor in the Department of Civil & Environmental Engineering at KAIST is heading the center.
KAIST held its opening ceremony on September 7, 2017 in the Applied Engineering Building located on the main campus.
Distinguished guests, including KAIST president Sung-Chul Shin, the President of the Korea Institute of Ocean Science and Technology Gi-Hoon Hong, the President of the Korean Society of Civil Engineering Young-Seok Park, and the Director in the Division of Engineering at the National Research Foundation of Korea Joong-Kon Park attended the ceremony.
The National Research Foundation of Korea provides Engineering Research Center (ERC) projects which find and foster groups with outstanding research performance in a field of engineering. The projects support these groups so that they can strengthen their global competitiveness while enhancing national competence in basic research.
The ‘Research Center for Smart Submerged Floating Tunnel Systems’ was selected as one of the ERC projects in 2017. For the next seven years, the research center will work to develop a submerged floating tunnel system resistant depths greater than 100 meters.
To achieve its goal, the center has defined crucial research topics including: i) a structural analysis program and integrated design technology specific for submerged floating tunnel systems, ii) high-durability marine construction materials and submerged construction integrated systems, and iii) safety and maintenance integrated technology for smart submerged floating tunnel systems.
The ‘Research Center for Smart Submerged Floating Tunnel Systems’ will devote itself to developing a variety of fundamental and applicable technology that will be leading global maritime construction. Moreover, it will concentrate on fostering professional research manpower in related areas.
The Director of the Center Lee said, “The center will cooperate with KAIST researchers who are experts in various fields, including structures, materials, construction, and maritime research. Based on this collaboration, the center will contribute to achieving autonomous technologies by developing fundamental and applicable technology related with submerged floating tunnel systems. It will also take the role of a leading global research hub in the field of submerged floating tunnels as well as construction technologies.”
2017.09.07 View 11095 -
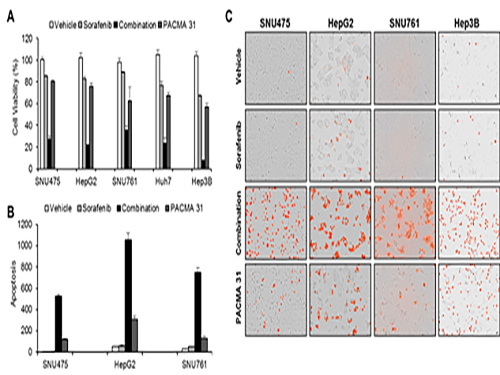 Discovery of an Optimal Drug Combination: Overcoming Resistance to Targeted Drugs for Liver Cancer
A KAIST research team presented a novel method for improving medication treatment for liver cancer using Systems Biology, combining research from information technology and the life sciences. Professor Kwang-Hyun Cho in the Department of Bio and Brain Engineering at KAIST conducted the research in collaboration with Professor Jung-Hwan Yoon in the Department of Internal Medicine at Seoul National University Hospital. This research was published in Hepatology in September 2017 (available online from August 24, 2017).
Liver cancer is the fifth and seventh most common cancer found in men and women throughout the world, which places it second in the cause of cancer deaths. In particular, Korea has 28.4 deaths from liver cancer per 100,000 persons, the highest death rate among OECD countries and twice that of Japan.
Each year in Korea, 16,000 people get liver cancer on average, yet the five-year survival rate stands below 12%. According to the National Cancer Information Center, lung cancer (17,399) took the highest portion of cancer-related deaths, followed by liver cancer (11,311) based on last year data.
Liver cancer is known to carry the highest social cost in comparison to other cancers and it causes the highest fatality in earlier age groups (40s-50s). In that sense, it is necessary to develop a new treatment that mitigates side effects yet elevates the survival rate.
There are ways in which liver cancer can be cured, such as surgery, embolization, and medication treatments; however, the options become limited for curing progressive cancer, a stage in which surgical methods cannot be executed.
Among anticancer medications, Sorafenib, a drug known for enhancing the survival rate of cancer patients, is a unique drug allowed for use as a targeted anticancer medication for progressive liver cancer patients. Its sales reached more than ten billion KRW annually in Korea, but its efficacy works on only about 20% of the treated patients. Also, acquired resistance to Sorafenib is emerging. Additionally, the action mechanism and resistance mechanism of Sorafenib is only vaguely identified.Although Sorafenib only extends the survival rate of terminal cancer patients less than three months on average, it is widely being used because drugs developed by global pharmaceutical companies failed to outperform its effectiveness.
Professor Cho’s research team analyzed the expression changes of genes in cell lines in response to Sorafenib in order to identify the effect and the resistance mechanism of Sorafenib.
As a result, the team discovered the resistance mechanism of Sorafenib using Systems Biology analysis. By combining computer simulations and biological experiments, it was revealed that protein disulfide isomerase (PDI) plays a crucial role in the resistance mechanism of Sorafenib and that its efficacy can be improved significantly by blocking PDI.
The research team used mice in the experiment and discovered the synergic effect of PDI inhibition with Sorafenib for reducing liver cancer cells, known as hepatocellular carcinoma. Also, more PDIs are shown in tissue from patients who possess a resistance to Sorafenib. From these findings, the team could identify the possibility of its clinical applications. The team also confirmed these findings from clinical data through a retrospective cohort study.
“Molecules that play an important role in cell lines are mostly put under complex regulation. For this reason, the existing biological research has a fundamental limitations for discovering its underlying principles,” Professor Cho said. “This research is a representative case of overcoming this limitation of traditional life science research by using a Systems Biology approach, combining IT and life science. It suggests the possibility of developing a new method that overcomes drug resistance with a network analysis of the targeted drug action mechanism of cancer.”
The research was supported by the National Research Foundation of Korea (NRF) and funded by the Ministry of Science and ICT.
(Figure 1. Simulation results from cellular experiments using hepatocellular carcinoma)
(Figure 2. Network analysis and computer simulation by using the endoplasmic reticulum (ER) stress network)
(Figure 3. ER stress network model)
2017.08.30 View 13138
Discovery of an Optimal Drug Combination: Overcoming Resistance to Targeted Drugs for Liver Cancer
A KAIST research team presented a novel method for improving medication treatment for liver cancer using Systems Biology, combining research from information technology and the life sciences. Professor Kwang-Hyun Cho in the Department of Bio and Brain Engineering at KAIST conducted the research in collaboration with Professor Jung-Hwan Yoon in the Department of Internal Medicine at Seoul National University Hospital. This research was published in Hepatology in September 2017 (available online from August 24, 2017).
Liver cancer is the fifth and seventh most common cancer found in men and women throughout the world, which places it second in the cause of cancer deaths. In particular, Korea has 28.4 deaths from liver cancer per 100,000 persons, the highest death rate among OECD countries and twice that of Japan.
Each year in Korea, 16,000 people get liver cancer on average, yet the five-year survival rate stands below 12%. According to the National Cancer Information Center, lung cancer (17,399) took the highest portion of cancer-related deaths, followed by liver cancer (11,311) based on last year data.
Liver cancer is known to carry the highest social cost in comparison to other cancers and it causes the highest fatality in earlier age groups (40s-50s). In that sense, it is necessary to develop a new treatment that mitigates side effects yet elevates the survival rate.
There are ways in which liver cancer can be cured, such as surgery, embolization, and medication treatments; however, the options become limited for curing progressive cancer, a stage in which surgical methods cannot be executed.
Among anticancer medications, Sorafenib, a drug known for enhancing the survival rate of cancer patients, is a unique drug allowed for use as a targeted anticancer medication for progressive liver cancer patients. Its sales reached more than ten billion KRW annually in Korea, but its efficacy works on only about 20% of the treated patients. Also, acquired resistance to Sorafenib is emerging. Additionally, the action mechanism and resistance mechanism of Sorafenib is only vaguely identified.Although Sorafenib only extends the survival rate of terminal cancer patients less than three months on average, it is widely being used because drugs developed by global pharmaceutical companies failed to outperform its effectiveness.
Professor Cho’s research team analyzed the expression changes of genes in cell lines in response to Sorafenib in order to identify the effect and the resistance mechanism of Sorafenib.
As a result, the team discovered the resistance mechanism of Sorafenib using Systems Biology analysis. By combining computer simulations and biological experiments, it was revealed that protein disulfide isomerase (PDI) plays a crucial role in the resistance mechanism of Sorafenib and that its efficacy can be improved significantly by blocking PDI.
The research team used mice in the experiment and discovered the synergic effect of PDI inhibition with Sorafenib for reducing liver cancer cells, known as hepatocellular carcinoma. Also, more PDIs are shown in tissue from patients who possess a resistance to Sorafenib. From these findings, the team could identify the possibility of its clinical applications. The team also confirmed these findings from clinical data through a retrospective cohort study.
“Molecules that play an important role in cell lines are mostly put under complex regulation. For this reason, the existing biological research has a fundamental limitations for discovering its underlying principles,” Professor Cho said. “This research is a representative case of overcoming this limitation of traditional life science research by using a Systems Biology approach, combining IT and life science. It suggests the possibility of developing a new method that overcomes drug resistance with a network analysis of the targeted drug action mechanism of cancer.”
The research was supported by the National Research Foundation of Korea (NRF) and funded by the Ministry of Science and ICT.
(Figure 1. Simulation results from cellular experiments using hepatocellular carcinoma)
(Figure 2. Network analysis and computer simulation by using the endoplasmic reticulum (ER) stress network)
(Figure 3. ER stress network model)
2017.08.30 View 13138