research
A new efficient oxide coating technology that can be applied in less than five minutes could lead to dramatic improvements in the lifetime and performance of fuel cells. The fundamental principle behind this approach is maximizing the oxygen reduction reaction site of a platinum thin-film electrode, preventing the electrodes from aggregating at high temperatures.
Fuel cells have emerged as a clean electricity generation system that does not pollute the air. In particular, solid oxide fuel cells (SOFCs) are beginning to gain a great deal of attention due to their higher power generation efficiency compared to other fuel cells. It is also advantageous to use other power sources than expensive hydrogen fuel.
However, the high costs and insufficient lifetimes caused by high temperatures needed to operate the solid oxide fuel cells have remained significant challenges to commercialization.
Recently, attempts to lower the operating temperature (< 600°C) of these devices by introducing thin-film processes have drew attention of researchers, with the resulting products known as thin-film-based solid oxide fuel cells.
In order to create enhanced device performance at lower temperatures, the research team, led by Professor WooChul Jung in the Department of Materials Science and Engineering, applied and developed oxide coating technology to maximize the oxygen reduction reaction sites of a platinum thin-film electrode and to prevent platinum electrodes from thermal aggregating.
The team succeeded in over-coating a platinum electrode with a new coating material called praseodymium-doped ceria (Pr,Ce)O2-, which has high conductivity for both electrons and oxygen ions and excellent catalytic properties for oxygen reduction reactions. As a result, electrode resistance was reduced by more than 1000 times, creating the potential for these electrodes to be used in high-temperature electrochemical cells.
In addition, they proposed that the high performance of thin-film-based oxide fuel cells’ oxygen electrodes could be realized through the nano-structuring of (Pr,Ce)O2-δ without any platinum.
Professor Jung said, “The electrode coating technology used in this study is of great technical value because of the utilization of affordable and mass-produced electrochemical deposition.” He added, “In the future, this technology will be feasible for replacing platinum electrodes in thin-film-based oxide fuel cells, and we expect that the affordable prices of this fuel cell will eventually boost market competitiveness.”
This research was described in Advanced Energy Materials in July and was featured as the Inside Front Cover and video abstract. It was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Korea Electric Power Corporation (KEPCO) Research Institute.
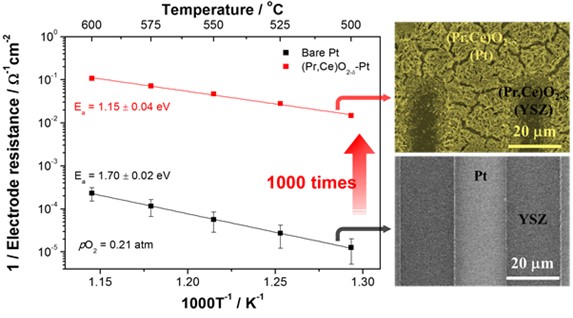
Figure 1. The change of electrode activity with and without overcoated (Pr,Ce)O2-δ nanostructures.
-
research Novel Material Properties of Hybrid Perovskite Nanostructures for Next-generation Non-linear Electronic Devices
(from left: Juho Lee, Dr. Muhammad Ejaz Khan and Professor Yong-Hoon Kim) A KAIST research team reported a novel non-linear device with the founding property coming from perovskite nanowires. They showed that hybrid perovskite-derived, inorganic-framework nanowires can acquire semi-metallicity, and proposed negative differential resistance (NDR) devices with excellent NDR characteristics that resulted from a novel quantum-hybridization NDR mechanism, implying the potential of perovskite nano
2019-02-22 -
research Hierarchical Porous Titanium Nitride Synthesized by Multiscale Phase Separation for LSBs
(from left: Professor Jinwoo Lee and PhD candidate Won-Gwang Lim) A KAIST research team developed ultra-stable, high-rate lithium-sulfur batteries (LSBs) by using hierarchical porous titanium nitride as a sulfur host, and achieved superior cycle stability and high rate performance for LSBs. The control of large amounts of energy is required for use in an electric vehicle or smart grid system. In this sense, the development of next-generation secondary batteries is in high
2019-01-28 -
research Highly Scalable Process to Obtain Stable 2D Nanosheet Dispersion
(Professor Do Hyun Kim and his team) A KAIST team developed technology that allows the mass production of two-dimensional (2D) nanomaterial dispersion by utilizing the characteristic shearing force of hydraulic power. The 2D nanosheet dispersion can be directly applied to solution-based processes to manufacture devices for electronics as well as energy storage and conversion. It is expected to be used in these devices with improved performance. There have been numero
2018-12-19 -
research Characteristics of Submesoscale Geophysical Turbulence Reported
A KAIST research team has reported some of unique characteristics and driving forces behind submesoscale geophysical turbulence. Using big data analysis on ocean surface currents and chlorophyll concentrations observed using coastal radars and satellites has brought better understanding of oceanic processes in space and time scales of O(1) kilometer and O(1) hour. The outcomes of this work will lead to improved tracking of water-borne materials and performance in global and regional climate
2018-12-13 -
research Silk Adhesive Paves the Way for Epidermal Electronics
(from left: Dr. Ji-Won Seo, Professor Hyunjoo Jenny Lee and PhD candidate, Hyojung Kim) Producing effective epidermal electronics requires a strong, biocompatible interface between a biological surface and a sensor. Here, a KAIST team employed a calcium-modified silk fibroin as a biocompatible and strong adhesive. This technology led to the development of epidermal electronics with strong adhesion for patients who need drug injections and physiological monitoring over a long
2018-11-21