Chem
-
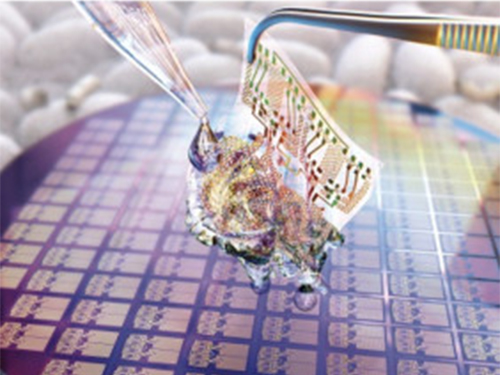 Wafer-Scale Multilayer Fabrication of Silk Fibroin-Based Microelectronics
A KAIST research team developed a novel fabrication method for the multilayer processing of silk-based microelectronics. This technology for creating a biodegradable silk fibroin film allows microfabrication with polymer or metal structures manufactured from photolithography. It can be a key technology in the implementation of silk fibroin-based biodegradable electronic devices or localized drug delivery through silk fibroin patterns.
Silk fibroins are biocompatible, biodegradable, transparent, and flexible, which makes them excellent candidates for implantable biomedical devices, and they have also been used as biodegradable films and functional microstructures in biomedical applications. However, conventional microfabrication processes require strong etching solutions and solvents to modify the structure of silk fibroins.
To prevent the silk fibroin from being damaged during the process, Professor Hyunjoo J. Lee from the School of Electrical Engineering and her team came up with a novel process, named aluminum hard mask on silk fibroin (AMoS), which is capable of micropatterning multiple layers composed of both fibroin and inorganic materials, such as metal and dielectrics with high-precision microscale alignment. The AMoS process can make silk fibroin patterns on devices, or make patterns on silk fibroin thin films with other materials by using photolithography, which is a core technology in the current microfabrication process.
The team successfully cultured primary neurons on the processed silk fibroin micro-patterns, and confirmed that silk fibroin has excellent biocompatibility before and after the fabrication process and that it also can be applied to implanted biological devices.
Through this technology, the team realized the multilayer micropatterning of fibroin films on a silk fibroin substrate and fabricated a biodegradable microelectric circuit consisting of resistors and silk fibroin dielectric capacitors in a silicon wafer with large areas.
They also used this technology to position the micro-pattern of the silk fibroin thin film closer to the flexible polymer-based brain electrode, and confirmed the dye molecules mounted on the silk fibroin were transferred successfully from the micropatterns.
Professor Lee said, “This technology facilitates wafer-scale, large-area processing of sensitive materials. We expect it to be applied to a wide range of biomedical devices in the future. Using the silk fibroin with micro-patterned brain electrodes can open up many new possibilities in research on brain circuits by mounting drugs that restrict or promote brain cell activities.”
This research, in collaboration with Dr. Nakwon Choi from KIST and led by PhD candidate Geon Kook, was published in ACS AMI (10.1021/acsami.8b13170) on January 16, 2019.
Figure 1. The cover page of ACS AMI
Figure 2. Fibroin microstructures and metal patterns on a fibroin produced by using the AMoS mask.
Figure 3. Biocompatibility assessment of the AMoS Process. Top: Schematics image of a) fibroin-coated silicon b) fibroin-pattered silicon and c) gold-patterned fibroin. Bottom: Representative confocal microscopy images of live (green) and dead (red) primary cortical neurons cultured on the substrates.
2019.03.15 View 23386
Wafer-Scale Multilayer Fabrication of Silk Fibroin-Based Microelectronics
A KAIST research team developed a novel fabrication method for the multilayer processing of silk-based microelectronics. This technology for creating a biodegradable silk fibroin film allows microfabrication with polymer or metal structures manufactured from photolithography. It can be a key technology in the implementation of silk fibroin-based biodegradable electronic devices or localized drug delivery through silk fibroin patterns.
Silk fibroins are biocompatible, biodegradable, transparent, and flexible, which makes them excellent candidates for implantable biomedical devices, and they have also been used as biodegradable films and functional microstructures in biomedical applications. However, conventional microfabrication processes require strong etching solutions and solvents to modify the structure of silk fibroins.
To prevent the silk fibroin from being damaged during the process, Professor Hyunjoo J. Lee from the School of Electrical Engineering and her team came up with a novel process, named aluminum hard mask on silk fibroin (AMoS), which is capable of micropatterning multiple layers composed of both fibroin and inorganic materials, such as metal and dielectrics with high-precision microscale alignment. The AMoS process can make silk fibroin patterns on devices, or make patterns on silk fibroin thin films with other materials by using photolithography, which is a core technology in the current microfabrication process.
The team successfully cultured primary neurons on the processed silk fibroin micro-patterns, and confirmed that silk fibroin has excellent biocompatibility before and after the fabrication process and that it also can be applied to implanted biological devices.
Through this technology, the team realized the multilayer micropatterning of fibroin films on a silk fibroin substrate and fabricated a biodegradable microelectric circuit consisting of resistors and silk fibroin dielectric capacitors in a silicon wafer with large areas.
They also used this technology to position the micro-pattern of the silk fibroin thin film closer to the flexible polymer-based brain electrode, and confirmed the dye molecules mounted on the silk fibroin were transferred successfully from the micropatterns.
Professor Lee said, “This technology facilitates wafer-scale, large-area processing of sensitive materials. We expect it to be applied to a wide range of biomedical devices in the future. Using the silk fibroin with micro-patterned brain electrodes can open up many new possibilities in research on brain circuits by mounting drugs that restrict or promote brain cell activities.”
This research, in collaboration with Dr. Nakwon Choi from KIST and led by PhD candidate Geon Kook, was published in ACS AMI (10.1021/acsami.8b13170) on January 16, 2019.
Figure 1. The cover page of ACS AMI
Figure 2. Fibroin microstructures and metal patterns on a fibroin produced by using the AMoS mask.
Figure 3. Biocompatibility assessment of the AMoS Process. Top: Schematics image of a) fibroin-coated silicon b) fibroin-pattered silicon and c) gold-patterned fibroin. Bottom: Representative confocal microscopy images of live (green) and dead (red) primary cortical neurons cultured on the substrates.
2019.03.15 View 23386 -
 KAIST Develops Analog Memristive Synapses for Neuromorphic Chips
(Professor Sung-Yool Choi from the School of Electrical Engineering)
A KAIST research team developed a technology that makes a transition of the operation mode of flexible memristors to synaptic analog switching by reducing the size of the formed filament. Through this technology, memristors can extend their role to memristive synapses for neuromorphic chips, which will lead to developing soft neuromorphic intelligent systems.
Brain-inspired neuromorphic chips have been gaining a great deal of attention for reducing the power consumption and integrating data processing, compared to conventional semiconductor chips. Similarly, memristors are known to be the most suitable candidate for making a crossbar array which is the most efficient architecture for realizing hardware-based artificial neural network (ANN) inside a neuromorphic chip.
A hardware-based ANN consists of a neuron circuit and synapse elements, the connecting pieces. In the neuromorphic system, the synaptic weight, which represents the connection strength between neurons, should be stored and updated as the type of analog data at each synapse.
However, most memristors have digital characteristics suitable for nonvolatile memory. These characteristics put a limitation on the analog operation of the memristors, which makes it difficult to apply them to synaptic devices.
Professor Sung-Yool Choi from the School of Electrical Engineering and his team fabricated a flexible polymer memristor on a plastic substrate, and found that changing the size of the conductive metal filaments formed inside the device on the scale of metal atoms can make a transition of the memristor behavior from digital to analog.
Using this phenomenon, the team developed flexible memristor-based electronic synapses, which can continuously and linearly update synaptic weight, and operate under mechanical deformations such as bending.
The team confirmed that the ANN based on these memristor synapses can effectively classify person’s facial images even when they were damaged. This research demonstrated the possibility of a neuromorphic chip that can efficiently recognize faces, numbers, and objects.
Professor Choi said, “We found the principles underlying the transition from digital to analog operation of the memristors. I believe that this research paves the way for applying various memristors to either digital memory or electronic synapses, and will accelerate the development of a high-performing neuromorphic chip.”
In a joint research project with Professor Sung Gap Im (KAIST) and Professor V. P. Dravid (Northwestern University), this study was led by Dr. Byung Chul Jang (Samsung Electronics), Dr. Sungkyu Kim (Northwestern University) and Dr. Sang Yoon Yang (KAIST), and was published online in Nano Letters (10.1021/acs.nanolett.8b04023) on January 4, 2019.
Figure 1. a) Schematic illustration of a flexible pV3D3 memristor-based electronic synapse array. b) Cross-sectional TEM image of the flexible pV3D3 memristor
2019.02.28 View 10634
KAIST Develops Analog Memristive Synapses for Neuromorphic Chips
(Professor Sung-Yool Choi from the School of Electrical Engineering)
A KAIST research team developed a technology that makes a transition of the operation mode of flexible memristors to synaptic analog switching by reducing the size of the formed filament. Through this technology, memristors can extend their role to memristive synapses for neuromorphic chips, which will lead to developing soft neuromorphic intelligent systems.
Brain-inspired neuromorphic chips have been gaining a great deal of attention for reducing the power consumption and integrating data processing, compared to conventional semiconductor chips. Similarly, memristors are known to be the most suitable candidate for making a crossbar array which is the most efficient architecture for realizing hardware-based artificial neural network (ANN) inside a neuromorphic chip.
A hardware-based ANN consists of a neuron circuit and synapse elements, the connecting pieces. In the neuromorphic system, the synaptic weight, which represents the connection strength between neurons, should be stored and updated as the type of analog data at each synapse.
However, most memristors have digital characteristics suitable for nonvolatile memory. These characteristics put a limitation on the analog operation of the memristors, which makes it difficult to apply them to synaptic devices.
Professor Sung-Yool Choi from the School of Electrical Engineering and his team fabricated a flexible polymer memristor on a plastic substrate, and found that changing the size of the conductive metal filaments formed inside the device on the scale of metal atoms can make a transition of the memristor behavior from digital to analog.
Using this phenomenon, the team developed flexible memristor-based electronic synapses, which can continuously and linearly update synaptic weight, and operate under mechanical deformations such as bending.
The team confirmed that the ANN based on these memristor synapses can effectively classify person’s facial images even when they were damaged. This research demonstrated the possibility of a neuromorphic chip that can efficiently recognize faces, numbers, and objects.
Professor Choi said, “We found the principles underlying the transition from digital to analog operation of the memristors. I believe that this research paves the way for applying various memristors to either digital memory or electronic synapses, and will accelerate the development of a high-performing neuromorphic chip.”
In a joint research project with Professor Sung Gap Im (KAIST) and Professor V. P. Dravid (Northwestern University), this study was led by Dr. Byung Chul Jang (Samsung Electronics), Dr. Sungkyu Kim (Northwestern University) and Dr. Sang Yoon Yang (KAIST), and was published online in Nano Letters (10.1021/acs.nanolett.8b04023) on January 4, 2019.
Figure 1. a) Schematic illustration of a flexible pV3D3 memristor-based electronic synapse array. b) Cross-sectional TEM image of the flexible pV3D3 memristor
2019.02.28 View 10634 -
 Stretchable Multi-functional Fiber for Energy Harvesting and Strain Sensing
(from left: Professor Steve Park, Jeongjae Ryu and Professor Seungbum Hong)
Fiber-based electronics are expected to play a vital role in next-generation wearable electronics. Woven into textiles, they can provide higher durability, comfort, and integrated multi-functionality. A KAIST team has developed a stretchable multi-functional fiber (SMF) that can harvest energy and detect strain, which can be applied to future wearable electronics.
With wearable electronics, health and physical conditions can be assessed by analyzing biological signals from the human body, such as pulse and muscle movements. Fibers are highly suitable for future wearable electronics because they can be easily integrated into textiles, which are designed to be conformable to curvilinear surfaces and comfortable to wear. Moreover, their weave structures offer support that makes them resistant to fatigue. Many research groups have developed fiber-based strain sensors to sense external biological signals. However, their sensitivities were relatively low.
The applicability of wearable devices is currently limited by their power source, as the size, weight, and lifetime of the battery lessens their versatility. Harvesting mechanical energy from the human body is a promising solution to overcome such limitations by utilizing various types of motions like bending, stretching, and pressing. However, previously reported, fiber-based energy harvesters were not stretchable and could not fully harvest the available mechanical energy.
Professor Seungbum Hong and Professor Steve Park from the Department of Materials Science and Engineering and their team fabricated a stretchable fiber by using a ferroelectric layer composed of P(VDF-TrFE)/PDMS sandwiched between stretchable electrodes composed of a composite of multi-walled carbon nanotubes (MWCNT) and poly 3,4-ethylenedioxythiophene polystyrenesulfonate (PEDOT:PSS).
Cracks formed in MWCNT/PEDOT:PSS layer help the fiber show high sensitivity compared to the previously reported fiber strain sensors. Furthermore, the new fiber can harvest mechanical energy under various mechanical stimuli such as stretching, tapping, and injecting water into the fiber using the piezoelectric effect of the P(VDF-TrFE)/PDMS layer.
Professor Hong said, “This new fiber has various functionalities and makes the device simple and compact. It is a core technology for developing wearable devices with energy harvesting and strain sensing capabilities.”
This article, led by PhD candidate Jeongjae Ryu, was published in the January 2019 issue of Nano Energy.
Figure 1.Schematic illustration of an SMF fiber and its piezoelectric voltage output and response to strain.
Figure 2. Photographs of a stretchable multi-functional fiber being stretched by 100%, bent, and twisted.
2019.01.31 View 9691
Stretchable Multi-functional Fiber for Energy Harvesting and Strain Sensing
(from left: Professor Steve Park, Jeongjae Ryu and Professor Seungbum Hong)
Fiber-based electronics are expected to play a vital role in next-generation wearable electronics. Woven into textiles, they can provide higher durability, comfort, and integrated multi-functionality. A KAIST team has developed a stretchable multi-functional fiber (SMF) that can harvest energy and detect strain, which can be applied to future wearable electronics.
With wearable electronics, health and physical conditions can be assessed by analyzing biological signals from the human body, such as pulse and muscle movements. Fibers are highly suitable for future wearable electronics because they can be easily integrated into textiles, which are designed to be conformable to curvilinear surfaces and comfortable to wear. Moreover, their weave structures offer support that makes them resistant to fatigue. Many research groups have developed fiber-based strain sensors to sense external biological signals. However, their sensitivities were relatively low.
The applicability of wearable devices is currently limited by their power source, as the size, weight, and lifetime of the battery lessens their versatility. Harvesting mechanical energy from the human body is a promising solution to overcome such limitations by utilizing various types of motions like bending, stretching, and pressing. However, previously reported, fiber-based energy harvesters were not stretchable and could not fully harvest the available mechanical energy.
Professor Seungbum Hong and Professor Steve Park from the Department of Materials Science and Engineering and their team fabricated a stretchable fiber by using a ferroelectric layer composed of P(VDF-TrFE)/PDMS sandwiched between stretchable electrodes composed of a composite of multi-walled carbon nanotubes (MWCNT) and poly 3,4-ethylenedioxythiophene polystyrenesulfonate (PEDOT:PSS).
Cracks formed in MWCNT/PEDOT:PSS layer help the fiber show high sensitivity compared to the previously reported fiber strain sensors. Furthermore, the new fiber can harvest mechanical energy under various mechanical stimuli such as stretching, tapping, and injecting water into the fiber using the piezoelectric effect of the P(VDF-TrFE)/PDMS layer.
Professor Hong said, “This new fiber has various functionalities and makes the device simple and compact. It is a core technology for developing wearable devices with energy harvesting and strain sensing capabilities.”
This article, led by PhD candidate Jeongjae Ryu, was published in the January 2019 issue of Nano Energy.
Figure 1.Schematic illustration of an SMF fiber and its piezoelectric voltage output and response to strain.
Figure 2. Photographs of a stretchable multi-functional fiber being stretched by 100%, bent, and twisted.
2019.01.31 View 9691 -
 A Comprehensive Metabolic Map for Bio-Based Chemicals Production
A KAIST research team completed a metabolic map that charts all available strategies and pathways of chemical reactions that lead to the production of various industrial bio-based chemicals.
The team was led by Distinguished Professor Sang Yup Lee, who has produced high-quality metabolic engineering and systems engineering research for decades, and made the hallmark chemicals map after seven years of studies.
The team presented a very detailed analysis on metabolic engineering for the production of a wide range of industrial chemicals, fuels, and materials. Surveying the current trends in the bio-based production of chemicals in industrial biotechnology, the team thoroughly examined the current status of industrial chemicals produced using biological and/or chemical reactions.
This comprehensive map is expected to serve as a blueprint for the visual and intuitive inspection of biological and/or chemical reactions for the production of interest from renewable resources. The team also compiled an accompanying poster to visually present the synthetic pathways of chemicals in the context of their microbial metabolism.
As metabolic engineering has become increasing powerful in addressing limited fossil resources, climate change, and other environmental issues, the number of microbially produced chemicals using biomass as a carbon source has increased substantially. The sustainable production of industrial chemicals and materials has been explored with micro-organisms as cell factories and renewable nonfood biomass as raw materials for alternative petroleum. The engineering of these micro-organism has increasingly become more efficient and effective with the help of metabolic engineering – a practice of engineering using the metabolism of living organisms to produce a desired metabolite.
With the establishment of systems metabolic engineering – the integration of metabolic engineering with tools and strategies from systems biology, synthetic biology and evolutionary engineering – the speed at which micro-organisms are being engineered has reached an unparalleled pace.
In order to evaluate the current state at which metabolically engineered micro-organisms can produce a large portfolio of industrial chemicals, the team conducted an extensive review of the literature and mapped them out on a poster. This resulting poster, termed the bio-based chemicals map, presents synthetic pathways for industrial chemicals, which consist of biological and/or chemical reactions.
Industrial chemicals and their production routes are presented in the context of central carbon metabolic pathways as these key metabolites serve as precursors for the chemicals to be produced. The resulting biochemical map allows the detection and analysis of optimal synthetic pathways for a given industrial chemical. In addition to the poster, the authors have compiled a list of chemicals that have successfully been produced using micro-organisms and a list of the corresponding companies producing them commercially. This thorough review of the literature and the accompanying analytical summary will be an important resource for researchers interested in the production of chemicals from renewable biomass sources.
Metabolically engineered micro-organisms have already made a huge contribution toward the sustainable production of chemicals using renewable resources. Professor Lee said he wanted a detailed survey of the current state and capacity of bio-based chemicals production.
“We are so excited that this review and poster will expand further discussion on the production of important chemicals through engineered micro-organisms and also combined biological and chemical means in a more sustainable manner,” he explained.
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biofineries from the Ministry of Science and ICT through the National Research Foundation of Korea.
For further information, Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST ( leesy@kaist.ac.kr , Tel: +82-42-350-3930)
Figure: Bio-based chemicals production through biological and chemical routes. This metabolic map describes representative chemicals that can be produced either by biological and/or chemical means. Red arrows represent chemical routes and blue arrows represent biological routes. Intermediate metabolites in the metabolism of a living organism can serve as a platform toward the production of industrially relevant chemicals. A more comprehensive map presented by the team can be found as a poster in the review.
2019.01.15 View 7939
A Comprehensive Metabolic Map for Bio-Based Chemicals Production
A KAIST research team completed a metabolic map that charts all available strategies and pathways of chemical reactions that lead to the production of various industrial bio-based chemicals.
The team was led by Distinguished Professor Sang Yup Lee, who has produced high-quality metabolic engineering and systems engineering research for decades, and made the hallmark chemicals map after seven years of studies.
The team presented a very detailed analysis on metabolic engineering for the production of a wide range of industrial chemicals, fuels, and materials. Surveying the current trends in the bio-based production of chemicals in industrial biotechnology, the team thoroughly examined the current status of industrial chemicals produced using biological and/or chemical reactions.
This comprehensive map is expected to serve as a blueprint for the visual and intuitive inspection of biological and/or chemical reactions for the production of interest from renewable resources. The team also compiled an accompanying poster to visually present the synthetic pathways of chemicals in the context of their microbial metabolism.
As metabolic engineering has become increasing powerful in addressing limited fossil resources, climate change, and other environmental issues, the number of microbially produced chemicals using biomass as a carbon source has increased substantially. The sustainable production of industrial chemicals and materials has been explored with micro-organisms as cell factories and renewable nonfood biomass as raw materials for alternative petroleum. The engineering of these micro-organism has increasingly become more efficient and effective with the help of metabolic engineering – a practice of engineering using the metabolism of living organisms to produce a desired metabolite.
With the establishment of systems metabolic engineering – the integration of metabolic engineering with tools and strategies from systems biology, synthetic biology and evolutionary engineering – the speed at which micro-organisms are being engineered has reached an unparalleled pace.
In order to evaluate the current state at which metabolically engineered micro-organisms can produce a large portfolio of industrial chemicals, the team conducted an extensive review of the literature and mapped them out on a poster. This resulting poster, termed the bio-based chemicals map, presents synthetic pathways for industrial chemicals, which consist of biological and/or chemical reactions.
Industrial chemicals and their production routes are presented in the context of central carbon metabolic pathways as these key metabolites serve as precursors for the chemicals to be produced. The resulting biochemical map allows the detection and analysis of optimal synthetic pathways for a given industrial chemical. In addition to the poster, the authors have compiled a list of chemicals that have successfully been produced using micro-organisms and a list of the corresponding companies producing them commercially. This thorough review of the literature and the accompanying analytical summary will be an important resource for researchers interested in the production of chemicals from renewable biomass sources.
Metabolically engineered micro-organisms have already made a huge contribution toward the sustainable production of chemicals using renewable resources. Professor Lee said he wanted a detailed survey of the current state and capacity of bio-based chemicals production.
“We are so excited that this review and poster will expand further discussion on the production of important chemicals through engineered micro-organisms and also combined biological and chemical means in a more sustainable manner,” he explained.
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biofineries from the Ministry of Science and ICT through the National Research Foundation of Korea.
For further information, Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST ( leesy@kaist.ac.kr , Tel: +82-42-350-3930)
Figure: Bio-based chemicals production through biological and chemical routes. This metabolic map describes representative chemicals that can be produced either by biological and/or chemical means. Red arrows represent chemical routes and blue arrows represent biological routes. Intermediate metabolites in the metabolism of a living organism can serve as a platform toward the production of industrially relevant chemicals. A more comprehensive map presented by the team can be found as a poster in the review.
2019.01.15 View 7939 -
 Fabrication of Shape-conformable Batteries with 3D-Printing
(from left: Dr. Bok Yeop Ahn, Dr. Chanhoon Kim, Professor Il-Doo Kim and Professor Jennifer A. Lewis)
Flexible, wireless electronic devices are rapidly emerging and have reached the level of commercialization; nevertheless, most of battery shapes are limited to either spherical and/or rectangular structures, which results in inefficient space use. Professor Il-Doo Kim’s team from the Department of Materials Science at KAIST has successfully developed technology to significantly enhance the variability of battery design through collaboration research with Professor Jennifer A. Lewis and her team from the School of Engineering and Applied Sciences at Harvard University.
Most of the battery shapes today are optimized for coin cell and/or pouch cells. Since the battery as an energy storage device occupies most of the space in microelectronic devices with different designs, new technology to freely change the shape of the battery is required.
The KAIST-Harvard research collaboration team has successfully manufactured various kinds of battery shapes, such as ring-type, H, and U shape, using 3D printing technology. And through the research collaboration with Dr. Youngmin Choi at the Korea Research Institute of Chemical Technology (KRICT), 3D-printed batteries were applied to small-scale wearable electronic devices (wearable light sensor rings).
The research group has adopted environmentally friendly aqueous Zn-ion batteries to make customized battery packs. This system, which uses Zn2+ instead of Li+ as charge carriers, is much safer compared with the conventional lithium rechargeable batteries that use highly inflammable organic electrolytes. Moreover, the processing conditions of lithium-ion batteries are very complicated because organic solvents can ignite upon exposure to moisture and oxygen.
As the aqueous Zn-ion batteries adopted by the research team are stable upon contact with atmospheric moisture and oxygen, they can be fabricated in the ambient air condition, and have advantages in packaging since packaged plastic does not dissolve in water even when plastic packaging is applied using a 3D printer.
To fabricate a stable cathode that can be modulated in various forms and allows high charge-discharge, the research team fabricated a carbon fiber current collector using electrospinning process and uniformly coated electrochemically active polyaniline conductive polymer on the surface of carbon fiber for a current collector-active layer integrated cathode. The cathode, based on conductive polyaniline consisting of a 3D structure, exhibits very fast charging speeds (50% of the charge in two minutes) and can be fabricated without the detachment of active cathode materials, so various battery forms with high mechanical stability can be manufactured.
Prof. Kim said, “Zn-ion batteries employing aqueous electrolytes have the advantage of fabrication under ambient conditions, so it is easy to fabricate the customized battery packs using 3D printing.”
“3D-printed batteries can be easily applied for niche applications such as wearable, personalized, miniaturized micro-robots, and implantable medical devices or microelectronic storage devices with unique designs,” added Professor Lewis.
With Dr. Chanhoon Kim in the Department of Materials Science and Engineering at KAIST and Dr. Bok Yeop Ahn School of Engineering and Applied Sciences at Harvard University participating as equally contributing first authors, this work was published in the December issue of ACS Nano.
This work was financially supported by the Global Research Laboratory (NRF-2015K1A1A2029679) and Wearable Platform Materials Technology Center (2016R1A5A1009926).
Figure 1.Fabrication of shape-conformable batteries based on 3D-printing technology and the application of polyaniline carbon nanofiber cathodes and wearable electronic devices
Figure 2.Fabricated shape-conformable batteries based on a 3D-printing method
Meanwhile, Professor Il-Doo Kim was recently appointed as an Associate Editor of ACS Nano, a highly renowned journal in the field of nanoscience.
Professor Kim said, “It is my great honor to be an Associate Editor of the highly renowned journal ACS Nano, which has an impact factor reaching 13.709 with 134,596 citations as of 2017. Through the editorial activities in the fields of energy, I will dedicate myself to improving the prominence of KAIST and expanding the scope of Korea’s science and technology. I will also contribute to carrying out more international collaborations with world-leading research groups.”
(Associate Editor of ACS Nano Professor Il-Doo Kim)
2018.12.20 View 11611
Fabrication of Shape-conformable Batteries with 3D-Printing
(from left: Dr. Bok Yeop Ahn, Dr. Chanhoon Kim, Professor Il-Doo Kim and Professor Jennifer A. Lewis)
Flexible, wireless electronic devices are rapidly emerging and have reached the level of commercialization; nevertheless, most of battery shapes are limited to either spherical and/or rectangular structures, which results in inefficient space use. Professor Il-Doo Kim’s team from the Department of Materials Science at KAIST has successfully developed technology to significantly enhance the variability of battery design through collaboration research with Professor Jennifer A. Lewis and her team from the School of Engineering and Applied Sciences at Harvard University.
Most of the battery shapes today are optimized for coin cell and/or pouch cells. Since the battery as an energy storage device occupies most of the space in microelectronic devices with different designs, new technology to freely change the shape of the battery is required.
The KAIST-Harvard research collaboration team has successfully manufactured various kinds of battery shapes, such as ring-type, H, and U shape, using 3D printing technology. And through the research collaboration with Dr. Youngmin Choi at the Korea Research Institute of Chemical Technology (KRICT), 3D-printed batteries were applied to small-scale wearable electronic devices (wearable light sensor rings).
The research group has adopted environmentally friendly aqueous Zn-ion batteries to make customized battery packs. This system, which uses Zn2+ instead of Li+ as charge carriers, is much safer compared with the conventional lithium rechargeable batteries that use highly inflammable organic electrolytes. Moreover, the processing conditions of lithium-ion batteries are very complicated because organic solvents can ignite upon exposure to moisture and oxygen.
As the aqueous Zn-ion batteries adopted by the research team are stable upon contact with atmospheric moisture and oxygen, they can be fabricated in the ambient air condition, and have advantages in packaging since packaged plastic does not dissolve in water even when plastic packaging is applied using a 3D printer.
To fabricate a stable cathode that can be modulated in various forms and allows high charge-discharge, the research team fabricated a carbon fiber current collector using electrospinning process and uniformly coated electrochemically active polyaniline conductive polymer on the surface of carbon fiber for a current collector-active layer integrated cathode. The cathode, based on conductive polyaniline consisting of a 3D structure, exhibits very fast charging speeds (50% of the charge in two minutes) and can be fabricated without the detachment of active cathode materials, so various battery forms with high mechanical stability can be manufactured.
Prof. Kim said, “Zn-ion batteries employing aqueous electrolytes have the advantage of fabrication under ambient conditions, so it is easy to fabricate the customized battery packs using 3D printing.”
“3D-printed batteries can be easily applied for niche applications such as wearable, personalized, miniaturized micro-robots, and implantable medical devices or microelectronic storage devices with unique designs,” added Professor Lewis.
With Dr. Chanhoon Kim in the Department of Materials Science and Engineering at KAIST and Dr. Bok Yeop Ahn School of Engineering and Applied Sciences at Harvard University participating as equally contributing first authors, this work was published in the December issue of ACS Nano.
This work was financially supported by the Global Research Laboratory (NRF-2015K1A1A2029679) and Wearable Platform Materials Technology Center (2016R1A5A1009926).
Figure 1.Fabrication of shape-conformable batteries based on 3D-printing technology and the application of polyaniline carbon nanofiber cathodes and wearable electronic devices
Figure 2.Fabricated shape-conformable batteries based on a 3D-printing method
Meanwhile, Professor Il-Doo Kim was recently appointed as an Associate Editor of ACS Nano, a highly renowned journal in the field of nanoscience.
Professor Kim said, “It is my great honor to be an Associate Editor of the highly renowned journal ACS Nano, which has an impact factor reaching 13.709 with 134,596 citations as of 2017. Through the editorial activities in the fields of energy, I will dedicate myself to improving the prominence of KAIST and expanding the scope of Korea’s science and technology. I will also contribute to carrying out more international collaborations with world-leading research groups.”
(Associate Editor of ACS Nano Professor Il-Doo Kim)
2018.12.20 View 11611 -
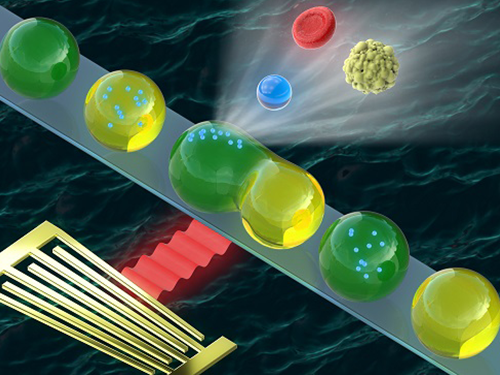 Washing and Enrichment of Micro-Particles Encapsulated in Droplets
Researchers developed microfluidic technology for the washing and enrichment of in-droplet micro-particles. They presented the technology using a microfluidic chip based on surface acoustic wave (SAW)-driven acoustic radiation force (ARF).
The team demonstrated the first instance of acoustic in-droplet micro-particle washing with a particle recovery rate of approximately 90 percent. They further extended the applicability of the proposed method to in-droplet particle enrichment with the unprecedented abilities to increase the in-droplet particle quantity and exchange the droplet dispersed phase.
This proposed method enabled on-chip, label-free, continuous, and selective in-droplet micro-particle manipulation. The team demonstrated the first instance of in-droplet micro-particle washing between two types of alternating droplets in a simple microchannel, proving that the method can increase the particle quantity, which has not been achieved by previously reported methods.
The study aimed to develop an in-droplet micro-particle washing and enrichment method based on SAW-driven ARF. When a droplet containing particles is exposed to an acoustic field, both the droplet and suspended particles experience ARF arising from inhomogeneous wave scattering at the liquid-liquid and liquid-solid interfaces. Unlike previous in-droplet particle manipulation methods, this method allows simultaneous and precise control over the droplets and suspended particles. Moreover, the proposed acoustic method does not require labelled particles, such as magnetic particles, and employs a simple microchannel geometry.
Microfluidic sample washing has emerged as an alternative to centrifugation because the limitations of centrifugation-based washing methods can be addressed using continuous washing processes. It also has considerable potential and importance in a variety of applications such as single-cell/particle assays, high-throughput screening of rare samples, and cell culture medium exchange.
Compared to continuous flow-based microfluidic methods, droplet-based microfluidic sample washing has been rarely explored due to technological difficulties. On-chip, in-droplet sample washing requires sample transfer across the droplet interface composed of two immiscible fluids. This process involves simultaneous and precise control over the encapsulated sample and droplet interface during the medium exchange of the in-droplet sample.
Sample encapsulation within individual microscale droplets offers isolated microenvironments for the samples. Experimental uncertainties due to cross-contamination and Taylor dispersion between multiple reagents can be reduced in droplet-based microfluidics.
This is the first research achievement made by the Acousto-Microfluidics Research Center for Next-Generation Healthcare, the cross-generation collaborative lab KAIST opened in May. This novel approach pairs senior and junior faculty members for sustaining the research legacy even after the senior researcher retires. The research center, which paired Chair Professor Hyung Jin Sung and Professors Hyoungsoo Kim and Yeunwoo Cho, made a breakthrough in microfluidics along with PhD candidate Jinsoo Park. The study was featured as the cover of Lab on a Chip published by Royal Society of Chemistry.
Jinsoo Park, first author of the study, believes this technology will may serve as an in-droplet sample preparation platform with in-line integration of other droplet microfluidic components. Chair Professor Sung said, “The proposed acoustic method will offer new perspectives on sample washing and enrichment by performing the operation in microscale droplets.”
Figure 1. (a) A microfluidic device for in-droplet micro-particle washing and enrichment; (b) alternatingly produced droplets of two kinds at a double T-junction; (c) a droplet and encapsulated micro-particles exposed to surface acoustic wave-driven acoustic radiation force; (d-h) sequential processes of in-droplet micro-particle washing and enrichment operation.
2018.10.19 View 8572
Washing and Enrichment of Micro-Particles Encapsulated in Droplets
Researchers developed microfluidic technology for the washing and enrichment of in-droplet micro-particles. They presented the technology using a microfluidic chip based on surface acoustic wave (SAW)-driven acoustic radiation force (ARF).
The team demonstrated the first instance of acoustic in-droplet micro-particle washing with a particle recovery rate of approximately 90 percent. They further extended the applicability of the proposed method to in-droplet particle enrichment with the unprecedented abilities to increase the in-droplet particle quantity and exchange the droplet dispersed phase.
This proposed method enabled on-chip, label-free, continuous, and selective in-droplet micro-particle manipulation. The team demonstrated the first instance of in-droplet micro-particle washing between two types of alternating droplets in a simple microchannel, proving that the method can increase the particle quantity, which has not been achieved by previously reported methods.
The study aimed to develop an in-droplet micro-particle washing and enrichment method based on SAW-driven ARF. When a droplet containing particles is exposed to an acoustic field, both the droplet and suspended particles experience ARF arising from inhomogeneous wave scattering at the liquid-liquid and liquid-solid interfaces. Unlike previous in-droplet particle manipulation methods, this method allows simultaneous and precise control over the droplets and suspended particles. Moreover, the proposed acoustic method does not require labelled particles, such as magnetic particles, and employs a simple microchannel geometry.
Microfluidic sample washing has emerged as an alternative to centrifugation because the limitations of centrifugation-based washing methods can be addressed using continuous washing processes. It also has considerable potential and importance in a variety of applications such as single-cell/particle assays, high-throughput screening of rare samples, and cell culture medium exchange.
Compared to continuous flow-based microfluidic methods, droplet-based microfluidic sample washing has been rarely explored due to technological difficulties. On-chip, in-droplet sample washing requires sample transfer across the droplet interface composed of two immiscible fluids. This process involves simultaneous and precise control over the encapsulated sample and droplet interface during the medium exchange of the in-droplet sample.
Sample encapsulation within individual microscale droplets offers isolated microenvironments for the samples. Experimental uncertainties due to cross-contamination and Taylor dispersion between multiple reagents can be reduced in droplet-based microfluidics.
This is the first research achievement made by the Acousto-Microfluidics Research Center for Next-Generation Healthcare, the cross-generation collaborative lab KAIST opened in May. This novel approach pairs senior and junior faculty members for sustaining the research legacy even after the senior researcher retires. The research center, which paired Chair Professor Hyung Jin Sung and Professors Hyoungsoo Kim and Yeunwoo Cho, made a breakthrough in microfluidics along with PhD candidate Jinsoo Park. The study was featured as the cover of Lab on a Chip published by Royal Society of Chemistry.
Jinsoo Park, first author of the study, believes this technology will may serve as an in-droplet sample preparation platform with in-line integration of other droplet microfluidic components. Chair Professor Sung said, “The proposed acoustic method will offer new perspectives on sample washing and enrichment by performing the operation in microscale droplets.”
Figure 1. (a) A microfluidic device for in-droplet micro-particle washing and enrichment; (b) alternatingly produced droplets of two kinds at a double T-junction; (c) a droplet and encapsulated micro-particles exposed to surface acoustic wave-driven acoustic radiation force; (d-h) sequential processes of in-droplet micro-particle washing and enrichment operation.
2018.10.19 View 8572 -
 The 1st Korea Toray Science and Technology Awardee, Prof. Sukbok Chang
(Distinguished Professor Sukbok Chang from the Department of Chemistry)
The Korea Toray Science Foundation (KTSF) awarded the first Korea Toray Science Technology Award in basic science to Distinguished Professor Sukbok Chang from the Department of Chemistry on September 19.
KTSF was established in January 2018, and its award goes to researchers who have significantly contributed to the development of chemistry and materials research with funds to support research projects.
Distinguished Professor Chang has devoted himself in organocatalysis research; in particular, his work on catalysts for effective lactam formation, which was an intricate problem, received great attention.
The award ceremony will take place in The Federation of Korean Industries Hall on October 31. KTFS board members, judges, and the CEO of Toray Industries Akihiro Nikkaku will attend the ceremony. Also, Dr. Ryoji Noyori, the Nobel Laureate in Chemistry, will give a talk on the role of chemistry and creative challenges as a researcher.
2018.10.04 View 10100
The 1st Korea Toray Science and Technology Awardee, Prof. Sukbok Chang
(Distinguished Professor Sukbok Chang from the Department of Chemistry)
The Korea Toray Science Foundation (KTSF) awarded the first Korea Toray Science Technology Award in basic science to Distinguished Professor Sukbok Chang from the Department of Chemistry on September 19.
KTSF was established in January 2018, and its award goes to researchers who have significantly contributed to the development of chemistry and materials research with funds to support research projects.
Distinguished Professor Chang has devoted himself in organocatalysis research; in particular, his work on catalysts for effective lactam formation, which was an intricate problem, received great attention.
The award ceremony will take place in The Federation of Korean Industries Hall on October 31. KTFS board members, judges, and the CEO of Toray Industries Akihiro Nikkaku will attend the ceremony. Also, Dr. Ryoji Noyori, the Nobel Laureate in Chemistry, will give a talk on the role of chemistry and creative challenges as a researcher.
2018.10.04 View 10100 -
 Spray Coated Tactile Sensor on a 3-D Surface for Robotic Skin
Robots will be able to conduct a wide variety of tasks as well as humans if they can be given tactile sensing capabilities.
A KAIST research team has reported a stretchable pressure insensitive strain sensor by using an all solution-based process. The solution-based process is easily scalable to accommodate for large areas and can be coated as a thin-film on 3-dimensional irregularly shaped objects via spray coating. These conditions make their processing technique unique and highly suitable for robotic electronic skin or wearable electronic applications.
The making of electronic skin to mimic the tactile sensing properties of human skin is an active area of research for various applications such as wearable electronics, robotics, and prosthetics. One of the major challenges in electronic skin research is differentiating various external stimuli, particularly between strain and pressure. Another issue is uniformly depositing electrical skin on 3-dimensional irregularly shaped objects.
To overcome these issues, the research team led by Professor Steve Park from the Department of Materials Science and Engineering and Professor Jung Kim from the Department of Mechanical Engineering developed electronic skin that can be uniformly coated on 3-dimensional surfaces and distinguish mechanical stimuli. The new electronic skin can also distinguish mechanical stimuli analogous to human skin. The structure of the electronic skin was designed to respond differently under applied pressure and strain. Under applied strain, conducting pathways undergo significant conformational changes, considerably changing the resistance. On the other hand, under applied pressure, negligible conformational change in the conducting pathway occurs; e-skin is therefore non-responsive to pressure. The research team is currently working on strain insensitive pressure sensors to use with the developed strain sensors.
The research team also spatially mapped the local strain without the use of patterned electrode arrays utilizing electrical impedance tomography (EIT). By using EIT, it is possible to minimize the number of electrodes, increase durability, and enable facile fabrication onto 3-dimensional surfaces.
Professor Park said, “Our electronic skin can be mass produced at a low cost and can easily be coated onto complex 3-dimensional surfaces. It is a key technology that can bring us closer to the commercialization of electronic skin for various applications in the near future.”
The result of this work entitled “Pressure Insensitive Strain Sensor with Facile Solution-based Process for Tactile Sensing Applications” was published in the August issue of ACS Nano as a cover article.
(Figure: Detecting mechanical stimuli using electrical impedance tomography.)
2018.09.21 View 9055
Spray Coated Tactile Sensor on a 3-D Surface for Robotic Skin
Robots will be able to conduct a wide variety of tasks as well as humans if they can be given tactile sensing capabilities.
A KAIST research team has reported a stretchable pressure insensitive strain sensor by using an all solution-based process. The solution-based process is easily scalable to accommodate for large areas and can be coated as a thin-film on 3-dimensional irregularly shaped objects via spray coating. These conditions make their processing technique unique and highly suitable for robotic electronic skin or wearable electronic applications.
The making of electronic skin to mimic the tactile sensing properties of human skin is an active area of research for various applications such as wearable electronics, robotics, and prosthetics. One of the major challenges in electronic skin research is differentiating various external stimuli, particularly between strain and pressure. Another issue is uniformly depositing electrical skin on 3-dimensional irregularly shaped objects.
To overcome these issues, the research team led by Professor Steve Park from the Department of Materials Science and Engineering and Professor Jung Kim from the Department of Mechanical Engineering developed electronic skin that can be uniformly coated on 3-dimensional surfaces and distinguish mechanical stimuli. The new electronic skin can also distinguish mechanical stimuli analogous to human skin. The structure of the electronic skin was designed to respond differently under applied pressure and strain. Under applied strain, conducting pathways undergo significant conformational changes, considerably changing the resistance. On the other hand, under applied pressure, negligible conformational change in the conducting pathway occurs; e-skin is therefore non-responsive to pressure. The research team is currently working on strain insensitive pressure sensors to use with the developed strain sensors.
The research team also spatially mapped the local strain without the use of patterned electrode arrays utilizing electrical impedance tomography (EIT). By using EIT, it is possible to minimize the number of electrodes, increase durability, and enable facile fabrication onto 3-dimensional surfaces.
Professor Park said, “Our electronic skin can be mass produced at a low cost and can easily be coated onto complex 3-dimensional surfaces. It is a key technology that can bring us closer to the commercialization of electronic skin for various applications in the near future.”
The result of this work entitled “Pressure Insensitive Strain Sensor with Facile Solution-based Process for Tactile Sensing Applications” was published in the August issue of ACS Nano as a cover article.
(Figure: Detecting mechanical stimuli using electrical impedance tomography.)
2018.09.21 View 9055 -
 KAIST Develops VRFB with Longer Durability
(from left: PhD candidate Soohyun Kim, Professor Hee-Tak Kim and PhD candidate Junghoon Choi)
There has been growing demand for large-scale storage for energy produced from renewable energy sources in an efficient and stable way. To meet this demand, a KAIST research team developed a new vanadium redox-flow battery (VRFB) with 15 times greater capacity retention and five times longer durability. This VRFB battery can be an excellent candidate for a large-scale rechargeable battery with no risk of explosion.
The VRFB has received much attention for its high efficiency and reliability with the absence of cross-contamination. However, it has the limitation of having insufficient charge and discharge efficiency and a low capacity retention rate because its perfluorinated membrane is very permeable to any active materials. To minimize energy loss, it needs a membrane that has low vanadium ion permeability and high ion conductivity. Hence, there was an attempt to incorporate a hydrocarbon membrane that has low cost and high ion selectivity but it turned out that the VO₂+ caused chemical degradation, which led to shortening the battery life drastically.
To develop a membrane with pore sizes smaller than the hydrated size of vanadium ions yet larger than that of the protons, a research team co-led by Professor Hee-Tae Jung and Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering implemented a graphene-oxide framework (GOF) membrane by cross-linking graphene oxide nanosheets. They believed that GOF, having strong ion selectivity, would be a good candidate for the membrane component for the VRFB. The interlayer spacing between the GO sheets limited moisture expansion and provided selective ion permeation.
The GOF membrane increased the capacity retention of the VRFB, which showed a 15 times higher rate than that of perfluorinated membranes. Its cycling stability was also enhanced up to five times, compared to conventional hydrocarbon membranes.
These pore-sized-tuned graphene oxide frameworks will allow pore-sized tuning of membranes and will be applicable to electrochemical systems that utilize ions of various sizes, such as rechargeable batteries and sensors.
Professor Kim said, “Developing a membrane that prevents the mixing of positive and negative active materials has been a chronic issue in the field of redox-flow batteries. Through this research, we showed that nanotechnology can prevent this crossover issue and membrane degradation. I believe that this technology can be applied to various rechargeable batteries requiring large-scale storage.”
This research was published in Nano Letters on May 3.
Figure 1. Electrochemical performances of the VRFBs with Nafion 115, SPAES (sulfonated poly), and GOF/SPAES: discharge capacity
Figure 2. Schematic of the selective ion transfer of hydrated vanadium ions and protons in the GOF membrane and the molecular structure of the GOF membrane, showing that the GO nanosheets are cross-linked with EDA (ethylenediamine)
2018.09.20 View 8031
KAIST Develops VRFB with Longer Durability
(from left: PhD candidate Soohyun Kim, Professor Hee-Tak Kim and PhD candidate Junghoon Choi)
There has been growing demand for large-scale storage for energy produced from renewable energy sources in an efficient and stable way. To meet this demand, a KAIST research team developed a new vanadium redox-flow battery (VRFB) with 15 times greater capacity retention and five times longer durability. This VRFB battery can be an excellent candidate for a large-scale rechargeable battery with no risk of explosion.
The VRFB has received much attention for its high efficiency and reliability with the absence of cross-contamination. However, it has the limitation of having insufficient charge and discharge efficiency and a low capacity retention rate because its perfluorinated membrane is very permeable to any active materials. To minimize energy loss, it needs a membrane that has low vanadium ion permeability and high ion conductivity. Hence, there was an attempt to incorporate a hydrocarbon membrane that has low cost and high ion selectivity but it turned out that the VO₂+ caused chemical degradation, which led to shortening the battery life drastically.
To develop a membrane with pore sizes smaller than the hydrated size of vanadium ions yet larger than that of the protons, a research team co-led by Professor Hee-Tae Jung and Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering implemented a graphene-oxide framework (GOF) membrane by cross-linking graphene oxide nanosheets. They believed that GOF, having strong ion selectivity, would be a good candidate for the membrane component for the VRFB. The interlayer spacing between the GO sheets limited moisture expansion and provided selective ion permeation.
The GOF membrane increased the capacity retention of the VRFB, which showed a 15 times higher rate than that of perfluorinated membranes. Its cycling stability was also enhanced up to five times, compared to conventional hydrocarbon membranes.
These pore-sized-tuned graphene oxide frameworks will allow pore-sized tuning of membranes and will be applicable to electrochemical systems that utilize ions of various sizes, such as rechargeable batteries and sensors.
Professor Kim said, “Developing a membrane that prevents the mixing of positive and negative active materials has been a chronic issue in the field of redox-flow batteries. Through this research, we showed that nanotechnology can prevent this crossover issue and membrane degradation. I believe that this technology can be applied to various rechargeable batteries requiring large-scale storage.”
This research was published in Nano Letters on May 3.
Figure 1. Electrochemical performances of the VRFBs with Nafion 115, SPAES (sulfonated poly), and GOF/SPAES: discharge capacity
Figure 2. Schematic of the selective ion transfer of hydrated vanadium ions and protons in the GOF membrane and the molecular structure of the GOF membrane, showing that the GO nanosheets are cross-linked with EDA (ethylenediamine)
2018.09.20 View 8031 -
 Transfering Nanowires onto a Flexible Substrate
(from left: PhD Min-Ho Seo and Professor Jun-Bo Yoon)
Boasting excellent physical and chemical properties, nanowires (NWs) are suitable for fabricating flexible electronics; therefore, technology to transfer well-aligned wires plays a crucial role in enhancing performance of the devices. A KAIST research team succeeded in developing NW-transfer technology that is expected to enhance the existing chemical reaction-based NW fabrication technology that has this far showed low performance in applicability and productivity.
NWs, one of the most well-known nanomaterials, have the structural advantage of being small and lightweight. Hence, NW-transfer technology has drawn attention because it can fabricate high-performance, flexible nanodevices with high simplicity and throughput.
A conventional nanowire-fabrication method generally has an irregularity issue since it mixes chemically synthesized nanowires in a solution and randomly distributes the NWs onto flexible substrates. Hence, numerous nanofabrication processes have emerged, and one of them is master-mold-based, which enables the fabrication of highly ordered NW arrays embedded onto substrates in a simple and cost-effective manner, but its employment is limited to only some materials because of its chemistry-based NW-transfer mechanism, which is complex and time consuming. For the successful transfer, it requires that adequate chemicals controlling the chemical interfacial adhesion between the master mold, NWs, and flexible substrate be present.
Here, Professor Jun-Bo Yoon and his team from the School of Electrical Engineering introduced a material-independent mechanical-interlocking-based nanowire-transfer (MINT) method to fabricate ultralong and fully aligned NWs on a large flexible substrate in a highly robust manner.
This method involves sequentially forming a nanosacrificial layer and NWs on a nanograting substrate that becomes the master mold for the transfer, then weakening the structure of the nanosacrificial layer through a dry etching process. The nanosacrificial layer very weakly holds the nanowires on the master mold. Therefore, when using a flexible substrate material, the nanowires are very easily transferred from the master mold to the substrate, just like a piece of tape lifting dust off a carpet.
This technology uses common physical vapor deposition and does not rely on NW materials, making it easy to fabricate NWs onto the flexible substrates.
Using this technology, the team was able to fabricate a variety of metal and metal-oxide NWs, including gold, platinum, and copper – all perfectly aligned on a flexible substrate. They also confirmed that it can be applied to creating stable and applicable devices in everyday life by successfully applying it to flexible heaters and gas sensors.
PhD Min-Ho Seo who led this research said, “We have successfully aligned various metals and semiconductor NWs with excellent physical properties onto flexible substrates and applied them to fabricated devices. As a platform-technology, it will contribute to developing high-performing and stable electronic devices.”
This research was published in ACS Nano on May 24.
Figure 1. Photograph of the fabricated wafer-scale fully aligned and ultralong Au nanowire array on a flexible substrate
2018.09.17 View 6772
Transfering Nanowires onto a Flexible Substrate
(from left: PhD Min-Ho Seo and Professor Jun-Bo Yoon)
Boasting excellent physical and chemical properties, nanowires (NWs) are suitable for fabricating flexible electronics; therefore, technology to transfer well-aligned wires plays a crucial role in enhancing performance of the devices. A KAIST research team succeeded in developing NW-transfer technology that is expected to enhance the existing chemical reaction-based NW fabrication technology that has this far showed low performance in applicability and productivity.
NWs, one of the most well-known nanomaterials, have the structural advantage of being small and lightweight. Hence, NW-transfer technology has drawn attention because it can fabricate high-performance, flexible nanodevices with high simplicity and throughput.
A conventional nanowire-fabrication method generally has an irregularity issue since it mixes chemically synthesized nanowires in a solution and randomly distributes the NWs onto flexible substrates. Hence, numerous nanofabrication processes have emerged, and one of them is master-mold-based, which enables the fabrication of highly ordered NW arrays embedded onto substrates in a simple and cost-effective manner, but its employment is limited to only some materials because of its chemistry-based NW-transfer mechanism, which is complex and time consuming. For the successful transfer, it requires that adequate chemicals controlling the chemical interfacial adhesion between the master mold, NWs, and flexible substrate be present.
Here, Professor Jun-Bo Yoon and his team from the School of Electrical Engineering introduced a material-independent mechanical-interlocking-based nanowire-transfer (MINT) method to fabricate ultralong and fully aligned NWs on a large flexible substrate in a highly robust manner.
This method involves sequentially forming a nanosacrificial layer and NWs on a nanograting substrate that becomes the master mold for the transfer, then weakening the structure of the nanosacrificial layer through a dry etching process. The nanosacrificial layer very weakly holds the nanowires on the master mold. Therefore, when using a flexible substrate material, the nanowires are very easily transferred from the master mold to the substrate, just like a piece of tape lifting dust off a carpet.
This technology uses common physical vapor deposition and does not rely on NW materials, making it easy to fabricate NWs onto the flexible substrates.
Using this technology, the team was able to fabricate a variety of metal and metal-oxide NWs, including gold, platinum, and copper – all perfectly aligned on a flexible substrate. They also confirmed that it can be applied to creating stable and applicable devices in everyday life by successfully applying it to flexible heaters and gas sensors.
PhD Min-Ho Seo who led this research said, “We have successfully aligned various metals and semiconductor NWs with excellent physical properties onto flexible substrates and applied them to fabricated devices. As a platform-technology, it will contribute to developing high-performing and stable electronic devices.”
This research was published in ACS Nano on May 24.
Figure 1. Photograph of the fabricated wafer-scale fully aligned and ultralong Au nanowire array on a flexible substrate
2018.09.17 View 6772 -
 Rh Ensemble Catalyst for Effective Automobile Exhaust Treatment
(from left: Professor Hyunjoo Lee and PhD candidate Hojin Jeong)
A KAIST research team has developed a fully dispersed Rh ensemble catalyst (ENS) that shows better performance than commercial diesel oxidation catalyst (DOC). This newly developed ENSs could improve low-temperature automobile exhaust treatment.
Precious metals have been used for various heterogeneous reactions, but it is crucial to maximize efficiency of catalysts due to their high cost. Single-atom catalysts (SACs) have received much attention because it is possible for all of the metal atoms to be used for reactions, yet they do not show catalytic activity for reactions that require ensemble sites.
Meanwhile, hydrocarbons, such as propylene (C3H6) and propane (C3H8) are typical automobile exhaust gas pollutants and must be converted to carbon dioxide (CO2) and water (H2O) before they are released as exhaust. Since the hydrocarbon oxidation reaction proceeds only during carbon-carbon (C-C) or carbon-hydrogen (C-H) bond cleavage, it is essential to secure the metal ensemble site for the catalytic reaction. Therefore, precious metal catalysts with high dispersion and ensemble sites are greatly needed.
To solve this issue, Professor Hyunjoo Lee from the Department of Chemical and Biomolecular Engineering and Professor Jeong Woo Han from POSTECH developed an Rh ensemble catalyst with 100% dispersion, and applied it to automobile after-treatment. Having a 100% dispersion means that every metal atom is used for the reaction since it is exposed on the surface.
SACs also have 100% dispersion, but the difference is that ENSs have the unique advantage of having an ensemble site with two or more atoms.
As a result of the experiment, the ENSs showed excellent catalytic performance in CO, NO, propylene, and propane oxidation at low temperatures. This complements the disadvantage of nanoparticle catalyst (NPs) that perform catalysis poorly at low temperatures due to low metal dispersion, or SACs without hydrocarbon oxidation.
In particular, the ENSs have superior low-temperature activity even better than commercial DOC, hence they are expected to be applied to automobile exhaust treatment.
Professor Lee said, “I believe that the ENSs have given academic contribution for proposing a new concept of metal catalysts, differentiating from conventional SACs and NPs. At the same time, they are of great value in the industry of exhaust treatment catalysts.”
This research, led by PhD candidate Hojin Jeong, was published in the Journal of the American Chemical Society on July 5.
Figure 1. Concept of Rh ensemble catalyst for automobile exhaust treatment
Figure 2. Structure and performance comparison of single-atom catalyst and ensemble catalyst
Figure 3. Energy-dispersive X-ray spectroscopy (EDS) mapping images for SAC, ENS, and NP, respectively (green, Eh; red, Ce)
2018.08.29 View 7855
Rh Ensemble Catalyst for Effective Automobile Exhaust Treatment
(from left: Professor Hyunjoo Lee and PhD candidate Hojin Jeong)
A KAIST research team has developed a fully dispersed Rh ensemble catalyst (ENS) that shows better performance than commercial diesel oxidation catalyst (DOC). This newly developed ENSs could improve low-temperature automobile exhaust treatment.
Precious metals have been used for various heterogeneous reactions, but it is crucial to maximize efficiency of catalysts due to their high cost. Single-atom catalysts (SACs) have received much attention because it is possible for all of the metal atoms to be used for reactions, yet they do not show catalytic activity for reactions that require ensemble sites.
Meanwhile, hydrocarbons, such as propylene (C3H6) and propane (C3H8) are typical automobile exhaust gas pollutants and must be converted to carbon dioxide (CO2) and water (H2O) before they are released as exhaust. Since the hydrocarbon oxidation reaction proceeds only during carbon-carbon (C-C) or carbon-hydrogen (C-H) bond cleavage, it is essential to secure the metal ensemble site for the catalytic reaction. Therefore, precious metal catalysts with high dispersion and ensemble sites are greatly needed.
To solve this issue, Professor Hyunjoo Lee from the Department of Chemical and Biomolecular Engineering and Professor Jeong Woo Han from POSTECH developed an Rh ensemble catalyst with 100% dispersion, and applied it to automobile after-treatment. Having a 100% dispersion means that every metal atom is used for the reaction since it is exposed on the surface.
SACs also have 100% dispersion, but the difference is that ENSs have the unique advantage of having an ensemble site with two or more atoms.
As a result of the experiment, the ENSs showed excellent catalytic performance in CO, NO, propylene, and propane oxidation at low temperatures. This complements the disadvantage of nanoparticle catalyst (NPs) that perform catalysis poorly at low temperatures due to low metal dispersion, or SACs without hydrocarbon oxidation.
In particular, the ENSs have superior low-temperature activity even better than commercial DOC, hence they are expected to be applied to automobile exhaust treatment.
Professor Lee said, “I believe that the ENSs have given academic contribution for proposing a new concept of metal catalysts, differentiating from conventional SACs and NPs. At the same time, they are of great value in the industry of exhaust treatment catalysts.”
This research, led by PhD candidate Hojin Jeong, was published in the Journal of the American Chemical Society on July 5.
Figure 1. Concept of Rh ensemble catalyst for automobile exhaust treatment
Figure 2. Structure and performance comparison of single-atom catalyst and ensemble catalyst
Figure 3. Energy-dispersive X-ray spectroscopy (EDS) mapping images for SAC, ENS, and NP, respectively (green, Eh; red, Ce)
2018.08.29 View 7855 -
 Levitating 2D Semiconductor for Better Performance
(from top: Professor Yeon Sik Jung and PhD candidate Soomin Yim)
Atomically thin 2D semiconductors have been drawing attention for their superior physical properties over silicon semiconductors; nevertheless, they are not the most appealing materials due to their structural instability and costly manufacturing process. To shed some light on these limitations, a KAIST research team suspended a 2D semiconductor on a dome-shaped nanostructure to produce a highly efficient semiconductor at a low cost.
2D semiconducting materials have emerged as alternatives for silicon-based semiconductors because of their inherent flexibility, high transparency, and excellent carrier transport properties, which are the important characteristics for flexible electronics.
Despite their outstanding physical and chemical properties, they are oversensitive to their environment due to their extremely thin nature. Hence, any irregularities in the supporting surface can affect the properties of 2D semiconductors and make it more difficult to produce reliable and well performing devices. In particular, it can result in serious degradation of charge-carrier mobility or light-emission yield.
To solve this problem, there have been continued efforts to fundamentally block the substrate effects. One way is to suspend a 2D semiconductor; however, this method will degrade mechanical durability due to the absence of a supporter underneath the 2D semiconducting materials.
Professor Yeon Sik Jung from the Department of Materials Science and Engineering and his team came up with a new strategy based on the insertion of high-density topographic patterns as a nanogap-containing supporter between 2D materials and the substrate in order to mitigate their contact and to block the substrate-induced unwanted effects.
More than 90% of the dome-shaped supporter is simply an empty space because of its nanometer scale size. Placing a 2D semiconductor on this structure creates a similar effect to levitating the layer. Hence, this method secures the mechanical durability of the device while minimizing the undesired effects from the substrate. By applying this method to the 2D semiconductor, the charge-carrier mobility was more than doubled, showing a significant improvement of the performance of the 2D semiconductor.
Additionally, the team reduced the price of manufacturing the semiconductor. In general, constructing an ultra-fine dome structure on a surface generally involves costly equipment to create individual patterns on the surface. However, the team employed a method of self-assembling nanopatterns in which molecules assemble themselves to form a nanostructure. This method led to reducing production costs and showed good compatibility with conventional semiconductor manufacturing processes.
Professor Jung said, “This research can be applied to improve devices using various 2D semiconducting materials as well as devices using graphene, a metallic 2D material. It will be useful in a broad range of applications, such as the material for the high speed transistor channels for next-generation flexible displays or for the active layer in light detectors.”
This research, led by PhD candidate Soomin Yim, was published in Nano Letters in April.
Figure 1. Image of a 2D semiconductor using dome structures
2018.08.28 View 7182
Levitating 2D Semiconductor for Better Performance
(from top: Professor Yeon Sik Jung and PhD candidate Soomin Yim)
Atomically thin 2D semiconductors have been drawing attention for their superior physical properties over silicon semiconductors; nevertheless, they are not the most appealing materials due to their structural instability and costly manufacturing process. To shed some light on these limitations, a KAIST research team suspended a 2D semiconductor on a dome-shaped nanostructure to produce a highly efficient semiconductor at a low cost.
2D semiconducting materials have emerged as alternatives for silicon-based semiconductors because of their inherent flexibility, high transparency, and excellent carrier transport properties, which are the important characteristics for flexible electronics.
Despite their outstanding physical and chemical properties, they are oversensitive to their environment due to their extremely thin nature. Hence, any irregularities in the supporting surface can affect the properties of 2D semiconductors and make it more difficult to produce reliable and well performing devices. In particular, it can result in serious degradation of charge-carrier mobility or light-emission yield.
To solve this problem, there have been continued efforts to fundamentally block the substrate effects. One way is to suspend a 2D semiconductor; however, this method will degrade mechanical durability due to the absence of a supporter underneath the 2D semiconducting materials.
Professor Yeon Sik Jung from the Department of Materials Science and Engineering and his team came up with a new strategy based on the insertion of high-density topographic patterns as a nanogap-containing supporter between 2D materials and the substrate in order to mitigate their contact and to block the substrate-induced unwanted effects.
More than 90% of the dome-shaped supporter is simply an empty space because of its nanometer scale size. Placing a 2D semiconductor on this structure creates a similar effect to levitating the layer. Hence, this method secures the mechanical durability of the device while minimizing the undesired effects from the substrate. By applying this method to the 2D semiconductor, the charge-carrier mobility was more than doubled, showing a significant improvement of the performance of the 2D semiconductor.
Additionally, the team reduced the price of manufacturing the semiconductor. In general, constructing an ultra-fine dome structure on a surface generally involves costly equipment to create individual patterns on the surface. However, the team employed a method of self-assembling nanopatterns in which molecules assemble themselves to form a nanostructure. This method led to reducing production costs and showed good compatibility with conventional semiconductor manufacturing processes.
Professor Jung said, “This research can be applied to improve devices using various 2D semiconducting materials as well as devices using graphene, a metallic 2D material. It will be useful in a broad range of applications, such as the material for the high speed transistor channels for next-generation flexible displays or for the active layer in light detectors.”
This research, led by PhD candidate Soomin Yim, was published in Nano Letters in April.
Figure 1. Image of a 2D semiconductor using dome structures
2018.08.28 View 7182