THE
-
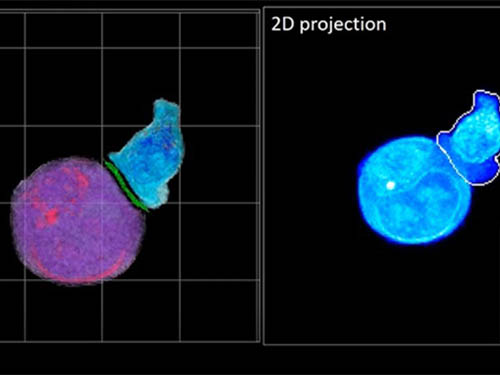 Deep-Learning and 3D Holographic Microscopy Beats Scientists at Analyzing Cancer Immunotherapy
Live tracking and analyzing of the dynamics of chimeric antigen receptor (CAR) T-cells targeting cancer cells can open new avenues for the development of cancer immunotherapy. However, imaging via conventional microscopy approaches can result in cellular damage, and assessments of cell-to-cell interactions are extremely difficult and labor-intensive. When researchers applied deep learning and 3D holographic microscopy to the task, however, they not only avoided these difficultues but found that AI was better at it than humans were.
Artificial intelligence (AI) is helping researchers decipher images from a new holographic microscopy technique needed to investigate a key process in cancer immunotherapy “live” as it takes place. The AI transformed work that, if performed manually by scientists, would otherwise be incredibly labor-intensive and time-consuming into one that is not only effortless but done better than they could have done it themselves. The research, conducted by the team of Professor YongKeun Park from the Department of Physics, appeared in the journal eLife last December.
A critical stage in the development of the human immune system’s ability to respond not just generally to any invader (such as pathogens or cancer cells) but specifically to that particular type of invader and remember it should it attempt to invade again is the formation of a junction between an immune cell called a T-cell and a cell that presents the antigen, or part of the invader that is causing the problem, to it. This process is like when a picture of a suspect is sent to a police car so that the officers can recognize the criminal they are trying to track down. The junction between the two cells, called the immunological synapse, or IS, is the key process in teaching the immune system how to recognize a specific type of invader.
Since the formation of the IS junction is such a critical step for the initiation of an antigen-specific immune response, various techniques allowing researchers to observe the process as it happens have been used to study its dynamics. Most of these live imaging techniques rely on fluorescence microscopy, where genetic tweaking causes part of a protein from a cell to fluoresce, in turn allowing the subject to be tracked via fluorescence rather than via the reflected light used in many conventional microscopy techniques.
However, fluorescence-based imaging can suffer from effects such as photo-bleaching and photo-toxicity, preventing the assessment of dynamic changes in the IS junction process over the long term. Fluorescence-based imaging still involves illumination, whereupon the fluorophores (chemical compounds that cause the fluorescence) emit light of a different color. Photo-bleaching or photo-toxicity occur when the subject is exposed to too much illumination, resulting in chemical alteration or cellular damage.
One recent option that does away with fluorescent labelling and thereby avoids such problems is 3D holographic microscopy or holotomography (HT). In this technique, the refractive index (the way that light changes direction when encountering a substance with a different density—why a straw looks like it bends in a glass of water) is recorded in 3D as a hologram.
Until now, HT has been used to study single cells, but never cell-cell interactions involved in immune responses. One of the main reasons is the difficulty of “segmentation,” or distinguishing the different parts of a cell and thus distinguishing between the interacting cells; in other words, deciphering which part belongs to which cell.
Manual segmentation, or marking out the different parts manually, is one option, but it is difficult and time-consuming, especially in three dimensions. To overcome this problem, automatic segmentation has been developed in which simple computer algorithms perform the identification.
“But these basic algorithms often make mistakes,” explained Professor YongKeun Park, “particularly with respect to adjoining segmentation, which of course is exactly what is occurring here in the immune response we’re most interested in.”
So, the researchers applied a deep learning framework to the HT segmentation problem. Deep learning is a type of machine learning in which artificial neural networks based on the human brain recognize patterns in a way that is similar to how humans do this. Regular machine learning requires data as an input that has already been labelled. The AI “learns” by understanding the labeled data and then recognizes the concept that has been labelled when it is fed novel data. For example, AI trained on a thousand images of cats labelled “cat” should be able to recognize a cat the next time it encounters an image with a cat in it. Deep learning involves multiple layers of artificial neural networks attacking much larger, but unlabeled datasets, in which the AI develops its own ‘labels’ for concepts it encounters.
In essence, the deep learning framework that KAIST researchers developed, called DeepIS, came up with its own concepts by which it distinguishes the different parts of the IS junction process. To validate this method, the research team applied it to the dynamics of a particular IS junction formed between chimeric antigen receptor (CAR) T-cells and target cancer cells. They then compared the results to what they would normally have done: the laborious process of performing the segmentation manually. They found not only that DeepIS was able to define areas within the IS with high accuracy, but that the technique was even able to capture information about the total distribution of proteins within the IS that may not have been easily measured using conventional techniques.
“In addition to allowing us to avoid the drudgery of manual segmentation and the problems of photo-bleaching and photo-toxicity, we found that the AI actually did a better job,” Professor Park added.
The next step will be to combine the technique with methods of measuring how much physical force is applied by different parts of the IS junction, such as holographic optical tweezers or traction force microscopy.
-Profile
Professor YongKeun Park
Department of Physics
Biomedical Optics Laboratory
http://bmol.kaist.ac.kr
KAIST
2021.02.24 View 12043
Deep-Learning and 3D Holographic Microscopy Beats Scientists at Analyzing Cancer Immunotherapy
Live tracking and analyzing of the dynamics of chimeric antigen receptor (CAR) T-cells targeting cancer cells can open new avenues for the development of cancer immunotherapy. However, imaging via conventional microscopy approaches can result in cellular damage, and assessments of cell-to-cell interactions are extremely difficult and labor-intensive. When researchers applied deep learning and 3D holographic microscopy to the task, however, they not only avoided these difficultues but found that AI was better at it than humans were.
Artificial intelligence (AI) is helping researchers decipher images from a new holographic microscopy technique needed to investigate a key process in cancer immunotherapy “live” as it takes place. The AI transformed work that, if performed manually by scientists, would otherwise be incredibly labor-intensive and time-consuming into one that is not only effortless but done better than they could have done it themselves. The research, conducted by the team of Professor YongKeun Park from the Department of Physics, appeared in the journal eLife last December.
A critical stage in the development of the human immune system’s ability to respond not just generally to any invader (such as pathogens or cancer cells) but specifically to that particular type of invader and remember it should it attempt to invade again is the formation of a junction between an immune cell called a T-cell and a cell that presents the antigen, or part of the invader that is causing the problem, to it. This process is like when a picture of a suspect is sent to a police car so that the officers can recognize the criminal they are trying to track down. The junction between the two cells, called the immunological synapse, or IS, is the key process in teaching the immune system how to recognize a specific type of invader.
Since the formation of the IS junction is such a critical step for the initiation of an antigen-specific immune response, various techniques allowing researchers to observe the process as it happens have been used to study its dynamics. Most of these live imaging techniques rely on fluorescence microscopy, where genetic tweaking causes part of a protein from a cell to fluoresce, in turn allowing the subject to be tracked via fluorescence rather than via the reflected light used in many conventional microscopy techniques.
However, fluorescence-based imaging can suffer from effects such as photo-bleaching and photo-toxicity, preventing the assessment of dynamic changes in the IS junction process over the long term. Fluorescence-based imaging still involves illumination, whereupon the fluorophores (chemical compounds that cause the fluorescence) emit light of a different color. Photo-bleaching or photo-toxicity occur when the subject is exposed to too much illumination, resulting in chemical alteration or cellular damage.
One recent option that does away with fluorescent labelling and thereby avoids such problems is 3D holographic microscopy or holotomography (HT). In this technique, the refractive index (the way that light changes direction when encountering a substance with a different density—why a straw looks like it bends in a glass of water) is recorded in 3D as a hologram.
Until now, HT has been used to study single cells, but never cell-cell interactions involved in immune responses. One of the main reasons is the difficulty of “segmentation,” or distinguishing the different parts of a cell and thus distinguishing between the interacting cells; in other words, deciphering which part belongs to which cell.
Manual segmentation, or marking out the different parts manually, is one option, but it is difficult and time-consuming, especially in three dimensions. To overcome this problem, automatic segmentation has been developed in which simple computer algorithms perform the identification.
“But these basic algorithms often make mistakes,” explained Professor YongKeun Park, “particularly with respect to adjoining segmentation, which of course is exactly what is occurring here in the immune response we’re most interested in.”
So, the researchers applied a deep learning framework to the HT segmentation problem. Deep learning is a type of machine learning in which artificial neural networks based on the human brain recognize patterns in a way that is similar to how humans do this. Regular machine learning requires data as an input that has already been labelled. The AI “learns” by understanding the labeled data and then recognizes the concept that has been labelled when it is fed novel data. For example, AI trained on a thousand images of cats labelled “cat” should be able to recognize a cat the next time it encounters an image with a cat in it. Deep learning involves multiple layers of artificial neural networks attacking much larger, but unlabeled datasets, in which the AI develops its own ‘labels’ for concepts it encounters.
In essence, the deep learning framework that KAIST researchers developed, called DeepIS, came up with its own concepts by which it distinguishes the different parts of the IS junction process. To validate this method, the research team applied it to the dynamics of a particular IS junction formed between chimeric antigen receptor (CAR) T-cells and target cancer cells. They then compared the results to what they would normally have done: the laborious process of performing the segmentation manually. They found not only that DeepIS was able to define areas within the IS with high accuracy, but that the technique was even able to capture information about the total distribution of proteins within the IS that may not have been easily measured using conventional techniques.
“In addition to allowing us to avoid the drudgery of manual segmentation and the problems of photo-bleaching and photo-toxicity, we found that the AI actually did a better job,” Professor Park added.
The next step will be to combine the technique with methods of measuring how much physical force is applied by different parts of the IS junction, such as holographic optical tweezers or traction force microscopy.
-Profile
Professor YongKeun Park
Department of Physics
Biomedical Optics Laboratory
http://bmol.kaist.ac.kr
KAIST
2021.02.24 View 12043 -
 Professor Bumjoon Kim Named Scientist of the Month
Professor Bumjoon Kim from the Department of Chemical and Biomolecular Engineering won January’s Scientist of the Month Award presented by the Ministry of Science and ICT (MSIT) and the National Research Foundation of Korea (NRF) on January 6. Professor Kim also received 10 million won in prize money.
Professor Kim was recognized for his research in the field of fuel cells. Since the first paper on fuel cells was published in 1839 by the German chemist Friedrich Schonbein, there has been an increase in the number of fields in which fuel cells are used, including national defense, aerospace engineering, and autonomous vehicles.
Professor Kim developed carbonized block copolymer particles with high durability and a high-performance fuel cell. Block copolymers are two different polymers cross-linked into a chain structure. Various nanostructures can be made effectively by using the attractive and repulsive forces between the chains.
Professor Kim used the membrane emulsification technique, employing a high-performance separation membrane to develop a platform that makes the mass production of highly durable carbonized particles possible, which he then used to develop high-performance energy devices like fuel cells.
The carbonized particles designed by Professor Kim and his research team were used to create the world’s more durable fuel cells that boast outstanding performance while using only five percent of the costly platinum needed for existing commercialized products.
The team’s research results were published in the Journal of the American Chemical Society and Energy Environmental Science in May and July of last year.
“We have developed a fuel cell that ticks all the boxes including performance, durability, and cost,” said Professor Kim. “Related techniques will not be limited to fuel cells, but could also be applied to the development of various energy devices like solar cells and secondary cells,” he added.
(END)
2021.01.22 View 11592
Professor Bumjoon Kim Named Scientist of the Month
Professor Bumjoon Kim from the Department of Chemical and Biomolecular Engineering won January’s Scientist of the Month Award presented by the Ministry of Science and ICT (MSIT) and the National Research Foundation of Korea (NRF) on January 6. Professor Kim also received 10 million won in prize money.
Professor Kim was recognized for his research in the field of fuel cells. Since the first paper on fuel cells was published in 1839 by the German chemist Friedrich Schonbein, there has been an increase in the number of fields in which fuel cells are used, including national defense, aerospace engineering, and autonomous vehicles.
Professor Kim developed carbonized block copolymer particles with high durability and a high-performance fuel cell. Block copolymers are two different polymers cross-linked into a chain structure. Various nanostructures can be made effectively by using the attractive and repulsive forces between the chains.
Professor Kim used the membrane emulsification technique, employing a high-performance separation membrane to develop a platform that makes the mass production of highly durable carbonized particles possible, which he then used to develop high-performance energy devices like fuel cells.
The carbonized particles designed by Professor Kim and his research team were used to create the world’s more durable fuel cells that boast outstanding performance while using only five percent of the costly platinum needed for existing commercialized products.
The team’s research results were published in the Journal of the American Chemical Society and Energy Environmental Science in May and July of last year.
“We have developed a fuel cell that ticks all the boxes including performance, durability, and cost,” said Professor Kim. “Related techniques will not be limited to fuel cells, but could also be applied to the development of various energy devices like solar cells and secondary cells,” he added.
(END)
2021.01.22 View 11592 -
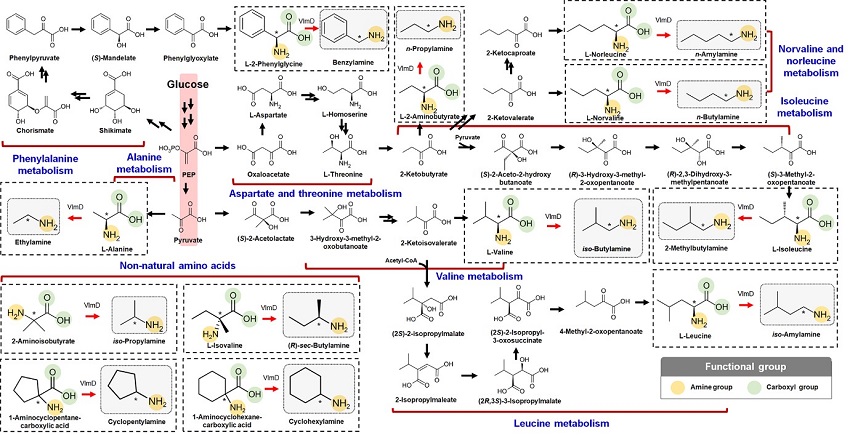 Expanding the Biosynthetic Pathway via Retrobiosynthesis
- Researchers reports a new strategy for the microbial production of multiple short-chain primary amines via retrobiosynthesis. -
KAIST metabolic engineers presented the bio-based production of multiple short-chain primary amines that have a wide range of applications in chemical industries for the first time. The research team led by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering designed the novel biosynthetic pathways for short-chain primary amines by combining retrobiosynthesis and a precursor selection step.
The research team verified the newly designed pathways by confirming the in vivo production of 10 short-chain primary amines by supplying the precursors. Furthermore, the platform Escherichia coli strains were metabolically engineered to produce three proof-of-concept short-chain primary amines from glucose, demonstrating the possibility of the bio-based production of diverse short-chain primary amines from renewable resources. The research team said this study expands the strategy of systematically designing biosynthetic pathways for the production of a group of related chemicals as demonstrated by multiple short-chain primary amines as examples.
Currently, most of the industrial chemicals used in our daily lives are produced with petroleum-based products. However, there are several serious issues with the petroleum industry such as the depletion of fossil fuel reserves and environmental problems including global warming. To solve these problems, the sustainable production of industrial chemicals and materials is being explored with microorganisms as cell factories and renewable non-food biomass as raw materials for alternative to petroleum-based products. The engineering of these microorganisms has increasingly become more efficient and effective with the help of systems metabolic engineering – a practice of engineering the metabolism of a living organism toward the production of a desired metabolite. In this regard, the number of chemicals produced using biomass as a raw material has substantially increased.
Although the scope of chemicals that are producible using microorganisms continues to expand through advances in systems metabolic engineering, the biological production of short-chain primary amines has not yet been reported despite their industrial importance. Short-chain primary amines are the chemicals that have an alkyl or aryl group in the place of a hydrogen atom in ammonia with carbon chain lengths ranging from C1 to C7. Short-chain primary amines have a wide range of applications in chemical industries, for example, as a precursor for pharmaceuticals (e.g., antidiabetic and antihypertensive drugs), agrochemicals (e.g., herbicides, fungicides and insecticides), solvents, and vulcanization accelerators for rubber and plasticizers. The market size of short-chain primary amines was estimated to be more than 4 billion US dollars in 2014.
The main reason why the bio-based production of short-chain primary amines was not yet possible was due to their unknown biosynthetic pathways. Therefore, the team designed synthetic biosynthetic pathways for short-chain primary amines by combining retrobiosynthesis and a precursor selection step. The retrobiosynthesis allowed the systematic design of a biosynthetic pathway for short-chain primary amines by using a set of biochemical reaction rules that describe chemical transformation patterns between a substrate and product molecules at an atomic level.
These multiple precursors predicted for the possible biosynthesis of each short-chain primary amine were sequentially narrowed down by using the precursor selection step for efficient metabolic engineering experiments.
“Our research demonstrates the possibility of the renewable production of short-chain primary amines for the first time. We are planning to increase production efficiencies of short-chain primary amines. We believe that our study will play an important role in the development of sustainable and eco-friendly bio-based industries and the reorganization of the chemical industry, which is mandatory for solving the environmental problems threating the survival of mankind,” said Professor Lee.
This paper titled “Microbial production of multiple short-chain primary amines via retrobiosynthesis” was published in Nature Communications. This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries from the Ministry of Science and ICT through the National Research Foundation (NRF) of Korea.
-Publication
Dong In Kim, Tong Un Chae, Hyun Uk Kim, Woo Dae Jang, and Sang Yup Lee. Microbial production of multiple short-chain primary amines via retrobiosynthesis. Nature Communications ( https://www.nature.com/articles/s41467-020-20423-6)
-Profile
Distinguished Professor Sang Yup Lee
leesy@kaist.ac.kr
Metabolic &Biomolecular Engineering National Research Laboratory
http://mbel.kaist.ac.kr
Department of Chemical and Biomolecular Engineering
KAIST
2021.01.14 View 11884
Expanding the Biosynthetic Pathway via Retrobiosynthesis
- Researchers reports a new strategy for the microbial production of multiple short-chain primary amines via retrobiosynthesis. -
KAIST metabolic engineers presented the bio-based production of multiple short-chain primary amines that have a wide range of applications in chemical industries for the first time. The research team led by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering designed the novel biosynthetic pathways for short-chain primary amines by combining retrobiosynthesis and a precursor selection step.
The research team verified the newly designed pathways by confirming the in vivo production of 10 short-chain primary amines by supplying the precursors. Furthermore, the platform Escherichia coli strains were metabolically engineered to produce three proof-of-concept short-chain primary amines from glucose, demonstrating the possibility of the bio-based production of diverse short-chain primary amines from renewable resources. The research team said this study expands the strategy of systematically designing biosynthetic pathways for the production of a group of related chemicals as demonstrated by multiple short-chain primary amines as examples.
Currently, most of the industrial chemicals used in our daily lives are produced with petroleum-based products. However, there are several serious issues with the petroleum industry such as the depletion of fossil fuel reserves and environmental problems including global warming. To solve these problems, the sustainable production of industrial chemicals and materials is being explored with microorganisms as cell factories and renewable non-food biomass as raw materials for alternative to petroleum-based products. The engineering of these microorganisms has increasingly become more efficient and effective with the help of systems metabolic engineering – a practice of engineering the metabolism of a living organism toward the production of a desired metabolite. In this regard, the number of chemicals produced using biomass as a raw material has substantially increased.
Although the scope of chemicals that are producible using microorganisms continues to expand through advances in systems metabolic engineering, the biological production of short-chain primary amines has not yet been reported despite their industrial importance. Short-chain primary amines are the chemicals that have an alkyl or aryl group in the place of a hydrogen atom in ammonia with carbon chain lengths ranging from C1 to C7. Short-chain primary amines have a wide range of applications in chemical industries, for example, as a precursor for pharmaceuticals (e.g., antidiabetic and antihypertensive drugs), agrochemicals (e.g., herbicides, fungicides and insecticides), solvents, and vulcanization accelerators for rubber and plasticizers. The market size of short-chain primary amines was estimated to be more than 4 billion US dollars in 2014.
The main reason why the bio-based production of short-chain primary amines was not yet possible was due to their unknown biosynthetic pathways. Therefore, the team designed synthetic biosynthetic pathways for short-chain primary amines by combining retrobiosynthesis and a precursor selection step. The retrobiosynthesis allowed the systematic design of a biosynthetic pathway for short-chain primary amines by using a set of biochemical reaction rules that describe chemical transformation patterns between a substrate and product molecules at an atomic level.
These multiple precursors predicted for the possible biosynthesis of each short-chain primary amine were sequentially narrowed down by using the precursor selection step for efficient metabolic engineering experiments.
“Our research demonstrates the possibility of the renewable production of short-chain primary amines for the first time. We are planning to increase production efficiencies of short-chain primary amines. We believe that our study will play an important role in the development of sustainable and eco-friendly bio-based industries and the reorganization of the chemical industry, which is mandatory for solving the environmental problems threating the survival of mankind,” said Professor Lee.
This paper titled “Microbial production of multiple short-chain primary amines via retrobiosynthesis” was published in Nature Communications. This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries from the Ministry of Science and ICT through the National Research Foundation (NRF) of Korea.
-Publication
Dong In Kim, Tong Un Chae, Hyun Uk Kim, Woo Dae Jang, and Sang Yup Lee. Microbial production of multiple short-chain primary amines via retrobiosynthesis. Nature Communications ( https://www.nature.com/articles/s41467-020-20423-6)
-Profile
Distinguished Professor Sang Yup Lee
leesy@kaist.ac.kr
Metabolic &Biomolecular Engineering National Research Laboratory
http://mbel.kaist.ac.kr
Department of Chemical and Biomolecular Engineering
KAIST
2021.01.14 View 11884 -
 Emeritus Professor Jae-Kyu Lee Wins the AIS LEO Award
Emeritus Professor Jae-Kyu Lee has won the Association for Information Systems LEO Award 2020. Professor Lee, the first Korean to receive the LEO Award, was recognized for his research and development in preventative cyber security, which is a major part of the efforts he leads to realize what Professor Lee has named "Bright Internet."
Established in 1999, this award was named after the world’s first business application of computing, the Lyons Electronic Office and recognizes outstanding individuals in the field of information systems. The LEO Award recognized four winners including Professor Lee this year.
He has been professor and HHI Chair Professor at KAIST from 1985 to 2016 since he has received his Ph.D. in information and operations management from the Wharton School, University of Pennsylvania. He served as the Dean of College of Business and supervised around 30 doctoral students. He is currently the Distinguished Professor of School of Management at Xi’an Jiaotong University.
His research mainly focused on the creation of Bright Internet for preventive cybersecurity, improving relevance of research from Axiomatic Theories, and development of AI for electronic commerce and managerial decision support.
He is a fellow and was the president of the Association for Information Systems, and co-chaired the International Conference on Information Systems in 2017. He was the founder of Principles for the Bright Internet and established the Bright Internet Research Center at KAIST and Xi’an Jiatong University. He also established the Bright Internet Global Summit since ICIS 2017 in Seoul, and organized the Bright Internet Project Consortium in 2019 as a combined effort of academia-industry partnership. (www.brightinternet.org.)
He was a charter member of the Pacific Asia Conference in Information Systems, and served as conference chair. He was the founder editor-in-chief of the journal, Electronic Commerce Research and Applications (Elsevier), and was the founding chair of the International Conference on Electronic Commerce. In Korea, her served as president of Korea Society of Management Information Systems and Korea Society of Intelligent Information Systems.
"I am honored to be designated the first Korean winner of the honorable LEO Award," Lee said. "Based on my life-long efforts for developments in the field, I will continue to contribute to the research and development of information media systems."
2020.12.16 View 6519
Emeritus Professor Jae-Kyu Lee Wins the AIS LEO Award
Emeritus Professor Jae-Kyu Lee has won the Association for Information Systems LEO Award 2020. Professor Lee, the first Korean to receive the LEO Award, was recognized for his research and development in preventative cyber security, which is a major part of the efforts he leads to realize what Professor Lee has named "Bright Internet."
Established in 1999, this award was named after the world’s first business application of computing, the Lyons Electronic Office and recognizes outstanding individuals in the field of information systems. The LEO Award recognized four winners including Professor Lee this year.
He has been professor and HHI Chair Professor at KAIST from 1985 to 2016 since he has received his Ph.D. in information and operations management from the Wharton School, University of Pennsylvania. He served as the Dean of College of Business and supervised around 30 doctoral students. He is currently the Distinguished Professor of School of Management at Xi’an Jiaotong University.
His research mainly focused on the creation of Bright Internet for preventive cybersecurity, improving relevance of research from Axiomatic Theories, and development of AI for electronic commerce and managerial decision support.
He is a fellow and was the president of the Association for Information Systems, and co-chaired the International Conference on Information Systems in 2017. He was the founder of Principles for the Bright Internet and established the Bright Internet Research Center at KAIST and Xi’an Jiatong University. He also established the Bright Internet Global Summit since ICIS 2017 in Seoul, and organized the Bright Internet Project Consortium in 2019 as a combined effort of academia-industry partnership. (www.brightinternet.org.)
He was a charter member of the Pacific Asia Conference in Information Systems, and served as conference chair. He was the founder editor-in-chief of the journal, Electronic Commerce Research and Applications (Elsevier), and was the founding chair of the International Conference on Electronic Commerce. In Korea, her served as president of Korea Society of Management Information Systems and Korea Society of Intelligent Information Systems.
"I am honored to be designated the first Korean winner of the honorable LEO Award," Lee said. "Based on my life-long efforts for developments in the field, I will continue to contribute to the research and development of information media systems."
2020.12.16 View 6519 -
 Mystery Solved with Math: Cytoplasmic Traffic Jam Disrupts Sleep-Wake Cycles
KAIST mathematicians and their collaborators at Florida State University have identified the principle of how aging and diseases like dementia and obesity cause sleep disorders. A combination of mathematical modelling and experiments demonstrated that the cytoplasmic congestion caused by aging, dementia, and/or obesity disrupts the circadian rhythms in the human body and leads to irregular sleep-wake cycles. This finding suggests new treatment strategies for addressing unstable sleep-wake cycles.
Human bodies adjust sleep schedules in accordance with the ‘circadian rhythms’, which are regulated by our time keeping system, the ‘circadian clock’. This clock tells our body when to rest by generating the 24-hour rhythms of a protein called PERIOD (PER) (See Figure 1).
The amount of the PER protein increases for half of the day and then decreases for the remaining half. The principle is that the PER protein accumulating in the cytoplasm for several hours enters the cell nucleus all at once, hindering the transcription of PER genes and thereby reducing the amount of PER.
However, it has remained a mystery how thousands of PER molecules can simultaneously enter into the nucleus in a complex cell environment where a variety of materials co-exist and can interfere with the motion of PER. This would be like finding a way for thousands of employees from all over New York City to enter an office building at the same time every day.
A group of researchers led by Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences solved the mystery by developing a spatiotemporal and probabilistic model that describes the motion of PER molecules in a cell environment.
This study was conducted in collaboration with Professor Choogon Lee’s group from Florida State University, where the experiments were carried out, and the results were published in the Proceedings of the National Academy of Sciences (PNAS) last month.
The joint research team’s spatial stochastic model (See Figure 2) described the motion of PER molecules in cells and demonstrated that the PER molecule should be sufficiently condensed around the cell nucleus to be phosphorylated simultaneously and enter the nucleus together (See Figure 3 Left). Thanks to this phosphorylation synchronization switch, thousands of PER molecules can enter the nucleus at the same time every day and maintain stable circadian rhythms.
However, when aging and/or diseases including dementia and obesity cause the cytoplasm to become congested with increased cytoplasmic obstacles such as protein aggregates and fat vacuoles, it hinders the timely condensation of PER molecules around the cell nucleus (See Figure 3 Right). As a result, the phosphorylation synchronization switch does not work and PER proteins enter into the nucleus at irregular times, making the circadian rhythms and sleep-wake cycles unstable, the study revealed.
Professor Kim said, “As a mathematician, I am excited to help enable the advancement of new treatment strategies that can improve the lives of so many patients who suffer from irregular sleep-wake cycles. Taking these findings as an opportunity, I hope to see more active interchanges of ideas and collaboration between mathematical and biological sciences.”
This work was supported by the National Institutes of Health and the National Science Foundation in the US, and the International Human Frontiers Science Program Organization and the National Research Foundation of Korea.
Publication:
Beesley, S. and Kim, D. W, et al. (2020) Wake-sleep cycles are severely disrupted by diseases affecting cytoplasmic homeostasis. Proceedings of the National Academy of Sciences (PNAS), Vol. 117, No. 45, 28402-28411. Available online at https://doi.org/10.1073/pnas.2003524117
Profile:
Jae Kyoung Kim, Ph.D.
Associate Professor
jaekkim@kaist.ac.kr
http://mathsci.kaist.ac.kr/~jaekkim
@umichkim on Twitter
Department of Mathematical Sciences
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile:
Choogon Lee, Ph.D.
Associate Professor
clee@neuro.fsu.edu
https://med.fsu.edu/biosci/lee-lab
Department of Biomedical Sciences
Florida State University
Florida, USA
(END)
2020.12.11 View 10067
Mystery Solved with Math: Cytoplasmic Traffic Jam Disrupts Sleep-Wake Cycles
KAIST mathematicians and their collaborators at Florida State University have identified the principle of how aging and diseases like dementia and obesity cause sleep disorders. A combination of mathematical modelling and experiments demonstrated that the cytoplasmic congestion caused by aging, dementia, and/or obesity disrupts the circadian rhythms in the human body and leads to irregular sleep-wake cycles. This finding suggests new treatment strategies for addressing unstable sleep-wake cycles.
Human bodies adjust sleep schedules in accordance with the ‘circadian rhythms’, which are regulated by our time keeping system, the ‘circadian clock’. This clock tells our body when to rest by generating the 24-hour rhythms of a protein called PERIOD (PER) (See Figure 1).
The amount of the PER protein increases for half of the day and then decreases for the remaining half. The principle is that the PER protein accumulating in the cytoplasm for several hours enters the cell nucleus all at once, hindering the transcription of PER genes and thereby reducing the amount of PER.
However, it has remained a mystery how thousands of PER molecules can simultaneously enter into the nucleus in a complex cell environment where a variety of materials co-exist and can interfere with the motion of PER. This would be like finding a way for thousands of employees from all over New York City to enter an office building at the same time every day.
A group of researchers led by Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences solved the mystery by developing a spatiotemporal and probabilistic model that describes the motion of PER molecules in a cell environment.
This study was conducted in collaboration with Professor Choogon Lee’s group from Florida State University, where the experiments were carried out, and the results were published in the Proceedings of the National Academy of Sciences (PNAS) last month.
The joint research team’s spatial stochastic model (See Figure 2) described the motion of PER molecules in cells and demonstrated that the PER molecule should be sufficiently condensed around the cell nucleus to be phosphorylated simultaneously and enter the nucleus together (See Figure 3 Left). Thanks to this phosphorylation synchronization switch, thousands of PER molecules can enter the nucleus at the same time every day and maintain stable circadian rhythms.
However, when aging and/or diseases including dementia and obesity cause the cytoplasm to become congested with increased cytoplasmic obstacles such as protein aggregates and fat vacuoles, it hinders the timely condensation of PER molecules around the cell nucleus (See Figure 3 Right). As a result, the phosphorylation synchronization switch does not work and PER proteins enter into the nucleus at irregular times, making the circadian rhythms and sleep-wake cycles unstable, the study revealed.
Professor Kim said, “As a mathematician, I am excited to help enable the advancement of new treatment strategies that can improve the lives of so many patients who suffer from irregular sleep-wake cycles. Taking these findings as an opportunity, I hope to see more active interchanges of ideas and collaboration between mathematical and biological sciences.”
This work was supported by the National Institutes of Health and the National Science Foundation in the US, and the International Human Frontiers Science Program Organization and the National Research Foundation of Korea.
Publication:
Beesley, S. and Kim, D. W, et al. (2020) Wake-sleep cycles are severely disrupted by diseases affecting cytoplasmic homeostasis. Proceedings of the National Academy of Sciences (PNAS), Vol. 117, No. 45, 28402-28411. Available online at https://doi.org/10.1073/pnas.2003524117
Profile:
Jae Kyoung Kim, Ph.D.
Associate Professor
jaekkim@kaist.ac.kr
http://mathsci.kaist.ac.kr/~jaekkim
@umichkim on Twitter
Department of Mathematical Sciences
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile:
Choogon Lee, Ph.D.
Associate Professor
clee@neuro.fsu.edu
https://med.fsu.edu/biosci/lee-lab
Department of Biomedical Sciences
Florida State University
Florida, USA
(END)
2020.12.11 View 10067 -
 A Comprehensive Review of Biosynthesis of Inorganic Nanomaterials Using Microorganisms and Bacteriophages
There are diverse methods for producing numerous inorganic nanomaterials involving many experimental variables. Among the numerous possible matches, finding the best pair for synthesizing in an environmentally friendly way has been a longstanding challenge for researchers and industries.
A KAIST bioprocess engineering research team led by Distinguished Professor Sang Yup Lee conducted a summary of 146 biosynthesized single and multi-element inorganic nanomaterials covering 55 elements in the periodic table synthesized using wild-type and genetically engineered microorganisms. Their research highlights the diverse applications of biogenic nanomaterials and gives strategies for improving the biosynthesis of nanomaterials in terms of their producibility, crystallinity, size, and shape.
The research team described a 10-step flow chart for developing the biosynthesis of inorganic nanomaterials using microorganisms and bacteriophages. The research was published at Nature Review Chemistry as a cover and hero paper on December 3.
“We suggest general strategies for microbial nanomaterial biosynthesis via a step-by-step flow chart and give our perspectives on the future of nanomaterial biosynthesis and applications. This flow chart will serve as a general guide for those wishing to prepare biosynthetic inorganic nanomaterials using microbial cells,” explained Dr.Yoojin Choi, a co-author of this research.
Most inorganic nanomaterials are produced using physical and chemical methods and biological synthesis has been gaining more and more attention. However, conventional synthesis processes have drawbacks in terms of high energy consumption and non-environmentally friendly processes. Meanwhile, microorganisms such as microalgae, yeasts, fungi, bacteria, and even viruses can be utilized as biofactories to produce single and multi-element inorganic nanomaterials under mild conditions.
After conducting a massive survey, the research team summed up that the development of genetically engineered microorganisms with increased inorganic-ion-binding affinity, inorganic-ion-reduction ability, and nanomaterial biosynthetic efficiency has enabled the synthesis of many inorganic nanomaterials.
Among the strategies, the team introduced their analysis of a Pourbaix diagram for controlling the size and morphology of a product. The research team said this Pourbaix diagram analysis can be widely employed for biosynthesizing new nanomaterials with industrial applications.Professor Sang Yup Lee added, “This research provides extensive information and perspectives on the biosynthesis of diverse inorganic nanomaterials using microorganisms and bacteriophages and their applications. We expect that biosynthetic inorganic nanomaterials will find more diverse and innovative applications across diverse fields of science and technology.”
Dr. Choi started this research in 2018 and her interview about completing this extensive research was featured in an article at Nature Career article on December 4.
-ProfileDistinguished Professor Sang Yup Lee leesy@kaist.ac.krMetabolic &Biomolecular Engineering National Research Laboratoryhttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.12.07 View 10596
A Comprehensive Review of Biosynthesis of Inorganic Nanomaterials Using Microorganisms and Bacteriophages
There are diverse methods for producing numerous inorganic nanomaterials involving many experimental variables. Among the numerous possible matches, finding the best pair for synthesizing in an environmentally friendly way has been a longstanding challenge for researchers and industries.
A KAIST bioprocess engineering research team led by Distinguished Professor Sang Yup Lee conducted a summary of 146 biosynthesized single and multi-element inorganic nanomaterials covering 55 elements in the periodic table synthesized using wild-type and genetically engineered microorganisms. Their research highlights the diverse applications of biogenic nanomaterials and gives strategies for improving the biosynthesis of nanomaterials in terms of their producibility, crystallinity, size, and shape.
The research team described a 10-step flow chart for developing the biosynthesis of inorganic nanomaterials using microorganisms and bacteriophages. The research was published at Nature Review Chemistry as a cover and hero paper on December 3.
“We suggest general strategies for microbial nanomaterial biosynthesis via a step-by-step flow chart and give our perspectives on the future of nanomaterial biosynthesis and applications. This flow chart will serve as a general guide for those wishing to prepare biosynthetic inorganic nanomaterials using microbial cells,” explained Dr.Yoojin Choi, a co-author of this research.
Most inorganic nanomaterials are produced using physical and chemical methods and biological synthesis has been gaining more and more attention. However, conventional synthesis processes have drawbacks in terms of high energy consumption and non-environmentally friendly processes. Meanwhile, microorganisms such as microalgae, yeasts, fungi, bacteria, and even viruses can be utilized as biofactories to produce single and multi-element inorganic nanomaterials under mild conditions.
After conducting a massive survey, the research team summed up that the development of genetically engineered microorganisms with increased inorganic-ion-binding affinity, inorganic-ion-reduction ability, and nanomaterial biosynthetic efficiency has enabled the synthesis of many inorganic nanomaterials.
Among the strategies, the team introduced their analysis of a Pourbaix diagram for controlling the size and morphology of a product. The research team said this Pourbaix diagram analysis can be widely employed for biosynthesizing new nanomaterials with industrial applications.Professor Sang Yup Lee added, “This research provides extensive information and perspectives on the biosynthesis of diverse inorganic nanomaterials using microorganisms and bacteriophages and their applications. We expect that biosynthetic inorganic nanomaterials will find more diverse and innovative applications across diverse fields of science and technology.”
Dr. Choi started this research in 2018 and her interview about completing this extensive research was featured in an article at Nature Career article on December 4.
-ProfileDistinguished Professor Sang Yup Lee leesy@kaist.ac.krMetabolic &Biomolecular Engineering National Research Laboratoryhttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.12.07 View 10596 -
 Three Professors Named to Highly Cited Researchers 2020 List
Distinguished Professor Sukbok Chang from the Department of Chemistry, Distinguished Professor Sang-Yup Lee from the Department of Chemical & Biomolecular Engineering, and Professor Jiyong Eom from the College of Business were named to Clarivate’s Highly Cited Researchers 2020 list.
Clarivate announced the researchers who rank in the top 1% of citations by field and publication year in the Web of Science citation index. A total of 6,167 researchers from more than 60 countries were listed this year and 37 Korean scholars made the list.
The methodology that determines the “Who’s Who” of influential researchers draws on data and analyses performed by bibliometric experts and data scientists at the Institute for Scientific Information at Clarivate. It also uses the tallies to identify the countries and research institutions where these scientific elite are based. More than 6,000 researchers from 21 fields in the sciences, social sciences, and cross field categories were selected based on the number of highly cited papers they produced over an 11-year period from January 2009 to December 2019.
Professor Chang made the list six years in a row, while Professor Lee made it for four consecutive years, and Professor Eom for the last two years. Professor Chang’s group (http://sbchang.kaist.ac.kr) investigates catalytic hydrocarbon functionalization. Professor Lee (http://mbel.kaist.ac.kr) is a pioneering scholar in the field of metabolic engineering, systems, and synthetic biology. Professor Eom’s (https://kaistceps.quv.kr) research extends to energy and environmental economics and management, energy big data, and green information systems.
2020.11.30 View 9330
Three Professors Named to Highly Cited Researchers 2020 List
Distinguished Professor Sukbok Chang from the Department of Chemistry, Distinguished Professor Sang-Yup Lee from the Department of Chemical & Biomolecular Engineering, and Professor Jiyong Eom from the College of Business were named to Clarivate’s Highly Cited Researchers 2020 list.
Clarivate announced the researchers who rank in the top 1% of citations by field and publication year in the Web of Science citation index. A total of 6,167 researchers from more than 60 countries were listed this year and 37 Korean scholars made the list.
The methodology that determines the “Who’s Who” of influential researchers draws on data and analyses performed by bibliometric experts and data scientists at the Institute for Scientific Information at Clarivate. It also uses the tallies to identify the countries and research institutions where these scientific elite are based. More than 6,000 researchers from 21 fields in the sciences, social sciences, and cross field categories were selected based on the number of highly cited papers they produced over an 11-year period from January 2009 to December 2019.
Professor Chang made the list six years in a row, while Professor Lee made it for four consecutive years, and Professor Eom for the last two years. Professor Chang’s group (http://sbchang.kaist.ac.kr) investigates catalytic hydrocarbon functionalization. Professor Lee (http://mbel.kaist.ac.kr) is a pioneering scholar in the field of metabolic engineering, systems, and synthetic biology. Professor Eom’s (https://kaistceps.quv.kr) research extends to energy and environmental economics and management, energy big data, and green information systems.
2020.11.30 View 9330 -
 Drawing the Line to Answer Art’s Big Questions
- KAIST scientists show how statistical physics can reveal art trends across time and culture. -
Algorithms have shown that the compositional structure of Western landscape paintings changed “suspiciously” smoothly between 1500 and 2000 AD, potentially indicating a selection bias by art curators or in art historical literature, physicists from the Korea Advanced Institute of Science and Technology (KAIST) and colleagues report in the Proceedings of the National Academy of Sciences (PNAS).
KAIST statistical physicist Hawoong Jeong worked with statisticians, digital analysts and art historians in Korea, Estonia and the US to clarify whether computer algorithms could help resolve long-standing questions about design principles used in landscape paintings, such as the placement of the horizon and other primary features.
“A foundational question among art historians is whether artwork contains organizing principles that transcend culture and time and, if yes, how these principles evolved over time,” explains Jeong. “We developed an information-theoretic approach that can capture compositional proportion in landscape paintings and found that the preferred compositional proportion systematically evolved over time.”
Digital versions of almost 15,000 canonical landscape paintings from the Western renaissance in the 1500s to the more recent contemporary art period were run through a computer algorithm. The algorithm progressively divides artwork into horizontal and vertical lines depending on the amount of information in each subsequent partition. It allows scientists to evaluate how artists and various art styles compose landscape artwork, in terms of placement of a piece’s most important components, in addition to how high or low the landscape’s horizon is placed.
The scientists started by analysing the first two partitioning lines identified by the algorithm in the paintings and found they could be categorized into four groups: an initial horizontal line followed by a second horizontal line (H-H); an initial horizontal line followed by a second vertical line (H-V); a vertical followed by horizontal line (V-H); or a vertical followed by a vertical line (V-V) (see image 1 and 2). They then looked at the categorizations over time.
They found that before the mid-nineteenth century, H-V was the dominant composition type, followed by H-H, V-H, and V-V. The mid-nineteenth century then brought change, with the H-V composition style decreasing in popularity with a rise in the H-H composition style. The other two styles remained relatively stable.
The scientists also looked at how the horizon line, which separates sky from land, changed over time. In the 16th century, the dominant horizon line of the painting was above the middle of the canvas, but it gradually descended to the lower middle of the canvas by the 17th century, where it remained until the mid-nineteenth century. After that, the horizon line began gradually rising again.
Interestingly, the algorithm showed that these findings were similar across cultures and artistic periods, even through periods dominated by a diversity in art styles. This similarity may well be a function, then, of a bias in the dataset.
“In recent decades, art historians have prioritized the argument that there is great diversity in the evolution of artistic expression rather than offering a relatively smoother consensus story in Western art,” Jeong says. “This study serves as a reminder that the available large-scale datasets might be perpetuating severe biases.”
The scientists next aim to broaden their analyses to include more diverse artwork, as this particular dataset was ultimately Western and male biased. Future analyses should also consider diagonal compositions in paintings, they say.
This work was supported by the National Research Foundation (NRF) of Korea.
Publication:
Lee, B, et al. (2020) Dissecting landscape art history with information theory. Proceedings of the National Academy of Sciences (PNAS), Vol. 117, No. 43, 26580-26590. Available online at https://doi.org/10.1073/pnas.2011927117
Profile:
Hawoong Jeong, Ph.D.
Professor
hjeong@kaist.ac.kr
https://www.kaist.ac.kr
Department of Physics
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
(END)
2020.11.13 View 11001
Drawing the Line to Answer Art’s Big Questions
- KAIST scientists show how statistical physics can reveal art trends across time and culture. -
Algorithms have shown that the compositional structure of Western landscape paintings changed “suspiciously” smoothly between 1500 and 2000 AD, potentially indicating a selection bias by art curators or in art historical literature, physicists from the Korea Advanced Institute of Science and Technology (KAIST) and colleagues report in the Proceedings of the National Academy of Sciences (PNAS).
KAIST statistical physicist Hawoong Jeong worked with statisticians, digital analysts and art historians in Korea, Estonia and the US to clarify whether computer algorithms could help resolve long-standing questions about design principles used in landscape paintings, such as the placement of the horizon and other primary features.
“A foundational question among art historians is whether artwork contains organizing principles that transcend culture and time and, if yes, how these principles evolved over time,” explains Jeong. “We developed an information-theoretic approach that can capture compositional proportion in landscape paintings and found that the preferred compositional proportion systematically evolved over time.”
Digital versions of almost 15,000 canonical landscape paintings from the Western renaissance in the 1500s to the more recent contemporary art period were run through a computer algorithm. The algorithm progressively divides artwork into horizontal and vertical lines depending on the amount of information in each subsequent partition. It allows scientists to evaluate how artists and various art styles compose landscape artwork, in terms of placement of a piece’s most important components, in addition to how high or low the landscape’s horizon is placed.
The scientists started by analysing the first two partitioning lines identified by the algorithm in the paintings and found they could be categorized into four groups: an initial horizontal line followed by a second horizontal line (H-H); an initial horizontal line followed by a second vertical line (H-V); a vertical followed by horizontal line (V-H); or a vertical followed by a vertical line (V-V) (see image 1 and 2). They then looked at the categorizations over time.
They found that before the mid-nineteenth century, H-V was the dominant composition type, followed by H-H, V-H, and V-V. The mid-nineteenth century then brought change, with the H-V composition style decreasing in popularity with a rise in the H-H composition style. The other two styles remained relatively stable.
The scientists also looked at how the horizon line, which separates sky from land, changed over time. In the 16th century, the dominant horizon line of the painting was above the middle of the canvas, but it gradually descended to the lower middle of the canvas by the 17th century, where it remained until the mid-nineteenth century. After that, the horizon line began gradually rising again.
Interestingly, the algorithm showed that these findings were similar across cultures and artistic periods, even through periods dominated by a diversity in art styles. This similarity may well be a function, then, of a bias in the dataset.
“In recent decades, art historians have prioritized the argument that there is great diversity in the evolution of artistic expression rather than offering a relatively smoother consensus story in Western art,” Jeong says. “This study serves as a reminder that the available large-scale datasets might be perpetuating severe biases.”
The scientists next aim to broaden their analyses to include more diverse artwork, as this particular dataset was ultimately Western and male biased. Future analyses should also consider diagonal compositions in paintings, they say.
This work was supported by the National Research Foundation (NRF) of Korea.
Publication:
Lee, B, et al. (2020) Dissecting landscape art history with information theory. Proceedings of the National Academy of Sciences (PNAS), Vol. 117, No. 43, 26580-26590. Available online at https://doi.org/10.1073/pnas.2011927117
Profile:
Hawoong Jeong, Ph.D.
Professor
hjeong@kaist.ac.kr
https://www.kaist.ac.kr
Department of Physics
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
(END)
2020.11.13 View 11001 -
 Professor Kyu-Young Whang Donates Toward the 50th Anniversary Memorial Building
Distinguished Professor Kyu-Young Whang from the School of Computing made a gift of 100 million KRW toward the construction of the 50th Anniversary Memorial Building during a ceremony on November 3 at the Daejeon campus. "As a member of the first class of KAIST, I feel very delighted to play a part in the fundraising campaign for the 50th anniversary celebration. This is also a token of appreciation to my alma mater and I look forward to alumni and the KAIST community joining this campaign," said Professor Emeritus Whang. KAIST will name the Kyu-Young Whang and Jonghae Song Christian Seminar Room at the 50th Anniversary Memorial Building. The ground will be broken in 2022 for construction of the building.
2020.11.04 View 6367
Professor Kyu-Young Whang Donates Toward the 50th Anniversary Memorial Building
Distinguished Professor Kyu-Young Whang from the School of Computing made a gift of 100 million KRW toward the construction of the 50th Anniversary Memorial Building during a ceremony on November 3 at the Daejeon campus. "As a member of the first class of KAIST, I feel very delighted to play a part in the fundraising campaign for the 50th anniversary celebration. This is also a token of appreciation to my alma mater and I look forward to alumni and the KAIST community joining this campaign," said Professor Emeritus Whang. KAIST will name the Kyu-Young Whang and Jonghae Song Christian Seminar Room at the 50th Anniversary Memorial Building. The ground will be broken in 2022 for construction of the building.
2020.11.04 View 6367 -
 'Mini-Lungs' Reveal Early Stages of SARS-CoV-2 Infection
Researchers in Korea and the UK have successfully grown miniature models of critical lung structures called alveoli, and used them to study how the coronavirus that causes COVID-19 infects the lungs.
To date, there have been more than 40 million cases of COVID-19 and almost 1.13 million deaths worldwide. The main target tissues of SARS-CoV-2, the virus that causes COVID-19, especially in patients that develop pneumonia, appear to be alveoli – tiny air sacs in the lungs that take up the oxygen we breathe and exchange it with carbon dioxide to exhale.
To better understand how SARS-CoV-2 infects the lungs and causes disease, a team of Professor Young Seok Ju from the Graduate School of Medical Science and Engineering at KAIST in collaboration with the Wellcome-MRC Cambridge Stem Cell Institute at the University of Cambridge turned to organoids – ‘mini-organs’ grown in three dimensions to mimic the behaviour of tissue and organs.
The team used tissue donated to tissue banks at the Royal Papworth Hospital NHS Foundation Trust and Addenbrooke’s Hospital, Cambridge University NHS Foundations Trust, UK, and Seoul National University Hospital to extract a type of lung cell known as human lung alveolar type 2 cells. By reprogramming these cells back to their earlier ‘stem cell’ stage, they were able to grow self-organizing alveolar-like 3D structures that mimic the behaviour of key lung tissue.
“The research community now has a powerful new platform to study precisely how the virus infects the lungs, as well as explore possible treatments,” said Professor Ju, co-senior author of the research.
Dr. Joo-Hyeon Lee, another co-senior author at the Wellcome-MRC Cambridge Stem Cell Institute, said: “We still know surprisingly little about how SARS-CoV-2 infects the lungs and causes disease. Our approach has allowed us to grow 3D models of key lung tissue – in a sense, ‘mini-lungs’ – in the lab and study what happens when they become infected.”
The team infected the organoids with a strain of SARS-CoV-2 taken from a patient in Korea who was diagnosed with COVID-19 on January 26 after traveling to Wuhan, China. Using a combination of fluorescence imaging and single cell genetic analysis, they were able to study how the cells responded to the virus.
When the 3D models were exposed to SARS-CoV-2, the virus began to replicate rapidly, reaching full cellular infection just six hours after infection. Replication enables the virus to spread throughout the body, infecting other cells and tissue.
Around the same time, the cells began to produce interferons – proteins that act as warning signals to neighbouring cells, telling them to activate their antiviral defences. After 48 hours, the interferons triggered the innate immune response – its first line of defence – and the cells started fighting back against infection.
Sixty hours after infection, a subset of alveolar cells began to disintegrate, leading to cell death and damage to the lung tissue.
Although the researchers observed changes to the lung cells within three days of infection, clinical symptoms of COVID-19 rarely occur so quickly and can sometimes take more than ten days after exposure to appear. The team say there are several possible reasons for this. It may take several days from the virus first infiltrating the upper respiratory tract to it reaching the alveoli. It may also require a substantial proportion of alveolar cells to be infected or for further interactions with immune cells resulting in inflammation before a patient displays symptoms.
“Based on our model we can tackle many unanswered key questions, such as understanding genetic susceptibility to SARS-CoV-2, assessing relative infectivity of viral mutants, and revealing the damage processes of the virus in human alveolar cells,” said Professor Ju. “Most importantly, it provides the opportunity to develop and screen potential therapeutic agents against SARS-CoV-2 infection.”
“We hope to use our technique to grow these 3D models from cells of patients who are particularly vulnerable to infection, such as the elderly or people with diseased lungs, and find out what happens to their tissue,” added Dr. Lee.
The research was a collaboration involving scientists from KAIST, the University of Cambridge, Korea National Institute of Health, Institute for Basic Science (IBS), Seoul National University Hospital and Genome Insight in Korea.
- ProfileProfessor Young Seok JuLaboratory of Cancer Genomics https://julab.kaist.ac.kr the Graduate School of Medical Science and EngineeringKAIST
2020.10.26 View 11414
'Mini-Lungs' Reveal Early Stages of SARS-CoV-2 Infection
Researchers in Korea and the UK have successfully grown miniature models of critical lung structures called alveoli, and used them to study how the coronavirus that causes COVID-19 infects the lungs.
To date, there have been more than 40 million cases of COVID-19 and almost 1.13 million deaths worldwide. The main target tissues of SARS-CoV-2, the virus that causes COVID-19, especially in patients that develop pneumonia, appear to be alveoli – tiny air sacs in the lungs that take up the oxygen we breathe and exchange it with carbon dioxide to exhale.
To better understand how SARS-CoV-2 infects the lungs and causes disease, a team of Professor Young Seok Ju from the Graduate School of Medical Science and Engineering at KAIST in collaboration with the Wellcome-MRC Cambridge Stem Cell Institute at the University of Cambridge turned to organoids – ‘mini-organs’ grown in three dimensions to mimic the behaviour of tissue and organs.
The team used tissue donated to tissue banks at the Royal Papworth Hospital NHS Foundation Trust and Addenbrooke’s Hospital, Cambridge University NHS Foundations Trust, UK, and Seoul National University Hospital to extract a type of lung cell known as human lung alveolar type 2 cells. By reprogramming these cells back to their earlier ‘stem cell’ stage, they were able to grow self-organizing alveolar-like 3D structures that mimic the behaviour of key lung tissue.
“The research community now has a powerful new platform to study precisely how the virus infects the lungs, as well as explore possible treatments,” said Professor Ju, co-senior author of the research.
Dr. Joo-Hyeon Lee, another co-senior author at the Wellcome-MRC Cambridge Stem Cell Institute, said: “We still know surprisingly little about how SARS-CoV-2 infects the lungs and causes disease. Our approach has allowed us to grow 3D models of key lung tissue – in a sense, ‘mini-lungs’ – in the lab and study what happens when they become infected.”
The team infected the organoids with a strain of SARS-CoV-2 taken from a patient in Korea who was diagnosed with COVID-19 on January 26 after traveling to Wuhan, China. Using a combination of fluorescence imaging and single cell genetic analysis, they were able to study how the cells responded to the virus.
When the 3D models were exposed to SARS-CoV-2, the virus began to replicate rapidly, reaching full cellular infection just six hours after infection. Replication enables the virus to spread throughout the body, infecting other cells and tissue.
Around the same time, the cells began to produce interferons – proteins that act as warning signals to neighbouring cells, telling them to activate their antiviral defences. After 48 hours, the interferons triggered the innate immune response – its first line of defence – and the cells started fighting back against infection.
Sixty hours after infection, a subset of alveolar cells began to disintegrate, leading to cell death and damage to the lung tissue.
Although the researchers observed changes to the lung cells within three days of infection, clinical symptoms of COVID-19 rarely occur so quickly and can sometimes take more than ten days after exposure to appear. The team say there are several possible reasons for this. It may take several days from the virus first infiltrating the upper respiratory tract to it reaching the alveoli. It may also require a substantial proportion of alveolar cells to be infected or for further interactions with immune cells resulting in inflammation before a patient displays symptoms.
“Based on our model we can tackle many unanswered key questions, such as understanding genetic susceptibility to SARS-CoV-2, assessing relative infectivity of viral mutants, and revealing the damage processes of the virus in human alveolar cells,” said Professor Ju. “Most importantly, it provides the opportunity to develop and screen potential therapeutic agents against SARS-CoV-2 infection.”
“We hope to use our technique to grow these 3D models from cells of patients who are particularly vulnerable to infection, such as the elderly or people with diseased lungs, and find out what happens to their tissue,” added Dr. Lee.
The research was a collaboration involving scientists from KAIST, the University of Cambridge, Korea National Institute of Health, Institute for Basic Science (IBS), Seoul National University Hospital and Genome Insight in Korea.
- ProfileProfessor Young Seok JuLaboratory of Cancer Genomics https://julab.kaist.ac.kr the Graduate School of Medical Science and EngineeringKAIST
2020.10.26 View 11414 -
 Experts to Help Asia Navigate the Post-COVID-19 and 4IR Eras
Risk Quotient 2020, an international conference co-hosted by KAIST and the National University of Singapore (NUS), will bring together world-leading experts from academia and industry to help Asia navigate the post-COVID-19 and Fourth Industrial Revolution (4IR) eras.
The online conference will be held on October 29 from 10 a.m. Korean time under the theme “COVID-19 Pandemic and A Brave New World”. It will be streamed live on YouTube at https://www.youtube.com/c/KAISTofficial and https://www.youtube.com/user/NUScast.
The Korea Policy Center for the Fourth Industrial Revolution (KPC4IR) at KAIST organized this conference in collaboration with the Lloyd's Register Foundation Institute for the Public Understanding of Risk (IPUR) at NUS.
During the conference, global leaders will examine the socioeconomic impacts of the COVID-19 pandemic on areas including digital innovation, education, the workforce, and the economy. They will then highlight digital and 4IR technologies that could be utilized to effectively mitigate the risks and challenges associated with the pandemic, while harnessing the opportunities that these socioeconomic effects may present. Their discussions will mainly focus on the Asian region.
In his opening remarks, KAIST President Sung-Chul Shin will express his appreciation for the Asian populations’ greater trust in and compliance with their governments, which have given the continent a leg up against the coronavirus. He will then emphasize that by working together through the exchange of ideas and global collaboration, we will be able to shape ‘a brave new world’ to better humanity. Welcoming remarks by Prof. Sang Yup Lee (Dean, KAIST Institutes) and Prof. Tze Yun Leong (Director, AI Technology at AI Singapore) will follow.
For the keynote speech, Prof. Lan Xue (Dean, Schwarzman College, Tsinghua University) will share China’s response to COVID-19 and lessons for crisis management. Prof. Danny Quah (Dean, Lee Kuan Yew School of Public Policy, NUS) will present possible ways to overcome these difficult times. Dr. Kak-Soo Shin (Senior Advisor, Shin & Kim LLC, Former Ambassador to the State of Israel and Japan, and Former First and Second Vice Minister of the Ministry of Foreign Affairs of the Republic of Korea) will stress the importance of the international community’s solidarity to ensure peace, prosperity, and safety in this new era.
Panel Session I will address the impact of COVID-19 on digital innovation. Dr. Carol Soon (Senior Research Fellow, Institute of Policy Studies, NUS) will present her interpretation of recent technological developments as both opportunities for our society as a whole and challenges for vulnerable groups such as low-income families. Dr. Christopher SungWook Chang (Managing Director, Kakao Mobility) will show how changes in mobility usage patterns can be captured by Kakao Mobility’s big data analysis. He will illustrate how the data can be used to interpret citizen’s behaviors and how risks can be transformed into opportunities by utilizing technology. Mr. Steve Ledzian’s (Vice President, Chief Technology Officer, FireEye) talk will discuss the dangers caused by threat actors and other cyber risk implications of COVID-19. Dr. June Sung Park (Chairman, Korea Software Technology Association (KOSTA)) will share how COVID-19 has accelerated digital transformations across all industries and why software education should be reformed to improve Korea’s competitiveness.
Panel Session II will examine the impact on education and the workforce. Dr. Sang-Jin Ban (President, Korean Educational Development Institute (KEDI)) will explain Korea’s educational response to the pandemic and the concept of “blended learning” as a new paradigm, and present both positive and negative impacts of online education on students’ learning experiences. Prof. Reuben Ng (Professor, Lee Kuan Yew School of Public Policy, NUS) will present on graduate underemployment, which seems to have worsened during COVID-19. Dr. Michael Fung’s presentation (Deputy Chief Executive (Industry), SkillsFuture SG) will introduce the promotion of lifelong learning in Singapore through a new national initiative known as the ‘SkillsFuture Movement’. This movement serves as an example of a national response to disruptions in the job market and the pace of skills obsolescence triggered by AI and COVID-19.
Panel Session III will touch on technology leadership and Asia’s digital economy and society. Prof. Naubahar Sharif (Professor, Division of Social Science and Division of Public Policy, Hong Kong University of Science and Technology (HKUST)) will share his views on the potential of China in taking over global technological leadership based on its massive domestic market, its government support, and the globalization process. Prof. Yee Kuang Heng (Professor, Graduate School of Public Policy, University of Tokyo) will illustrate how different legal and political needs in China and Japan have shaped the ways technologies have been deployed in responding to COVID-19. Dr. Hayun Kang (Head, International Cooperation Research Division, Korea Information Society Development Institute (KISDI)) will explain Korea’s relative success containing the pandemic compared to other countries, and how policy leaders and institutions that embrace digital technologies in the pursuit of public welfare objectives can produce positive outcomes while minimizing the side effects.
Prof. Kyung Ryul Park (Graduate School of Science and Technology Policy, KAIST) will be hosting the entire conference, whereas Prof. Alice Hae Yun Oh (Director, MARS Artificial Intelligence Research Center, KAIST), Prof. Wonjoon Kim (Dean, Graduate School of Innovation and Technology Management, College of Business, KAIST), Prof. Youngsun Kwon (Dean, KAIST Academy), and Prof. Taejun Lee (Korea Development Institute (KDI) School of Public Policy and Management) are to chair discussions with the keynote speakers and panelists.
Closing remarks will be delivered by Prof. Chan Ghee Koh (Director, NUS IPUR), Prof. So Young Kim (Director, KAIST KPC4IR), and Prof. Joungho Kim (Director, KAIST Global Strategy Institute (GSI)).
“This conference is expected to serve as a springboard to help Asian countries recover from global crises such as the COVID-19 pandemic through active cooperation and joint engagement among scholars, experts, and policymakers,” according to Director So Young Kim.
(END)
2020.10.22 View 14182
Experts to Help Asia Navigate the Post-COVID-19 and 4IR Eras
Risk Quotient 2020, an international conference co-hosted by KAIST and the National University of Singapore (NUS), will bring together world-leading experts from academia and industry to help Asia navigate the post-COVID-19 and Fourth Industrial Revolution (4IR) eras.
The online conference will be held on October 29 from 10 a.m. Korean time under the theme “COVID-19 Pandemic and A Brave New World”. It will be streamed live on YouTube at https://www.youtube.com/c/KAISTofficial and https://www.youtube.com/user/NUScast.
The Korea Policy Center for the Fourth Industrial Revolution (KPC4IR) at KAIST organized this conference in collaboration with the Lloyd's Register Foundation Institute for the Public Understanding of Risk (IPUR) at NUS.
During the conference, global leaders will examine the socioeconomic impacts of the COVID-19 pandemic on areas including digital innovation, education, the workforce, and the economy. They will then highlight digital and 4IR technologies that could be utilized to effectively mitigate the risks and challenges associated with the pandemic, while harnessing the opportunities that these socioeconomic effects may present. Their discussions will mainly focus on the Asian region.
In his opening remarks, KAIST President Sung-Chul Shin will express his appreciation for the Asian populations’ greater trust in and compliance with their governments, which have given the continent a leg up against the coronavirus. He will then emphasize that by working together through the exchange of ideas and global collaboration, we will be able to shape ‘a brave new world’ to better humanity. Welcoming remarks by Prof. Sang Yup Lee (Dean, KAIST Institutes) and Prof. Tze Yun Leong (Director, AI Technology at AI Singapore) will follow.
For the keynote speech, Prof. Lan Xue (Dean, Schwarzman College, Tsinghua University) will share China’s response to COVID-19 and lessons for crisis management. Prof. Danny Quah (Dean, Lee Kuan Yew School of Public Policy, NUS) will present possible ways to overcome these difficult times. Dr. Kak-Soo Shin (Senior Advisor, Shin & Kim LLC, Former Ambassador to the State of Israel and Japan, and Former First and Second Vice Minister of the Ministry of Foreign Affairs of the Republic of Korea) will stress the importance of the international community’s solidarity to ensure peace, prosperity, and safety in this new era.
Panel Session I will address the impact of COVID-19 on digital innovation. Dr. Carol Soon (Senior Research Fellow, Institute of Policy Studies, NUS) will present her interpretation of recent technological developments as both opportunities for our society as a whole and challenges for vulnerable groups such as low-income families. Dr. Christopher SungWook Chang (Managing Director, Kakao Mobility) will show how changes in mobility usage patterns can be captured by Kakao Mobility’s big data analysis. He will illustrate how the data can be used to interpret citizen’s behaviors and how risks can be transformed into opportunities by utilizing technology. Mr. Steve Ledzian’s (Vice President, Chief Technology Officer, FireEye) talk will discuss the dangers caused by threat actors and other cyber risk implications of COVID-19. Dr. June Sung Park (Chairman, Korea Software Technology Association (KOSTA)) will share how COVID-19 has accelerated digital transformations across all industries and why software education should be reformed to improve Korea’s competitiveness.
Panel Session II will examine the impact on education and the workforce. Dr. Sang-Jin Ban (President, Korean Educational Development Institute (KEDI)) will explain Korea’s educational response to the pandemic and the concept of “blended learning” as a new paradigm, and present both positive and negative impacts of online education on students’ learning experiences. Prof. Reuben Ng (Professor, Lee Kuan Yew School of Public Policy, NUS) will present on graduate underemployment, which seems to have worsened during COVID-19. Dr. Michael Fung’s presentation (Deputy Chief Executive (Industry), SkillsFuture SG) will introduce the promotion of lifelong learning in Singapore through a new national initiative known as the ‘SkillsFuture Movement’. This movement serves as an example of a national response to disruptions in the job market and the pace of skills obsolescence triggered by AI and COVID-19.
Panel Session III will touch on technology leadership and Asia’s digital economy and society. Prof. Naubahar Sharif (Professor, Division of Social Science and Division of Public Policy, Hong Kong University of Science and Technology (HKUST)) will share his views on the potential of China in taking over global technological leadership based on its massive domestic market, its government support, and the globalization process. Prof. Yee Kuang Heng (Professor, Graduate School of Public Policy, University of Tokyo) will illustrate how different legal and political needs in China and Japan have shaped the ways technologies have been deployed in responding to COVID-19. Dr. Hayun Kang (Head, International Cooperation Research Division, Korea Information Society Development Institute (KISDI)) will explain Korea’s relative success containing the pandemic compared to other countries, and how policy leaders and institutions that embrace digital technologies in the pursuit of public welfare objectives can produce positive outcomes while minimizing the side effects.
Prof. Kyung Ryul Park (Graduate School of Science and Technology Policy, KAIST) will be hosting the entire conference, whereas Prof. Alice Hae Yun Oh (Director, MARS Artificial Intelligence Research Center, KAIST), Prof. Wonjoon Kim (Dean, Graduate School of Innovation and Technology Management, College of Business, KAIST), Prof. Youngsun Kwon (Dean, KAIST Academy), and Prof. Taejun Lee (Korea Development Institute (KDI) School of Public Policy and Management) are to chair discussions with the keynote speakers and panelists.
Closing remarks will be delivered by Prof. Chan Ghee Koh (Director, NUS IPUR), Prof. So Young Kim (Director, KAIST KPC4IR), and Prof. Joungho Kim (Director, KAIST Global Strategy Institute (GSI)).
“This conference is expected to serve as a springboard to help Asian countries recover from global crises such as the COVID-19 pandemic through active cooperation and joint engagement among scholars, experts, and policymakers,” according to Director So Young Kim.
(END)
2020.10.22 View 14182 -
 Scientist of October: Professor Jungwon Kim
Professor Jungwon Kim from the Department of Mechanical Engineering was selected as the ‘Scientist of the Month’ for October 2020 by the Ministry of Science and ICT and the National Research Foundation of Korea. Professor Kim was recognized for his contributions to expanding the horizons of the basics of precision engineering through his research on multifunctional ultrahigh-speed, high-resolution sensors. He received 10 million KRW in prize money.
Professor Kim was selected as the recipient of this award in celebration of “Measurement Day”, which commemorates October 26 as the day in which King Sejong the Great established a volume measurement system.
Professor Kim discovered that the time difference between the pulse of light created by a laser and the pulse of the current produced by a light-emitting diode was as small as 100 attoseconds (10-16 seconds). He then developed a unique multifunctional ultrahigh-speed, high-resolution Time-of-Flight (TOF) sensor that could take measurements of multiple points at the same time by sampling electric light. The sensor, with a measurement speed of 100 megahertz (100 million vibrations per second), a resolution of 180 picometers (1/5.5 billion meters), and a dynamic range of 150 decibels, overcame the limitations of both existing TOF techniques and laser interferometric techniques at the same time. The results of this research were published in Nature Photonics on February 10, 2020.
Professor Kim said, “I’d like to thank the graduate students who worked passionately with me, and KAIST for providing an environment in which I could fully focus on research. I am looking forward to the new and diverse applications in the field of machine manufacturing, such as studying the dynamic phenomena in microdevices, or taking ultraprecision measurement of shapes for advanced manufacturing.”
(END)
2020.10.15 View 11407
Scientist of October: Professor Jungwon Kim
Professor Jungwon Kim from the Department of Mechanical Engineering was selected as the ‘Scientist of the Month’ for October 2020 by the Ministry of Science and ICT and the National Research Foundation of Korea. Professor Kim was recognized for his contributions to expanding the horizons of the basics of precision engineering through his research on multifunctional ultrahigh-speed, high-resolution sensors. He received 10 million KRW in prize money.
Professor Kim was selected as the recipient of this award in celebration of “Measurement Day”, which commemorates October 26 as the day in which King Sejong the Great established a volume measurement system.
Professor Kim discovered that the time difference between the pulse of light created by a laser and the pulse of the current produced by a light-emitting diode was as small as 100 attoseconds (10-16 seconds). He then developed a unique multifunctional ultrahigh-speed, high-resolution Time-of-Flight (TOF) sensor that could take measurements of multiple points at the same time by sampling electric light. The sensor, with a measurement speed of 100 megahertz (100 million vibrations per second), a resolution of 180 picometers (1/5.5 billion meters), and a dynamic range of 150 decibels, overcame the limitations of both existing TOF techniques and laser interferometric techniques at the same time. The results of this research were published in Nature Photonics on February 10, 2020.
Professor Kim said, “I’d like to thank the graduate students who worked passionately with me, and KAIST for providing an environment in which I could fully focus on research. I am looking forward to the new and diverse applications in the field of machine manufacturing, such as studying the dynamic phenomena in microdevices, or taking ultraprecision measurement of shapes for advanced manufacturing.”
(END)
2020.10.15 View 11407