Science
-
 KAIST's Masters of Science Journalism Presents the 4th Annual Moon-Sul Chung Award
The Masters of Science Journalism (MSJ) program at the Graduate School of Future Strategy, KAIST, hosted a conference and presented its fourth annual Moon-Sul Chung Award to the Hankyoreh newspaper at KAIST’s Seoul campus on October 11, 2014.
MSJ organized the conference to review major issues reported in the Korean news in the context of the sciences, for example, how the Korean media covered the tragic accident of the Sewol Ferry, a passenger ship that capsized and eventually sank with hundreds of passengers on board in the waters off the southern coast of Korea on April 16, 2014. Participants of the conference discussed the approaches taken by the media to report science-related issues such as mechanical problems of the ship and technical errors made by the operator.
MSJ conferred its annual award to a Hankyoreh news team which covered the Sewol incident. The award is named for Moon-Sul Chung, a philanthropist who created a scholarship fund for the school in 2011 to recognize news organizations that have striven to report social issues in a fair and balanced manner.
2014.10.14 View 7170
KAIST's Masters of Science Journalism Presents the 4th Annual Moon-Sul Chung Award
The Masters of Science Journalism (MSJ) program at the Graduate School of Future Strategy, KAIST, hosted a conference and presented its fourth annual Moon-Sul Chung Award to the Hankyoreh newspaper at KAIST’s Seoul campus on October 11, 2014.
MSJ organized the conference to review major issues reported in the Korean news in the context of the sciences, for example, how the Korean media covered the tragic accident of the Sewol Ferry, a passenger ship that capsized and eventually sank with hundreds of passengers on board in the waters off the southern coast of Korea on April 16, 2014. Participants of the conference discussed the approaches taken by the media to report science-related issues such as mechanical problems of the ship and technical errors made by the operator.
MSJ conferred its annual award to a Hankyoreh news team which covered the Sewol incident. The award is named for Moon-Sul Chung, a philanthropist who created a scholarship fund for the school in 2011 to recognize news organizations that have striven to report social issues in a fair and balanced manner.
2014.10.14 View 7170 -
 Thomson Reuters Nominates Distinguished Professor Ryong Ryoo for Its 2014 Nobel Citation Laureates in Chemistry
The Intellectual Property & Science business of Thomson Reuters announced on September 25th its “2014 Citation Laureates,” a list of candidates considered likely to win the Nobel Prize in the fields of physics, chemistry, physiology or medicine, and economics. The annual Thomson Reuters Citation Laureates will be recognized in perpetuity as contenders for a Nobel Prize.
Distinguished Professor Ryong Ryoo of the Department of Chemistry, KAIST, has been nominated for the 2014 Thomson Reuters Citation Laureates in Chemistry. He is the first Korean scientist who has made the list. In addition to Professor Ryoo, seven other scientists were selected as possible contenders for the 2014 Nobel Prize in Chemistry, or in the future.
Professor Ryoo was named alongside Charles T. Kresge, Chief Technology Officer of Saudi Aramco, Dhahran, and Galen D. Stucky, Professor of the Department Chemistry and Biochemistry at the University of California, Santa Barbara, for their research on the design of functional mesoporous materials (http://sciencewatch.com/nobel/2014-predictions/chemistry-laureates). Mesoporous materials have high surface areas with narrow pore-sized distribution and tunable pores diameters, offering promising properties and applications in various areas including adsorption, separation, sensing, and catalysis.
Professor Ryoo has focused his research interest in the synthesis of new functional nanoporous materials such as hierarchical zeolites, mesoporous silicas, carbons, and organic-inorganic composite materials that can be used for advanced applications in the production of alternative energy sources and in green chemical processes.
According to the press release by the Thomson Reuters, the list of the 2014 Nobel predictions includes 27 researchers representing 27 distinct academic and research organizations across nine different countries.
The annual Thomson Reuters Citation Laureates study is based on the analysis of proprietary data from the research and citation database, identifying the most influential researchers in the categories of chemistry, physics, physiology or medicine, and economics. Since its inception in 2002, the study has accurately forecasted 35 Nobel Prize winners. For the full text of the press release, please go to: http://thomsonreuters.com/press-releases/092014/2014-nobel-laureates-predictions.
2014.09.29 View 12922
Thomson Reuters Nominates Distinguished Professor Ryong Ryoo for Its 2014 Nobel Citation Laureates in Chemistry
The Intellectual Property & Science business of Thomson Reuters announced on September 25th its “2014 Citation Laureates,” a list of candidates considered likely to win the Nobel Prize in the fields of physics, chemistry, physiology or medicine, and economics. The annual Thomson Reuters Citation Laureates will be recognized in perpetuity as contenders for a Nobel Prize.
Distinguished Professor Ryong Ryoo of the Department of Chemistry, KAIST, has been nominated for the 2014 Thomson Reuters Citation Laureates in Chemistry. He is the first Korean scientist who has made the list. In addition to Professor Ryoo, seven other scientists were selected as possible contenders for the 2014 Nobel Prize in Chemistry, or in the future.
Professor Ryoo was named alongside Charles T. Kresge, Chief Technology Officer of Saudi Aramco, Dhahran, and Galen D. Stucky, Professor of the Department Chemistry and Biochemistry at the University of California, Santa Barbara, for their research on the design of functional mesoporous materials (http://sciencewatch.com/nobel/2014-predictions/chemistry-laureates). Mesoporous materials have high surface areas with narrow pore-sized distribution and tunable pores diameters, offering promising properties and applications in various areas including adsorption, separation, sensing, and catalysis.
Professor Ryoo has focused his research interest in the synthesis of new functional nanoporous materials such as hierarchical zeolites, mesoporous silicas, carbons, and organic-inorganic composite materials that can be used for advanced applications in the production of alternative energy sources and in green chemical processes.
According to the press release by the Thomson Reuters, the list of the 2014 Nobel predictions includes 27 researchers representing 27 distinct academic and research organizations across nine different countries.
The annual Thomson Reuters Citation Laureates study is based on the analysis of proprietary data from the research and citation database, identifying the most influential researchers in the categories of chemistry, physics, physiology or medicine, and economics. Since its inception in 2002, the study has accurately forecasted 35 Nobel Prize winners. For the full text of the press release, please go to: http://thomsonreuters.com/press-releases/092014/2014-nobel-laureates-predictions.
2014.09.29 View 12922 -
 The College of Information Science & Technology names its Alumnus of the Year 2014
The College of Information Science & Technology (CIST), KAIST, selected Tae-Kyung Yoo, the Chief Executive Officer of Lumens, Inc., a Korean company producing semiconductors and light emitting diodes (LEDs), as its Alumnus of the Year 2014.
The award ceremony took place on September 19, 2014 at the KAIST Institute with the participation of the university’s senior management and students.
Mr. Yoo was recognized for his pioneering work to develop the LED industry in Korea as the next-generation growth engine for the nation’s economy.
After the ceremony, he gave a talk entitled “The Past and Future of the LED Industry: Its Important Role in the Change of Korean Industry.”
The CIST created the Alumnus of the Year 2014 award, for the first time this year, to appreciate its alumni’s contribution to the advancement of the industrial and academic sectors of Korean information science and technology, and it will continue presenting the award from this year onwards.
2014.09.22 View 8316
The College of Information Science & Technology names its Alumnus of the Year 2014
The College of Information Science & Technology (CIST), KAIST, selected Tae-Kyung Yoo, the Chief Executive Officer of Lumens, Inc., a Korean company producing semiconductors and light emitting diodes (LEDs), as its Alumnus of the Year 2014.
The award ceremony took place on September 19, 2014 at the KAIST Institute with the participation of the university’s senior management and students.
Mr. Yoo was recognized for his pioneering work to develop the LED industry in Korea as the next-generation growth engine for the nation’s economy.
After the ceremony, he gave a talk entitled “The Past and Future of the LED Industry: Its Important Role in the Change of Korean Industry.”
The CIST created the Alumnus of the Year 2014 award, for the first time this year, to appreciate its alumni’s contribution to the advancement of the industrial and academic sectors of Korean information science and technology, and it will continue presenting the award from this year onwards.
2014.09.22 View 8316 -
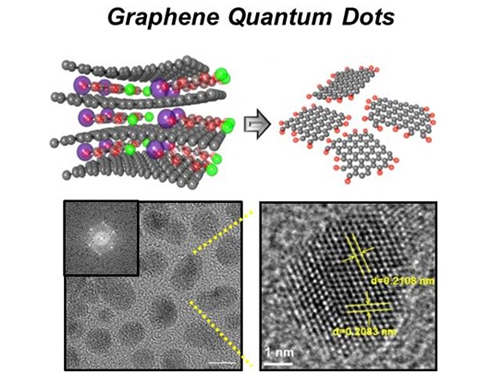 News Article on the Development of Synthesis Process for Graphene Quantum Dots
Before It's News, an international online news agency, highlighted the recent research conducted by KAIST professors (Seokwoo Jeon of the Department of Materials Science and Engineering, Yong-Hoon Cho of the Department of Physics, and Seunghyup Yoo of the Department of Electrical Engineering) on the development of synthesis process for graphene quantum dots, nanometer-sized round semiconductor nanoparticles that are very efficient at emitting photons. If commercialized, this synthetic technology will lead the way to the development of paper-thin displays in the future.
For the article, please go to the link below:
Before It’s News, September 3, 2014“Graphene quantum dots prove highly efficient in emitting light”
http://beforeitsnews.com/science-and-technology/2014/09/graphene-quantum-dots-prove-highly-efficient-in-emitting-light-2718190.html
2014.09.07 View 16067
News Article on the Development of Synthesis Process for Graphene Quantum Dots
Before It's News, an international online news agency, highlighted the recent research conducted by KAIST professors (Seokwoo Jeon of the Department of Materials Science and Engineering, Yong-Hoon Cho of the Department of Physics, and Seunghyup Yoo of the Department of Electrical Engineering) on the development of synthesis process for graphene quantum dots, nanometer-sized round semiconductor nanoparticles that are very efficient at emitting photons. If commercialized, this synthetic technology will lead the way to the development of paper-thin displays in the future.
For the article, please go to the link below:
Before It’s News, September 3, 2014“Graphene quantum dots prove highly efficient in emitting light”
http://beforeitsnews.com/science-and-technology/2014/09/graphene-quantum-dots-prove-highly-efficient-in-emitting-light-2718190.html
2014.09.07 View 16067 -
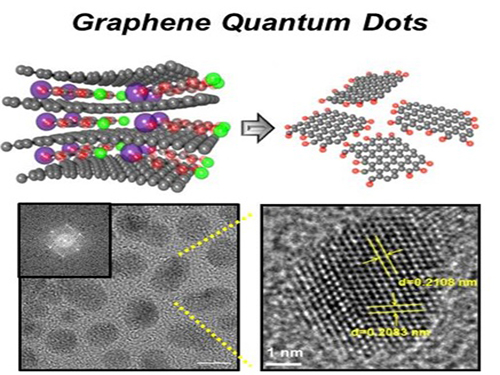 Extracting Light from Graphite: Core Technology of Graphene Quantum Dots Display Developed
Professor Seokwoo Jeon of the Department of Materials Science and Engineering, Professor Yong-Hoon Cho of the Department of Physics, and Professor Seunghyup Yoo of the Department of Electrical Engineering announced that they were able to develop topnotch graphene quantum dots from graphite.
Using the method of synthesizing graphite intercalation compound from graphite with salt and water, the research team developed graphene quantum dots in an ecofriendly way.
The quantum dots have a diameter of 5 nanometers with their sizes equal and yield high quantum efficiency. Unlike conventional quantum dots, they are not comprised of toxic materials such as lead or cadmium. As the quantum dots can be developed from materials which can be easily found in the nature, researchers look forward to putting these into mass production at low cost.
The research team also discovered a luminescence mechanism of graphene quantum dots and confirmed the possibility of commercial use by developing quantum dot light-emitting diodes with brightness of 1,000 cd/m2, which is greater than that of cellphone displays.
Professor Seokwoo Jeon said, “Although quantum dot LEDs have a lower luminous efficiency than existing ones, their luminescent property can be further improved” and emphasized that “using quantum dot displays will allow us to develop not only paper-thin displays but also flexible ones.”
Sponsored by Graphene Research Center in KAIST Institute for NanoCentury, the research finding was published online in the April 20th issue of Advanced Optical Materials.
Picture 1: Graphene quantum dots and their synthesis
Picture 2: Luminescence mechanism of graphene quantum dots
Picture 3: Structure of graphene quantum dots LED and its emission
2014.09.06 View 20657
Extracting Light from Graphite: Core Technology of Graphene Quantum Dots Display Developed
Professor Seokwoo Jeon of the Department of Materials Science and Engineering, Professor Yong-Hoon Cho of the Department of Physics, and Professor Seunghyup Yoo of the Department of Electrical Engineering announced that they were able to develop topnotch graphene quantum dots from graphite.
Using the method of synthesizing graphite intercalation compound from graphite with salt and water, the research team developed graphene quantum dots in an ecofriendly way.
The quantum dots have a diameter of 5 nanometers with their sizes equal and yield high quantum efficiency. Unlike conventional quantum dots, they are not comprised of toxic materials such as lead or cadmium. As the quantum dots can be developed from materials which can be easily found in the nature, researchers look forward to putting these into mass production at low cost.
The research team also discovered a luminescence mechanism of graphene quantum dots and confirmed the possibility of commercial use by developing quantum dot light-emitting diodes with brightness of 1,000 cd/m2, which is greater than that of cellphone displays.
Professor Seokwoo Jeon said, “Although quantum dot LEDs have a lower luminous efficiency than existing ones, their luminescent property can be further improved” and emphasized that “using quantum dot displays will allow us to develop not only paper-thin displays but also flexible ones.”
Sponsored by Graphene Research Center in KAIST Institute for NanoCentury, the research finding was published online in the April 20th issue of Advanced Optical Materials.
Picture 1: Graphene quantum dots and their synthesis
Picture 2: Luminescence mechanism of graphene quantum dots
Picture 3: Structure of graphene quantum dots LED and its emission
2014.09.06 View 20657 -
 Regulatory T Cells Influence Liver Damage of Hepatitis A Patients
Liver damage becomes more severe with the decrease of regulatory T cells
“This research will aid the development of hepatitis A targeted drug,” said a KAIST researcher.
The KAIST Graduate School of Medical Science and Engineering’s Professor Eui-Cheol Shin and his research team have identified the mechanism, explaining how the regulatory T cells are responsible for the body’s immune system and how they have induced liver damage of hepatitis A patients.
The research results were published online in the July 9th edition of ‘Gut,’ the world’s most prominent journal in the field of gastroenterology.
Hepatitis A is an acute form of hepatitis caused by hepatitis A virus. The virus spreads through oral contact and enters the body via digestive organs.
Regulatory T cells play an important role in maintaining the homeostasis of the body’s immune system by inhibiting the activation of other immune cells. In the case of chronic viral infections, regulatory T cells are known to contribute to the duration of the infection, weakening the immune response to virus infections. However, there has been no information on what roles the regulatory T cells perform in the case of acute viral infections.
The research team used the fluorescence flow cytometry technique to determine the number and characteristics of a variety of immune cells, including regulatory T cells, in the blood of hepatitis A patients.
Consequently, the researchers confirmed that the decrease in the regulatory T cells immune inhibitory ability was consistent with a significant reduction in the number of regulatory T cells in the blood of hepatitis A patients. Furthermore, it was identified that the more noticeable decrease of regulatory T cells led to the occurrence of a more severe liver injury.
The analysis of hepatitis A patient’s blood proved that the cause of the decrease in the number and function of regulatory T cells was the increased expression of cell surface protein ‘Fas,’ which induces cell death.
Professor Shin said, “This study is the first case which proposes the mechanism for clinical aspects in not only hepatitis A, but also acute virus infection.” He added on the future prospect of the research that: “In the future, we can prevent tissue damage by inhibiting cell death of regulatory T cells for severe acute viral infections that do not have an effective treatment for the virus itself.”
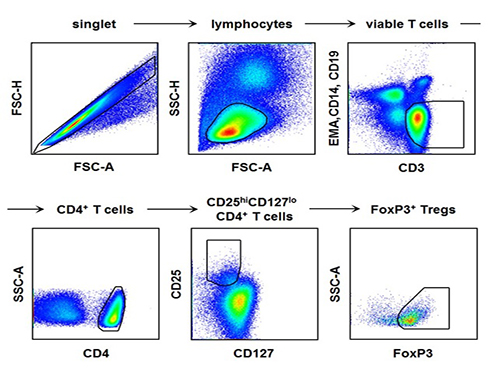
[Picture]
The picture shows the process of fluorescence flow cytometry technique to study regulatory T cell in the blood of hepatitis A patients.
2014.08.11 View 11570
Regulatory T Cells Influence Liver Damage of Hepatitis A Patients
Liver damage becomes more severe with the decrease of regulatory T cells
“This research will aid the development of hepatitis A targeted drug,” said a KAIST researcher.
The KAIST Graduate School of Medical Science and Engineering’s Professor Eui-Cheol Shin and his research team have identified the mechanism, explaining how the regulatory T cells are responsible for the body’s immune system and how they have induced liver damage of hepatitis A patients.
The research results were published online in the July 9th edition of ‘Gut,’ the world’s most prominent journal in the field of gastroenterology.
Hepatitis A is an acute form of hepatitis caused by hepatitis A virus. The virus spreads through oral contact and enters the body via digestive organs.
Regulatory T cells play an important role in maintaining the homeostasis of the body’s immune system by inhibiting the activation of other immune cells. In the case of chronic viral infections, regulatory T cells are known to contribute to the duration of the infection, weakening the immune response to virus infections. However, there has been no information on what roles the regulatory T cells perform in the case of acute viral infections.
The research team used the fluorescence flow cytometry technique to determine the number and characteristics of a variety of immune cells, including regulatory T cells, in the blood of hepatitis A patients.
Consequently, the researchers confirmed that the decrease in the regulatory T cells immune inhibitory ability was consistent with a significant reduction in the number of regulatory T cells in the blood of hepatitis A patients. Furthermore, it was identified that the more noticeable decrease of regulatory T cells led to the occurrence of a more severe liver injury.
The analysis of hepatitis A patient’s blood proved that the cause of the decrease in the number and function of regulatory T cells was the increased expression of cell surface protein ‘Fas,’ which induces cell death.
Professor Shin said, “This study is the first case which proposes the mechanism for clinical aspects in not only hepatitis A, but also acute virus infection.” He added on the future prospect of the research that: “In the future, we can prevent tissue damage by inhibiting cell death of regulatory T cells for severe acute viral infections that do not have an effective treatment for the virus itself.”
[Picture]
The picture shows the process of fluorescence flow cytometry technique to study regulatory T cell in the blood of hepatitis A patients.
2014.08.11 View 11570 -
 The Journal of Clinical Investigation: Researchers Uncover the Secret Lymphatic Identity of the Schlemm's Canal
The Journal of Clinical Investigation (JCI), a peer-reviewed, top-tier medical journal published by the American Society for Clinical Investigation, carried a commentary entitled “Schlemm’s Canal: More Than Meets the Eye, Lymphatics in Disguise” in the July 25, 2014 issue.
In the commentary, the authors compared a research paper (“Lymphatic regular PROX1 determines Schlemm’s canal integrity and identity”) by Professor Gou-Young Koh of the Graduate School of Medical Science and Engineering at KAIST with research work from the University of Helsinki (article entitled “The Schlemm’s canal is a VEGF-C/VEGFR-3 responsive lymphatic-like vessel”).
The JCI released a press statement dated July 25, 2014 on its commentary. It mentioned that glaucoma, one of the leading causes of blindness worldwide, elevates eye pressure owing to poor drainage of aqueous humor. A specialized structure called “Schlemm’s canal” funnels aqueous humor from the eye back into circulation, which is critical to prevent pressure buildup in the eye. The article discussed the role of Schlemm’s canal in the context of lymphatic vascular characteristics by reviewing two research group’s papers back-to-back.
For the full text of the press release, please visit the link below:
Press Release from the Journal of Clinical Investigation, July 25, 2014
“Researchers uncover the secret lymphatic identity of the Schlemm’s canal”
http://www.eurekalert.org/pub_releases/2014-07/joci-rut072414.php
2014.07.28 View 9504
The Journal of Clinical Investigation: Researchers Uncover the Secret Lymphatic Identity of the Schlemm's Canal
The Journal of Clinical Investigation (JCI), a peer-reviewed, top-tier medical journal published by the American Society for Clinical Investigation, carried a commentary entitled “Schlemm’s Canal: More Than Meets the Eye, Lymphatics in Disguise” in the July 25, 2014 issue.
In the commentary, the authors compared a research paper (“Lymphatic regular PROX1 determines Schlemm’s canal integrity and identity”) by Professor Gou-Young Koh of the Graduate School of Medical Science and Engineering at KAIST with research work from the University of Helsinki (article entitled “The Schlemm’s canal is a VEGF-C/VEGFR-3 responsive lymphatic-like vessel”).
The JCI released a press statement dated July 25, 2014 on its commentary. It mentioned that glaucoma, one of the leading causes of blindness worldwide, elevates eye pressure owing to poor drainage of aqueous humor. A specialized structure called “Schlemm’s canal” funnels aqueous humor from the eye back into circulation, which is critical to prevent pressure buildup in the eye. The article discussed the role of Schlemm’s canal in the context of lymphatic vascular characteristics by reviewing two research group’s papers back-to-back.
For the full text of the press release, please visit the link below:
Press Release from the Journal of Clinical Investigation, July 25, 2014
“Researchers uncover the secret lymphatic identity of the Schlemm’s canal”
http://www.eurekalert.org/pub_releases/2014-07/joci-rut072414.php
2014.07.28 View 9504 -
 Discovery of New Therapeutic Targets for Alzheimer's Disease
A Korean research team headed by Professor Dae-Soo Kim of Biological Sciences at KAIST and Dr. Chang-Jun Lee from the Korea Institute of Science and Technology (KIST) successfully identified that reactive astrocytes, commonly observed in brains affected by Alzheimer’s disease, produce abnormal amounts of inhibitory neurotransmitter gamma-Aminobutyric acid (GABA) in reaction to the enzyme Monoamine oxidase B (Mao-B) and release GABA through the Bestrophin-1 channel to suppress the normal signal transmission of brain nerve cells.
By suppressing the GABA production or release from reactive astrocytes, the research team was able to restore the model mice's memory and learning impairment caused by Alzheimer’s disease. This discovery will allow the development of new drugs to treat Alzheimer’s and other related diseases.
The research result was published in the June 29, 2014 edition of Nature Medicine (Title: GABA from Reactive Astrocytes Impairs Memory in Mouse Models of Alzheimer’s Disease).
For details, please read the article below:
Technology News, July 10, 2014
"Discovery of New Drug Targets for Memory Impairment in Alzheimer’s Disease"
http://technews.tmcnet.com/news/2014/07/10/7917811.htm
2014.07.16 View 10738
Discovery of New Therapeutic Targets for Alzheimer's Disease
A Korean research team headed by Professor Dae-Soo Kim of Biological Sciences at KAIST and Dr. Chang-Jun Lee from the Korea Institute of Science and Technology (KIST) successfully identified that reactive astrocytes, commonly observed in brains affected by Alzheimer’s disease, produce abnormal amounts of inhibitory neurotransmitter gamma-Aminobutyric acid (GABA) in reaction to the enzyme Monoamine oxidase B (Mao-B) and release GABA through the Bestrophin-1 channel to suppress the normal signal transmission of brain nerve cells.
By suppressing the GABA production or release from reactive astrocytes, the research team was able to restore the model mice's memory and learning impairment caused by Alzheimer’s disease. This discovery will allow the development of new drugs to treat Alzheimer’s and other related diseases.
The research result was published in the June 29, 2014 edition of Nature Medicine (Title: GABA from Reactive Astrocytes Impairs Memory in Mouse Models of Alzheimer’s Disease).
For details, please read the article below:
Technology News, July 10, 2014
"Discovery of New Drug Targets for Memory Impairment in Alzheimer’s Disease"
http://technews.tmcnet.com/news/2014/07/10/7917811.htm
2014.07.16 View 10738 -
 Artificial Antibody-based Therapeutic Candidate for Lung Cancer Developed
Professor Hak-Sung Kim of Biological Sciences at KAIST publishes a cover article on artificial antibody in "Molecular Therapy".
Repebody-based lung cancer therapeutic drug candidate developed Repebody-based protein demonstrates the possibility of the development of a new drug
KAIST Biological Sciences Department’s Professor Hak-Sung Kim, in collaboration with Professor Eun-Kyung Cho from the College of Medicine at Chungnam National University, has successfully developed an artificial antibody-based, or repebody, cancer therapeutic candidate. These research results were published as a cover paper of the July edition of Molecular Therapy.
The repebody developed by Professor Kim and his team strongly binds to interleukin-6, a cancer-causing factor. It has also been confirmed that the repebody can significantly inhibit the proliferation of cancer cells in non-small-cell lung cancer animal model.
Numerous multinational pharmaceutical and biotechnology companies have invested astronomical amounts of money in research for the development of protein therapeutics with low side effects and high efficacy. More than 20 kinds of such therapeutics are currently under clinical trials, and over 100 drugs are under clinical demonstration. Among these, the majority is antibody-based therapeutics, and most of the investments are heavily concentrated in this field. However, antibody production cost is very high because it has large molecular weights and complex structural properties, and this makes it difficult to engineer. Consequently, the development costs a great deal of time and money.
In order to overcome the existing limitations of antibody-based therapeutics, Professor Kim and his team have developed a new artificial antibody, or repebody, which was published in Proceedings of the National Academy of Sciences (PNAS) in 2012. Based on this research, they have succeeded in developing a therapeutic candidate for treating non-small-cell lung cancer with a specifically strong cohesion to the cancer-causing factor, interleukin-6.
Interleukin-6 is a crucial substance within the body that is involved in immune and inflammatory-related signals. When abnormally expressed, it activates various carcinogenic pathways and promotes tumor growth and metastasis. Because of its importance, multinational pharmaceutical companies are heavily investing in developing therapeutics that can inhibit the signaling of interleukin-6.
In this study, Professor Kim and his team observed that a repebody consists of repeated modules, and they conceived a module-based affinity amplification technology that can effectively increase the binding affinity with the disease target. The developed therapeutic candidate has been confirmed in cell and animal experiments to show low immunogenicity, as well as to strongly inhibit the proliferation of non-small-cell lung cancer.
Furthermore, by investigating the complex structure of the repebody with interleukin-6, Professor Kim has identified its mechanism, which demonstrated the potential for therapeutic development. The researchers are currently carrying out pre-clinical trials for acquiring permission to perform clinical trials on animals with non-small-cell lung cancer. The repebody can be developed into a new protein drug after demonstrating its safety and efficacy.
Professor Hak-Sung Kim and his team have confirmed that the repebody can be utilized as a new protein drug, and this will be a significant contribution to Korea’s protein drugs and biotechnology industry development.
The research was supported by the Future Pioneer Industry project and sponsored by the Ministry of Science, ICT and Future Planning.
Figure 1. Professor Kim’s article published as the cover article of July edition of Molecular Therapy
Figure 2. Clinical proof of the repebody’s inhibition of cancer growth using animal models
2014.07.14 View 14848
Artificial Antibody-based Therapeutic Candidate for Lung Cancer Developed
Professor Hak-Sung Kim of Biological Sciences at KAIST publishes a cover article on artificial antibody in "Molecular Therapy".
Repebody-based lung cancer therapeutic drug candidate developed Repebody-based protein demonstrates the possibility of the development of a new drug
KAIST Biological Sciences Department’s Professor Hak-Sung Kim, in collaboration with Professor Eun-Kyung Cho from the College of Medicine at Chungnam National University, has successfully developed an artificial antibody-based, or repebody, cancer therapeutic candidate. These research results were published as a cover paper of the July edition of Molecular Therapy.
The repebody developed by Professor Kim and his team strongly binds to interleukin-6, a cancer-causing factor. It has also been confirmed that the repebody can significantly inhibit the proliferation of cancer cells in non-small-cell lung cancer animal model.
Numerous multinational pharmaceutical and biotechnology companies have invested astronomical amounts of money in research for the development of protein therapeutics with low side effects and high efficacy. More than 20 kinds of such therapeutics are currently under clinical trials, and over 100 drugs are under clinical demonstration. Among these, the majority is antibody-based therapeutics, and most of the investments are heavily concentrated in this field. However, antibody production cost is very high because it has large molecular weights and complex structural properties, and this makes it difficult to engineer. Consequently, the development costs a great deal of time and money.
In order to overcome the existing limitations of antibody-based therapeutics, Professor Kim and his team have developed a new artificial antibody, or repebody, which was published in Proceedings of the National Academy of Sciences (PNAS) in 2012. Based on this research, they have succeeded in developing a therapeutic candidate for treating non-small-cell lung cancer with a specifically strong cohesion to the cancer-causing factor, interleukin-6.
Interleukin-6 is a crucial substance within the body that is involved in immune and inflammatory-related signals. When abnormally expressed, it activates various carcinogenic pathways and promotes tumor growth and metastasis. Because of its importance, multinational pharmaceutical companies are heavily investing in developing therapeutics that can inhibit the signaling of interleukin-6.
In this study, Professor Kim and his team observed that a repebody consists of repeated modules, and they conceived a module-based affinity amplification technology that can effectively increase the binding affinity with the disease target. The developed therapeutic candidate has been confirmed in cell and animal experiments to show low immunogenicity, as well as to strongly inhibit the proliferation of non-small-cell lung cancer.
Furthermore, by investigating the complex structure of the repebody with interleukin-6, Professor Kim has identified its mechanism, which demonstrated the potential for therapeutic development. The researchers are currently carrying out pre-clinical trials for acquiring permission to perform clinical trials on animals with non-small-cell lung cancer. The repebody can be developed into a new protein drug after demonstrating its safety and efficacy.
Professor Hak-Sung Kim and his team have confirmed that the repebody can be utilized as a new protein drug, and this will be a significant contribution to Korea’s protein drugs and biotechnology industry development.
The research was supported by the Future Pioneer Industry project and sponsored by the Ministry of Science, ICT and Future Planning.
Figure 1. Professor Kim’s article published as the cover article of July edition of Molecular Therapy
Figure 2. Clinical proof of the repebody’s inhibition of cancer growth using animal models
2014.07.14 View 14848 -
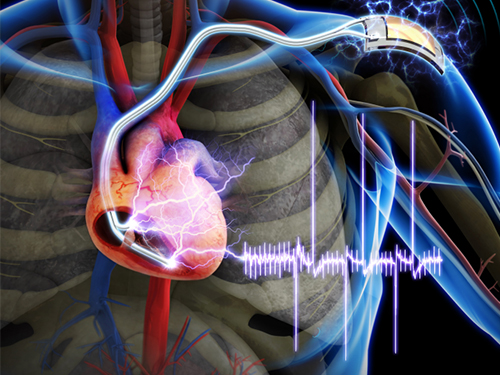 The First Demonstration of a Self-powered Cardiac Pacemaker
As the number of pacemakers implanted each year reaches into the millions worldwide, improving the lifespan of pacemaker batteries has been of great concern for developers and manufacturers. Currently, pacemaker batteries last seven years on average, requiring frequent replacements, which may pose patients to a potential risk involved in medical procedures.
A research team from the Korea Advanced Institute of Science and Technology (KAIST), headed by Professor Keon Jae Lee of the Department of Materials Science and Engineering at KAIST and Professor Boyoung Joung, M.D. of the Division of Cardiology at Severance Hospital of Yonsei University, has developed a self-powered artificial cardiac pacemaker that is operated semi-permanently by a flexible piezoelectric nanogenerator.
The artificial cardiac pacemaker is widely acknowledged as medical equipment that is integrated into the human body to regulate the heartbeats through electrical stimulation to contract the cardiac muscles of people who suffer from arrhythmia. However, repeated surgeries to replace pacemaker batteries have exposed elderly patients to health risks such as infections or severe bleeding during operations.
The team’s newly designed flexible piezoelectric nanogenerator directly stimulated a living rat’s heart using electrical energy converted from the small body movements of the rat. This technology could facilitate the use of self-powered flexible energy harvesters, not only prolonging the lifetime of cardiac pacemakers but also realizing real-time heart monitoring.
The research team fabricated high-performance flexible nanogenerators utilizing a bulk single-crystal PMN-PT thin film (iBULe Photonics). The harvested energy reached up to 8.2 V and 0.22 mA by bending and pushing motions, which were high enough values to directly stimulate the rat’s heart.
Professor Keon Jae Lee said:
“For clinical purposes, the current achievement will benefit the development of self-powered cardiac pacemakers as well as prevent heart attacks via the real-time diagnosis of heart arrhythmia. In addition, the flexible piezoelectric nanogenerator could also be utilized as an electrical source for various implantable medical devices.”
This research result was described in the April online issue of Advanced Materials (“Self-Powered Cardiac Pacemaker Enabled by Flexible Single Crystalline PMN-PT Piezoelectric Energy Harvester”: http://onlinelibrary.wiley.com/doi/10.1002/adma.201400562/abstract).
Youtube link: http://www.youtube.com/watch?v=ZWYT2cU_Mog&feature=youtu.be
Picture Caption: A self-powered cardiac pacemaker is enabled by a flexible piezoelectric energy harvester.
2014.06.25 View 18534
The First Demonstration of a Self-powered Cardiac Pacemaker
As the number of pacemakers implanted each year reaches into the millions worldwide, improving the lifespan of pacemaker batteries has been of great concern for developers and manufacturers. Currently, pacemaker batteries last seven years on average, requiring frequent replacements, which may pose patients to a potential risk involved in medical procedures.
A research team from the Korea Advanced Institute of Science and Technology (KAIST), headed by Professor Keon Jae Lee of the Department of Materials Science and Engineering at KAIST and Professor Boyoung Joung, M.D. of the Division of Cardiology at Severance Hospital of Yonsei University, has developed a self-powered artificial cardiac pacemaker that is operated semi-permanently by a flexible piezoelectric nanogenerator.
The artificial cardiac pacemaker is widely acknowledged as medical equipment that is integrated into the human body to regulate the heartbeats through electrical stimulation to contract the cardiac muscles of people who suffer from arrhythmia. However, repeated surgeries to replace pacemaker batteries have exposed elderly patients to health risks such as infections or severe bleeding during operations.
The team’s newly designed flexible piezoelectric nanogenerator directly stimulated a living rat’s heart using electrical energy converted from the small body movements of the rat. This technology could facilitate the use of self-powered flexible energy harvesters, not only prolonging the lifetime of cardiac pacemakers but also realizing real-time heart monitoring.
The research team fabricated high-performance flexible nanogenerators utilizing a bulk single-crystal PMN-PT thin film (iBULe Photonics). The harvested energy reached up to 8.2 V and 0.22 mA by bending and pushing motions, which were high enough values to directly stimulate the rat’s heart.
Professor Keon Jae Lee said:
“For clinical purposes, the current achievement will benefit the development of self-powered cardiac pacemakers as well as prevent heart attacks via the real-time diagnosis of heart arrhythmia. In addition, the flexible piezoelectric nanogenerator could also be utilized as an electrical source for various implantable medical devices.”
This research result was described in the April online issue of Advanced Materials (“Self-Powered Cardiac Pacemaker Enabled by Flexible Single Crystalline PMN-PT Piezoelectric Energy Harvester”: http://onlinelibrary.wiley.com/doi/10.1002/adma.201400562/abstract).
Youtube link: http://www.youtube.com/watch?v=ZWYT2cU_Mog&feature=youtu.be
Picture Caption: A self-powered cardiac pacemaker is enabled by a flexible piezoelectric energy harvester.
2014.06.25 View 18534 -
 2014 Conference on Korean Sociology Held at KAIST
The Korean Sociological Association (KSA) hosted a two-day conference in 2014 entitled “In the age of anxiety, sociology can present answers” at the College of Liberal Arts and Convergence Science, KAIST, on June 20-21, 2014. Professor Jung-Ro Yoon of the Department of Humanities and Social Sciences at KAIST is the President of KSA. The conference addressed issues such as big data and risk society. During the conference, 40 sessions took place, and 150 research papers were released.
Professor Yoon said, “The conference will offer a great opportunity for Korean sociologists to discuss anxiety, chaos, risk, and the uncertainty that Korean society experiences and suggest answers and a new vision upon which Korean society should move forward.”
2014.06.22 View 9092
2014 Conference on Korean Sociology Held at KAIST
The Korean Sociological Association (KSA) hosted a two-day conference in 2014 entitled “In the age of anxiety, sociology can present answers” at the College of Liberal Arts and Convergence Science, KAIST, on June 20-21, 2014. Professor Jung-Ro Yoon of the Department of Humanities and Social Sciences at KAIST is the President of KSA. The conference addressed issues such as big data and risk society. During the conference, 40 sessions took place, and 150 research papers were released.
Professor Yoon said, “The conference will offer a great opportunity for Korean sociologists to discuss anxiety, chaos, risk, and the uncertainty that Korean society experiences and suggest answers and a new vision upon which Korean society should move forward.”
2014.06.22 View 9092 -
 Professor Won Do Heo on LED Light Technology for Controlling Proteins in Living Cells
With the newly developed LED technology, Professor Won Do Heo at the College of Life Science and Bioengineering, KAIST, was able to suppress cell migration and division when cells are exposed to LED light. This suggests a breakthrough to apply in future cancer cell research.
Professor Heo talked about the impact of his research in the following excerpt from a news article:
“We are already conducting research on the spread of cancer, as well as brain science in animal models with the Light-Activated Reversible Inhibition by Assembled Trap. I believe this technology will be a breakthrough in investigating cancer treatments and the function of neurons in a complex neural network, which existing technologies have not been able to do.”
From EE Times Europe, June 19, 2014
“LED Light Technology Controls Proteins in Living Cells”
http://www.ledlighting-eetimes.com/en/led-light-technology-controls-proteins-in-living-cells.html?cmp_id=7&news_id=222909336
2014.06.22 View 8782
Professor Won Do Heo on LED Light Technology for Controlling Proteins in Living Cells
With the newly developed LED technology, Professor Won Do Heo at the College of Life Science and Bioengineering, KAIST, was able to suppress cell migration and division when cells are exposed to LED light. This suggests a breakthrough to apply in future cancer cell research.
Professor Heo talked about the impact of his research in the following excerpt from a news article:
“We are already conducting research on the spread of cancer, as well as brain science in animal models with the Light-Activated Reversible Inhibition by Assembled Trap. I believe this technology will be a breakthrough in investigating cancer treatments and the function of neurons in a complex neural network, which existing technologies have not been able to do.”
From EE Times Europe, June 19, 2014
“LED Light Technology Controls Proteins in Living Cells”
http://www.ledlighting-eetimes.com/en/led-light-technology-controls-proteins-in-living-cells.html?cmp_id=7&news_id=222909336
2014.06.22 View 8782