MIS
-
 Professor Jae-Kyu Lee Elected to Head the Association for Information Systems
Jae Kyu Lee, HHI (Hyundai Heavy Industries, Co., Ltd.) Chair Professor, College of Business at KAIST, has been elected to lead the world major academic society, Association for Information Systems (AIS), from July 2015 to June 2016. Professor Lee will be the first Korean to serve the organization as president. From July 2014 to June 2015, he will serve as president-elect.
Currently, Professor Lee is the Director of EEWS (Energy, Environment, Water, and Sustainability) Research Center at KAIST, focusing on research and development in finding solutions to critical issues facing humanity. He also played a pivotal role in the conclusion of a memorandum of understanding between HHI and KAIST in June 2013 to establish HHI-KAIST EEWS Research Center within the KAIST campus.
The AIS is the premier professional association for individuals and organizations who lead the research, teaching, practice, and study of information systems worldwide.
2014.05.14 View 11571
Professor Jae-Kyu Lee Elected to Head the Association for Information Systems
Jae Kyu Lee, HHI (Hyundai Heavy Industries, Co., Ltd.) Chair Professor, College of Business at KAIST, has been elected to lead the world major academic society, Association for Information Systems (AIS), from July 2015 to June 2016. Professor Lee will be the first Korean to serve the organization as president. From July 2014 to June 2015, he will serve as president-elect.
Currently, Professor Lee is the Director of EEWS (Energy, Environment, Water, and Sustainability) Research Center at KAIST, focusing on research and development in finding solutions to critical issues facing humanity. He also played a pivotal role in the conclusion of a memorandum of understanding between HHI and KAIST in June 2013 to establish HHI-KAIST EEWS Research Center within the KAIST campus.
The AIS is the premier professional association for individuals and organizations who lead the research, teaching, practice, and study of information systems worldwide.
2014.05.14 View 11571 -
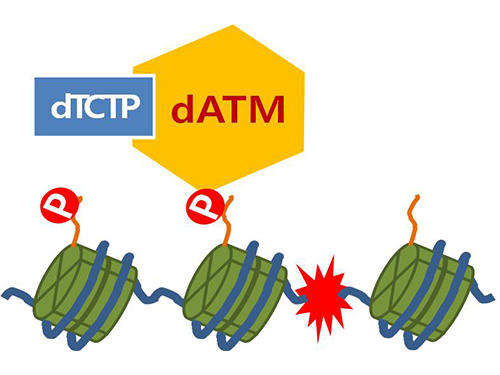 Mechanism in regulation of cancer-related key enzyme, ATM, for DNA damage and repair revealed
Professor Kwang-Wook Choi
A research team led by Professor Kwang-Wook Choi and Dr. Seong-Tae Hong from the Department of Biological Sciences at KAIST has successfully investigated the operational mechanism of the protein Ataxia Telangiectasia Mutated (ATM), an essential protein to the function of a crucial key enzyme that repairs the damaged DNA which stores biometric information. The results were published on December 19th Nature Communications online edition.
All organisms, including humans, constantly strive to protect the information within their DNA from damages posed by a number of factors, such as carbonized materials in our daily food intake, radioactive materials such as radon emitting from the cement of buildings or ultraviolet of the sunlight, which could be a trigger for cancer.
In order to keep the DNA information safe, the organisms are always carrying out complex and sophisticated DNA repair work, which involves the crucial DNA damage repair protein ATM. Consequently, a faulty ATM leads to higher risks of cancer.
Until now, academia predicted that the Translationally Controlled Tumor Protein (TCTP) will play an important role in regulating the function of ATM. However, since most of main research regarding TCTP has only been conducted in cultured cells, it was unable to identify exactly what mechanisms TCTP employs to control ATM.
The KAIST research team identified that TCTP can combine with ATM or increase the enzymatic activity of ATM. In addition, Drosophilia, one of the most widely used model organisms for molecular genetics, has been used to identify that TCTP and ATM play a very important role in repairing the DNA damaged by radiation. This information has allowed the researchers to establish TCTP’s essential function in maintaining the DNA information in cell cultures and even in higher organisms, and to provide specific and important clues to the regulation of ATM by TCTP.
Professor Kwang-Wook Choi said, “Our research is a good example that basic research using Drosophilia can make important contributions to understanding the process of diseases, such as cancer, and to developing adequate treatment.”
The research has been funded by the Ministry of Science, ICT and Future Planning, Republic of Korea, and the National Research Foundation of Korea.
Figure 1. When the amount of TCTP protein is reduced, cells of the Drosophila's eye are abnormally deformed by radiation. Scale bars = 200mm
Figure 2. When the amount of TCTP protein is reduced, the chromosomes of Drosophilia are easily broken by radiation. Scale bars = 10 mm.
Figure 3. When gene expressions of TCTP and ATM are reduced, large defects occur in the normal development of the eye. (Left: normal Drosophilia's eye, right: development-deficient eye)
Figure 4. ATM marks the position of the broken DNA, with TCTP helping to facilitate this reaction. DNA (blue line) within the cell nucleus is coiled around the histone protein (green cylinder). When DNA is broken, ATM protein attaches a phosphate group (P). Multiple DNA repair protein recognizes the phosphate as a signal that requires repair and gathers at the site.
2014.01.07 View 15425
Mechanism in regulation of cancer-related key enzyme, ATM, for DNA damage and repair revealed
Professor Kwang-Wook Choi
A research team led by Professor Kwang-Wook Choi and Dr. Seong-Tae Hong from the Department of Biological Sciences at KAIST has successfully investigated the operational mechanism of the protein Ataxia Telangiectasia Mutated (ATM), an essential protein to the function of a crucial key enzyme that repairs the damaged DNA which stores biometric information. The results were published on December 19th Nature Communications online edition.
All organisms, including humans, constantly strive to protect the information within their DNA from damages posed by a number of factors, such as carbonized materials in our daily food intake, radioactive materials such as radon emitting from the cement of buildings or ultraviolet of the sunlight, which could be a trigger for cancer.
In order to keep the DNA information safe, the organisms are always carrying out complex and sophisticated DNA repair work, which involves the crucial DNA damage repair protein ATM. Consequently, a faulty ATM leads to higher risks of cancer.
Until now, academia predicted that the Translationally Controlled Tumor Protein (TCTP) will play an important role in regulating the function of ATM. However, since most of main research regarding TCTP has only been conducted in cultured cells, it was unable to identify exactly what mechanisms TCTP employs to control ATM.
The KAIST research team identified that TCTP can combine with ATM or increase the enzymatic activity of ATM. In addition, Drosophilia, one of the most widely used model organisms for molecular genetics, has been used to identify that TCTP and ATM play a very important role in repairing the DNA damaged by radiation. This information has allowed the researchers to establish TCTP’s essential function in maintaining the DNA information in cell cultures and even in higher organisms, and to provide specific and important clues to the regulation of ATM by TCTP.
Professor Kwang-Wook Choi said, “Our research is a good example that basic research using Drosophilia can make important contributions to understanding the process of diseases, such as cancer, and to developing adequate treatment.”
The research has been funded by the Ministry of Science, ICT and Future Planning, Republic of Korea, and the National Research Foundation of Korea.
Figure 1. When the amount of TCTP protein is reduced, cells of the Drosophila's eye are abnormally deformed by radiation. Scale bars = 200mm
Figure 2. When the amount of TCTP protein is reduced, the chromosomes of Drosophilia are easily broken by radiation. Scale bars = 10 mm.
Figure 3. When gene expressions of TCTP and ATM are reduced, large defects occur in the normal development of the eye. (Left: normal Drosophilia's eye, right: development-deficient eye)
Figure 4. ATM marks the position of the broken DNA, with TCTP helping to facilitate this reaction. DNA (blue line) within the cell nucleus is coiled around the histone protein (green cylinder). When DNA is broken, ATM protein attaches a phosphate group (P). Multiple DNA repair protein recognizes the phosphate as a signal that requires repair and gathers at the site.
2014.01.07 View 15425 -
 Secondary, High Capacity Battery developed from Rice Husks
Rice husks, a waste product from rice polishing, has been successfully utilized as the silicon anode for use in high capacity lithium ion secondary batteries. The new silicon anode derived from rice husks exhibit superior output and lifespan.
Professor Choi Jang Wook (The Graduate School of Energy, Environment, Water and Sustainability (EEWS)) and Professor Park Seung Min (Department of Biochemistry) and their respective research teams separated naturally occurring, highly porous silica material within the rice husks and developed a 3-dimensional, highly porous silicon anode material.
The result of the research effort was published in the online edition of the Proceedings of the National Academy of Sciences (PNAS) journal, a world renowned journal in the field of natural sciences.
Silicon has attracted much attention as anode material for next generation lithium ion secondary batteries because it exhibits 3~5 times higher capacity than conventional graphene. The high capacity will pave the way to lithium secondary batteries with higher energy densities than conventional batteries. It is anticipated that the application of silicon batteries will yield electronic devices with a longer duration for use in addition to electronic vehicles boasting longer mileage.
The silicon anode is based on the 3-dimensional, highly porous structure of rice husks which remedies the problematic extreme volume expansion of conventional silicon anodes.
Utilization of inexpensive rice husks to create high value silicon anodes will cause a ripple effect on the industry and academia.
2013.08.23 View 12114
Secondary, High Capacity Battery developed from Rice Husks
Rice husks, a waste product from rice polishing, has been successfully utilized as the silicon anode for use in high capacity lithium ion secondary batteries. The new silicon anode derived from rice husks exhibit superior output and lifespan.
Professor Choi Jang Wook (The Graduate School of Energy, Environment, Water and Sustainability (EEWS)) and Professor Park Seung Min (Department of Biochemistry) and their respective research teams separated naturally occurring, highly porous silica material within the rice husks and developed a 3-dimensional, highly porous silicon anode material.
The result of the research effort was published in the online edition of the Proceedings of the National Academy of Sciences (PNAS) journal, a world renowned journal in the field of natural sciences.
Silicon has attracted much attention as anode material for next generation lithium ion secondary batteries because it exhibits 3~5 times higher capacity than conventional graphene. The high capacity will pave the way to lithium secondary batteries with higher energy densities than conventional batteries. It is anticipated that the application of silicon batteries will yield electronic devices with a longer duration for use in addition to electronic vehicles boasting longer mileage.
The silicon anode is based on the 3-dimensional, highly porous structure of rice husks which remedies the problematic extreme volume expansion of conventional silicon anodes.
Utilization of inexpensive rice husks to create high value silicon anodes will cause a ripple effect on the industry and academia.
2013.08.23 View 12114 -
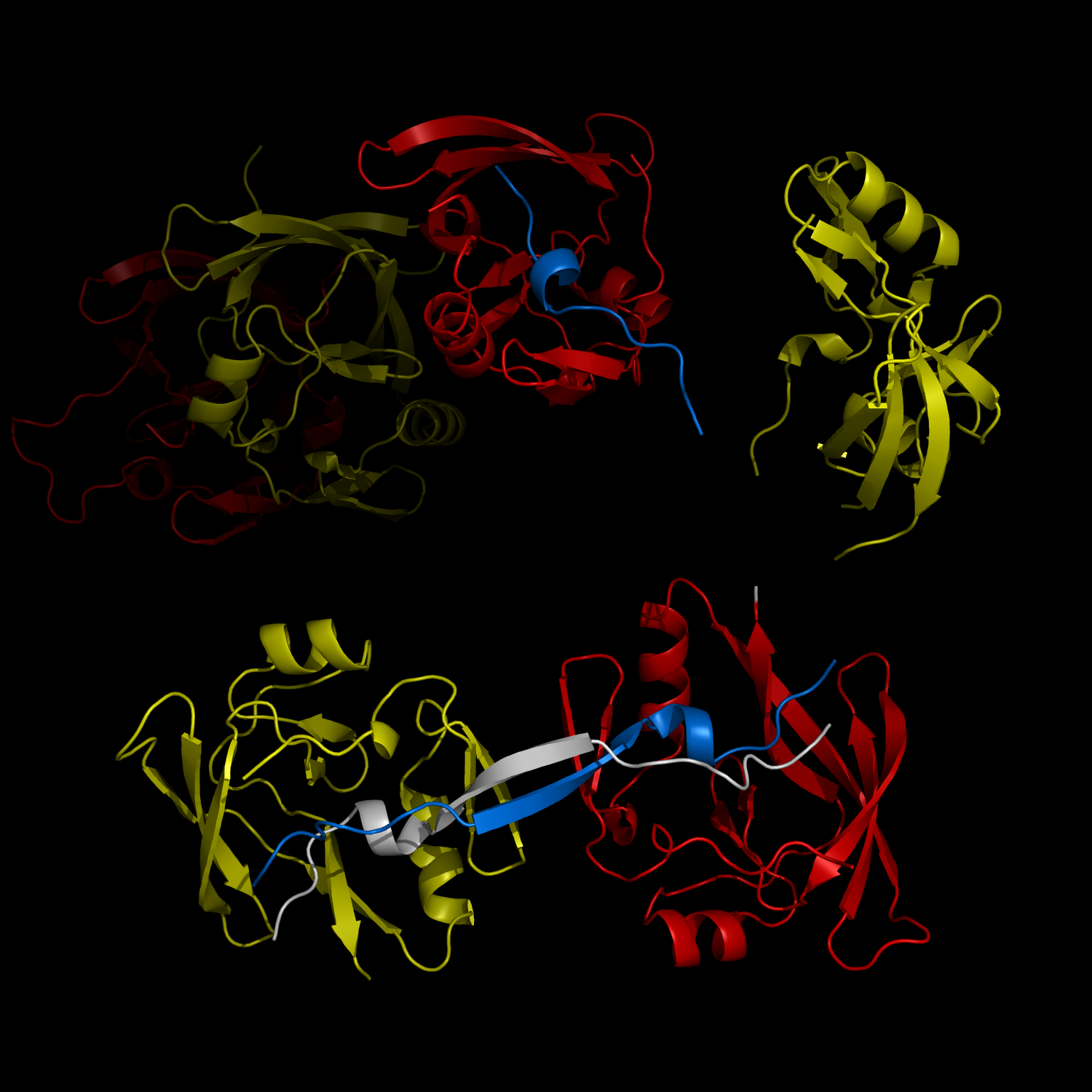 New Structural Insight into Neurodegenerative Disease
A research team from the Korea Advanced Institute of Science and Technology (KAIST) released their results on the structure and molecular details of the neurodegenerative disease-associated protein Ataxin-1. Mutations in Ataxin-1 cause the neurological disease, Spinocerebella Ataxia Type 1 (SCA1), which is characterized by a loss of muscular coordination and balance (ataxia), as is seen in Parkinson’s, Alzheimer’s, and Huntington’s diseases.
SCA1-causing mutations in the ATAXIN1 gene alter the length of a glutamine stretch in the Ataxin-1 protein. The research team provides the first structural insight into the complex formation of ATAXIN-1 with its binding partner, Capicua (CIC). The team, led by Professor Ji-Joon Song from the Department of Biological Sciences at KAIST, solved the structure of Ataxin-1 and CIC complex in atomic level revealing molecular details of the interaction between Ataxin-1 and CIC.
Professor Song explained his recent research work,
“We are able to see the intricate process of complex formation and reconfiguration of the two proteins when they interact with each other. Our work, we expect, will provide a new therapeutic target to modulate SCA1 neurodegenerative disease.”
Understanding structural and molecular details of proteins at the atomic level will help researchers to track the molecular pathogenesis of the disease and, ultimately, design targeted therapies or treatments for patients, rather than just relieving the symptoms of diseases.
Professor Song’s research paper, entitled “Structural Basis of Protein Complex Formation and Reconfiguration by Polyglutamine Disease Protein ATAXIN-1 and Capicua,” will be published in the March 15th issue of Genes & Development (www.genesdev.org).
Complex Formation and Reconfiguration of ATAXIN-1 and Capicua
The complex formation between a polyglutamine disease protein, ATXIN-1 and the transcriptional repressor Capicua (CIC) plays a critical role in SCA 1 pathogenesis. The image shows that the homodimerization of ATXIN-1 (yellow and red) is disrupted upon binding of CIC (blue). Furthermore, the binding of CIC to the ATXIN-1 induces a new form of ATXIN-1 dimerization mediated by CICs (ATXIN-1 AXH domains are shown in yellow and red, and CIC peptides shown in blue and white).
2013.04.02 View 10284
New Structural Insight into Neurodegenerative Disease
A research team from the Korea Advanced Institute of Science and Technology (KAIST) released their results on the structure and molecular details of the neurodegenerative disease-associated protein Ataxin-1. Mutations in Ataxin-1 cause the neurological disease, Spinocerebella Ataxia Type 1 (SCA1), which is characterized by a loss of muscular coordination and balance (ataxia), as is seen in Parkinson’s, Alzheimer’s, and Huntington’s diseases.
SCA1-causing mutations in the ATAXIN1 gene alter the length of a glutamine stretch in the Ataxin-1 protein. The research team provides the first structural insight into the complex formation of ATAXIN-1 with its binding partner, Capicua (CIC). The team, led by Professor Ji-Joon Song from the Department of Biological Sciences at KAIST, solved the structure of Ataxin-1 and CIC complex in atomic level revealing molecular details of the interaction between Ataxin-1 and CIC.
Professor Song explained his recent research work,
“We are able to see the intricate process of complex formation and reconfiguration of the two proteins when they interact with each other. Our work, we expect, will provide a new therapeutic target to modulate SCA1 neurodegenerative disease.”
Understanding structural and molecular details of proteins at the atomic level will help researchers to track the molecular pathogenesis of the disease and, ultimately, design targeted therapies or treatments for patients, rather than just relieving the symptoms of diseases.
Professor Song’s research paper, entitled “Structural Basis of Protein Complex Formation and Reconfiguration by Polyglutamine Disease Protein ATAXIN-1 and Capicua,” will be published in the March 15th issue of Genes & Development (www.genesdev.org).
Complex Formation and Reconfiguration of ATAXIN-1 and Capicua
The complex formation between a polyglutamine disease protein, ATXIN-1 and the transcriptional repressor Capicua (CIC) plays a critical role in SCA 1 pathogenesis. The image shows that the homodimerization of ATXIN-1 (yellow and red) is disrupted upon binding of CIC (blue). Furthermore, the binding of CIC to the ATXIN-1 induces a new form of ATXIN-1 dimerization mediated by CICs (ATXIN-1 AXH domains are shown in yellow and red, and CIC peptides shown in blue and white).
2013.04.02 View 10284 -
 Synthesis of a New Organic Supermolecule Succeeded
From left to right: Prof.Stoddart, Prof.Goddard and Prof.Jang Wook Choi
KAIST EEWS graduate school’s research team led by Prof. Stoddart, Prof. Goddard and Prof. Jang Wook Choi has succeeded the synthesis of a new organic supermolecule that is stable in a radical condition under room temperature.
Prof. Stoddart, who mainly led this research, is the world’s great scholar on orgaic molecular structure especially on catenane with an interconnection of several ring structures. Catenane is originated from Latin “catenane” referring to “chain”. The brief structure of the synthesized catenane is as following:
Usually radicals are known to be unstable since they are electronically neutral and have very high reactivity. However, the radicals from this research showed air- and water- stability. It also showed a reversible change in oxidation number from o to +8 through chemical/electrochemical oxidation-reduction reaction. The phenomenon where paramagnetic and diamagnetic characteristics change according to the oxidation number has also been observed.
Thus, the research like this - on the molecules showing various characteristics with stable radical - is expected to give a new direction to the next-generation electromemory system, semiconductor and energy storage system research.
Meanwhile, this research, led by Prof.Stoddart team with Prof.Goddard and Prof. Jang Wook Choi’s team, is conducted under the support of Science and Technology’s World Class University project by Ministry of Education and published in ‘Science’ on 25th of Jan.
2013.02.24 View 12755
Synthesis of a New Organic Supermolecule Succeeded
From left to right: Prof.Stoddart, Prof.Goddard and Prof.Jang Wook Choi
KAIST EEWS graduate school’s research team led by Prof. Stoddart, Prof. Goddard and Prof. Jang Wook Choi has succeeded the synthesis of a new organic supermolecule that is stable in a radical condition under room temperature.
Prof. Stoddart, who mainly led this research, is the world’s great scholar on orgaic molecular structure especially on catenane with an interconnection of several ring structures. Catenane is originated from Latin “catenane” referring to “chain”. The brief structure of the synthesized catenane is as following:
Usually radicals are known to be unstable since they are electronically neutral and have very high reactivity. However, the radicals from this research showed air- and water- stability. It also showed a reversible change in oxidation number from o to +8 through chemical/electrochemical oxidation-reduction reaction. The phenomenon where paramagnetic and diamagnetic characteristics change according to the oxidation number has also been observed.
Thus, the research like this - on the molecules showing various characteristics with stable radical - is expected to give a new direction to the next-generation electromemory system, semiconductor and energy storage system research.
Meanwhile, this research, led by Prof.Stoddart team with Prof.Goddard and Prof. Jang Wook Choi’s team, is conducted under the support of Science and Technology’s World Class University project by Ministry of Education and published in ‘Science’ on 25th of Jan.
2013.02.24 View 12755 -
 Prof. Jang-Uk Choi develops Strong, Long-lasting Lithium-ion Battery
Lithium-ion secondary battery with high power, as well asmuch longer life span, has been developed using nanotechnology. Professor Jang-Uk Choi and his colleagues at KAIST University EEWS graduate school has succeeded in developing a new lithium-ion secondary battery that has more than five times the output and three times the life span of the conventional batteries.
The industry expects the new battery to significantly improve the acceleration performance and solve the drawbacks of slow electric cars, which occurred due to failure of battery performance to keep up with the output of the motors during acceleration.
It is also expected that the new battery could be utilized in various fields that require high power batteries such as Smart Grid, which is the next generation intelligent electrical grid, as well as electric tools and many others.
Currently, the most widely used commercial lithium ion batteries’ lithium-cobalt-based cathode material has the disadvantage of expensive cost, high toxicity, short life expectancy and long-charge/discharge time. Also, it has been difficult to apply in electric cars that require a large current density and are vulnerable to heat generated during charging/discharging.
On the other hand, Professor Choi and his colleagues’ lithium-manganese based cathode material is gaining popularity for having the advantages such as abundant raw materials, cheap prices, eco-friendliness and especially excellent high-temperature stability and high output, which are suitable for use as electrode material in electric cars.
The pure lithium manganese based cathode material has a critical drawback of a very short life expectancy, only lasting about average of 1-2 years, which is due to the elution when the melted manganese flows out into the electrolyte. There have been various studies to solve this problem; however, the unique crystal structure of the material remained as a challenge for many scientists.
Professor Choi’s team analyzed the structure of the crystal at the time shortly before manganese oxides were formed, while controlling the reaction temperature at the step of synthesizing nanomaterial. It has been found that, at 220℃, there are simultaneously existing two crystal faces, one that inhibits the dissolution of manganese ions and the other that enables lithium ions to move smoothly.
Each of these crystal faces improves both the life span and output, increasing the output more than five times and life expectancy over three times. In addition, the existing high temperature life span, that was known to be especially vulnerable, has improved ten-fold.
“By controlling the crystal face of lithium manganese anode material, which has previously existed in the battery as chunks of about 10 micro-meter particles, both output and life span has significantly improved,” said Professor Choi, “Domestic and international patent application for the regarding technology has been finished and we have plans to work with companies in the future for commercialization within 2-3 years.”
Professor Yi Cui of Stanford University, the world’s leading scholar on the secondary battery, has evaluated that “This research exemplifies how nanotechnology can innovatively develop the field of secondary battery.”
Meanwhile, the research led by Professor Jang-Uk Choi and participated by researcher Ju-Seong Kim has been published on the online edition (dated Nov 27th) of Nanoletters, the world’s leading authority on Nanoscience.
2012.12.21 View 10672
Prof. Jang-Uk Choi develops Strong, Long-lasting Lithium-ion Battery
Lithium-ion secondary battery with high power, as well asmuch longer life span, has been developed using nanotechnology. Professor Jang-Uk Choi and his colleagues at KAIST University EEWS graduate school has succeeded in developing a new lithium-ion secondary battery that has more than five times the output and three times the life span of the conventional batteries.
The industry expects the new battery to significantly improve the acceleration performance and solve the drawbacks of slow electric cars, which occurred due to failure of battery performance to keep up with the output of the motors during acceleration.
It is also expected that the new battery could be utilized in various fields that require high power batteries such as Smart Grid, which is the next generation intelligent electrical grid, as well as electric tools and many others.
Currently, the most widely used commercial lithium ion batteries’ lithium-cobalt-based cathode material has the disadvantage of expensive cost, high toxicity, short life expectancy and long-charge/discharge time. Also, it has been difficult to apply in electric cars that require a large current density and are vulnerable to heat generated during charging/discharging.
On the other hand, Professor Choi and his colleagues’ lithium-manganese based cathode material is gaining popularity for having the advantages such as abundant raw materials, cheap prices, eco-friendliness and especially excellent high-temperature stability and high output, which are suitable for use as electrode material in electric cars.
The pure lithium manganese based cathode material has a critical drawback of a very short life expectancy, only lasting about average of 1-2 years, which is due to the elution when the melted manganese flows out into the electrolyte. There have been various studies to solve this problem; however, the unique crystal structure of the material remained as a challenge for many scientists.
Professor Choi’s team analyzed the structure of the crystal at the time shortly before manganese oxides were formed, while controlling the reaction temperature at the step of synthesizing nanomaterial. It has been found that, at 220℃, there are simultaneously existing two crystal faces, one that inhibits the dissolution of manganese ions and the other that enables lithium ions to move smoothly.
Each of these crystal faces improves both the life span and output, increasing the output more than five times and life expectancy over three times. In addition, the existing high temperature life span, that was known to be especially vulnerable, has improved ten-fold.
“By controlling the crystal face of lithium manganese anode material, which has previously existed in the battery as chunks of about 10 micro-meter particles, both output and life span has significantly improved,” said Professor Choi, “Domestic and international patent application for the regarding technology has been finished and we have plans to work with companies in the future for commercialization within 2-3 years.”
Professor Yi Cui of Stanford University, the world’s leading scholar on the secondary battery, has evaluated that “This research exemplifies how nanotechnology can innovatively develop the field of secondary battery.”
Meanwhile, the research led by Professor Jang-Uk Choi and participated by researcher Ju-Seong Kim has been published on the online edition (dated Nov 27th) of Nanoletters, the world’s leading authority on Nanoscience.
2012.12.21 View 10672 -
 Professor Lee Jae Kyu : Appointed Fellow at Association of Information Systems
Professor Lee Jae Kyu of the Graduate School of Information Media Management was made Fellow of the Association of Information Systems.
Professor Less was the Chief Editor of Electronic Commerce Research and Applications, Chairman of Asia Pacific Information System Symposium, and Chairman of Korea Academy of Management Information, in addition to Chairman of the Academy of Korea Intelligence Information System.
The ‘Electronic Commerce’ co-written by Professor Lee is being used as primary MBA textbook in many universities around the world.
Homepage : http://www.business.kaist.ac.kr/faculty/jklee/
2012.01.31 View 10043
Professor Lee Jae Kyu : Appointed Fellow at Association of Information Systems
Professor Lee Jae Kyu of the Graduate School of Information Media Management was made Fellow of the Association of Information Systems.
Professor Less was the Chief Editor of Electronic Commerce Research and Applications, Chairman of Asia Pacific Information System Symposium, and Chairman of Korea Academy of Management Information, in addition to Chairman of the Academy of Korea Intelligence Information System.
The ‘Electronic Commerce’ co-written by Professor Lee is being used as primary MBA textbook in many universities around the world.
Homepage : http://www.business.kaist.ac.kr/faculty/jklee/
2012.01.31 View 10043 -
 Spintronics: A high wire act by Nanowerk News
An article by Nanowerk News on the integration of ferromagnetic nanowire arrays on grapheme substrates was published. Professor Bong-Soo Kim from the Department of Chemistry, KAIST, led the research in conjunction with Hanyang University and Samsung in Korea.
http://www.nanowerk.com/news/newsid=22204.php
Posted: Jul 25th, 2011
Spintronics: A high wire act
(Nanowerk News) Graphene is a promising material for a wide range of applications due to its remarkable mechanical and electronic properties. An application of particular interest is spin-based electronics, or spintronics, in which the spin orientation of an electron is used to perform circuit functions in addition to its charge. Bongsoo Kim and colleagues from KAIST, Hanyang University and Samsung in Korea now report the integration of ferromagnetic nanowire arrays on graphene substrates, opening up a route for the construction of graphene-based spintronic devices using nanowires as spin-injecting contacts ("Epitaxially Integrating Ferromagnetic Fe1.3Ge Nanowire Arrays on Few-Layer Graphene").
The spin of an electron is a property that, like charge, can be used to encode, process and transport information. However, spin information is easily lost in most media, which has made spintronics difficult to realize in practice. In graphene, on the other hand, spin can be preserved for longer due to its peculiar electron transport properties. "Low intrinsic spin–orbit coupling, long spin diffusion lengths and vanishing hyperfine interaction are features of graphene that make it a promising medium for spin transport," explains Kim.
Scanning electron microscopy image of vertical iron germanide nanowires grown on graphene. (© ACS 2011)
A prerequisite for the realization of spintronic devices based on graphene is its integration with ferromagnetic contacts to allow spin injection. Kim and his co-workers found that nanowires of iron germanide (Fe1.3Ge) serve as efficient contacts for this purpose. "Iron germanide nanowires show low resistivity and room-temperature ferromagnetism, and they are compatible with existing complementary metal–oxide–semiconductor technologies," says Kim.
To produce the atomically well-defined interfacial contact between the nanowires and the graphene surface needed for optimum device performance, the researchers deposited the contacts by an epitaxial method based on chemical vapor transport. Through careful adjustment of deposition parameters such as carrier gas flow rate and reaction temperature, the researchers produced vertically aligned nanowires that are closely lattice-matched to the graphene sheets (see image).
Initially preparing the graphene sheets on a substrate of silicon oxide allowed the researchers to isolate the final nanowire–graphene structure by etching and then transfer it to another substrate, greatly expanding the versatility of the approach. It is a delicate process, however. "It is necessary to transfer the graphene films onto the substrate very carefully in order to avoid folding and wrinkling of the graphene," says Kim.
Source: Tokyo Institute of Technology
2011.07.26 View 11838
Spintronics: A high wire act by Nanowerk News
An article by Nanowerk News on the integration of ferromagnetic nanowire arrays on grapheme substrates was published. Professor Bong-Soo Kim from the Department of Chemistry, KAIST, led the research in conjunction with Hanyang University and Samsung in Korea.
http://www.nanowerk.com/news/newsid=22204.php
Posted: Jul 25th, 2011
Spintronics: A high wire act
(Nanowerk News) Graphene is a promising material for a wide range of applications due to its remarkable mechanical and electronic properties. An application of particular interest is spin-based electronics, or spintronics, in which the spin orientation of an electron is used to perform circuit functions in addition to its charge. Bongsoo Kim and colleagues from KAIST, Hanyang University and Samsung in Korea now report the integration of ferromagnetic nanowire arrays on graphene substrates, opening up a route for the construction of graphene-based spintronic devices using nanowires as spin-injecting contacts ("Epitaxially Integrating Ferromagnetic Fe1.3Ge Nanowire Arrays on Few-Layer Graphene").
The spin of an electron is a property that, like charge, can be used to encode, process and transport information. However, spin information is easily lost in most media, which has made spintronics difficult to realize in practice. In graphene, on the other hand, spin can be preserved for longer due to its peculiar electron transport properties. "Low intrinsic spin–orbit coupling, long spin diffusion lengths and vanishing hyperfine interaction are features of graphene that make it a promising medium for spin transport," explains Kim.
Scanning electron microscopy image of vertical iron germanide nanowires grown on graphene. (© ACS 2011)
A prerequisite for the realization of spintronic devices based on graphene is its integration with ferromagnetic contacts to allow spin injection. Kim and his co-workers found that nanowires of iron germanide (Fe1.3Ge) serve as efficient contacts for this purpose. "Iron germanide nanowires show low resistivity and room-temperature ferromagnetism, and they are compatible with existing complementary metal–oxide–semiconductor technologies," says Kim.
To produce the atomically well-defined interfacial contact between the nanowires and the graphene surface needed for optimum device performance, the researchers deposited the contacts by an epitaxial method based on chemical vapor transport. Through careful adjustment of deposition parameters such as carrier gas flow rate and reaction temperature, the researchers produced vertically aligned nanowires that are closely lattice-matched to the graphene sheets (see image).
Initially preparing the graphene sheets on a substrate of silicon oxide allowed the researchers to isolate the final nanowire–graphene structure by etching and then transfer it to another substrate, greatly expanding the versatility of the approach. It is a delicate process, however. "It is necessary to transfer the graphene films onto the substrate very carefully in order to avoid folding and wrinkling of the graphene," says Kim.
Source: Tokyo Institute of Technology
2011.07.26 View 11838 -
 Scientists develop highly efficient industrial catalyst
http://english.yonhapnews.co.kr/business/2011/07/14/48/0501000000AEN20110714009600320F.HTML
SEOUL, July 15 (Yonhap) -- South Korean scientists said Friday that they have developed a highly efficient nanoporous industrial catalyst that can have a considerable impact on chemical and oil-refining sectors.
The team of scientists led by Ryoo Ryong, a chemistry professor at the Korea Advanced Institute of Science and Technology (KAIST), said the solid zeolite compound developed in the laboratory has a reaction speed five to 10 times faster than that of conventional materials.
Zeolite, which is made from silica and aluminium, is frequently used as an absorbent, water purifier and in nuclear reprocessing, although it is mainly employed in the chemical industry.
The annual size of the zeolite market is estimated at US$2.5 billion with output using the material topping $30 billion. At present, 41 percent of all catalysts used in the chemical sector are nano-scale zeolite materials.
The KAIST team said that because the new zeolite is made up of different sized pores, the material can be used as a catalyst when existing materials are unable to act as a changing agent.
"Existing zeolites only have pores under 1 nanometer in diameter, but the new material has holes that range from 1 nanometer to 3.5 nanometers, which are all arranged in a regular honeycomb arrangement," Ryoo said. A nanometer is one-billionth of a meter.
He said the ability to have both micro- and meso-sized pores is key to the faster reaction speed that is an integral part of raising efficiency. The South Korean researchers used a so-called surfactant process to make the different sizes of pores.
The development is a breakthrough because researchers and companies such as Exxon Mobil Corp. have been trying to build zeolite with different sizes of pores for the past two decades without making serious headway. There are more than 200 different types of zeolites in the world.
Ryoo, who received funding from the government, has requested intellectual property rights for the discovery, which has been published in the latest issue of Science magazine. He also developed another zeolite in the past that can transform methanol to gasoline up to 10 times more efficiently than existing catalysts.
Exxon Mobil has expressed interest in the two zeolites made by Ryoo"s team. Undisclosed South Korean petrochemical companies have also made inquiries that may lead to commercial development in the future.
"There are some technical issues to resolve, mainly related with mass production and stability," the scientist said.
He said full-fledge production will be determined by how much companies are willing to spend on research to speed up development that can bring down overall production costs.
The KAIST team said it took two years to make the new zeolite, which can be custom made to meet specific needs.
(END)
2011.07.15 View 13809
Scientists develop highly efficient industrial catalyst
http://english.yonhapnews.co.kr/business/2011/07/14/48/0501000000AEN20110714009600320F.HTML
SEOUL, July 15 (Yonhap) -- South Korean scientists said Friday that they have developed a highly efficient nanoporous industrial catalyst that can have a considerable impact on chemical and oil-refining sectors.
The team of scientists led by Ryoo Ryong, a chemistry professor at the Korea Advanced Institute of Science and Technology (KAIST), said the solid zeolite compound developed in the laboratory has a reaction speed five to 10 times faster than that of conventional materials.
Zeolite, which is made from silica and aluminium, is frequently used as an absorbent, water purifier and in nuclear reprocessing, although it is mainly employed in the chemical industry.
The annual size of the zeolite market is estimated at US$2.5 billion with output using the material topping $30 billion. At present, 41 percent of all catalysts used in the chemical sector are nano-scale zeolite materials.
The KAIST team said that because the new zeolite is made up of different sized pores, the material can be used as a catalyst when existing materials are unable to act as a changing agent.
"Existing zeolites only have pores under 1 nanometer in diameter, but the new material has holes that range from 1 nanometer to 3.5 nanometers, which are all arranged in a regular honeycomb arrangement," Ryoo said. A nanometer is one-billionth of a meter.
He said the ability to have both micro- and meso-sized pores is key to the faster reaction speed that is an integral part of raising efficiency. The South Korean researchers used a so-called surfactant process to make the different sizes of pores.
The development is a breakthrough because researchers and companies such as Exxon Mobil Corp. have been trying to build zeolite with different sizes of pores for the past two decades without making serious headway. There are more than 200 different types of zeolites in the world.
Ryoo, who received funding from the government, has requested intellectual property rights for the discovery, which has been published in the latest issue of Science magazine. He also developed another zeolite in the past that can transform methanol to gasoline up to 10 times more efficiently than existing catalysts.
Exxon Mobil has expressed interest in the two zeolites made by Ryoo"s team. Undisclosed South Korean petrochemical companies have also made inquiries that may lead to commercial development in the future.
"There are some technical issues to resolve, mainly related with mass production and stability," the scientist said.
He said full-fledge production will be determined by how much companies are willing to spend on research to speed up development that can bring down overall production costs.
The KAIST team said it took two years to make the new zeolite, which can be custom made to meet specific needs.
(END)
2011.07.15 View 13809 -
 A KAIST graduate to become a professor at a prestigious university in UAE
A KAIST graduate to become a professor at a prestigious university in UAE
Dr. Jerald Yoo, a KAIST graduate, has been appointed as an assistant professor at the Masdar Institute of Science and Technology (MIST) in Abu Dhabi, United Arab Emirates (UAE), by the recommendation of the Massachusetts Institute of Technology (MIT) since April 1, 2010.
The MIST is a private, not-for-profit, independent, research-driven institute developed with the support and cooperation of MIT and the Abu Dhabi government, which was opened in September 2009. Currently, at the school, there are 25 professors and 100 students from 22 countries around the world. The institute has a campus in Masdar City where the Abu Dhabi government plans to nurture it as a “place for zero carbon emissions.”
According to an agreement between the MIST and MIT, Professor Yoo will teach and work on co-research projects at MIT for one year beginning in May 2010 and then working at the MIST thereafter.
Professor Yoo received all of his degrees (BS, MS, and Ph.D.) from KAIST majoring in electrical engineering and earned his doctoral degree in January 2010. His research works included developing a wearable patch to monitor bio signals with an application of wearable sensor networks and low energy electronic circuit technologies. During his doctoral study, Professor Yoo published papers at the IEEE International Solid-State Circuits Conference (ISSCC) and in journals of IEEE Solid-State Circuits Society (SSCS).
Professor Yoo said, "The wearable health care system is certainly necessary to improve the quality of our lives, and the field should receive a sustaining support for further research. I will do my best to continuously produce valuable research results and hope that my research works will be helpful for an academic exchange between South Korea and Abu Dhabi.”
About the Masdar Institute of Science and Technology (MIST) in Abu Dhabi:
http://www.masdar.ac.ae/
The Masdar Institute is the centerpiece of the Masdar Initiative, a landmark program announced in April 2006 by the government of Abu Dhabi to establish an entirely new economic sector dedicated to alternative and sustainable energy. Masdar is a highly-strategic initiative with primary objectives of: helping drive the economic diversification of Abu Dhabi; maintaining and expanding Abu Dhabi"s position in evolving global energy markets; positioning Abu Dhabi as a developer of technology; and making a meaningful contribution towards sustainable human development.
The Masdar Institute is a private, not-for-profit, independent, research-driven institute developed with the support and cooperation of the Massachusetts Institute of Technology (MIT). The Institute offers Masters and (eventually) PhD programs in science and engineering disciplines, with a focus on advanced energy and sustainable technologies. It welcomes and encourages applications from qualified local and international students and provides fellowships to talented students who meet its high admission standards. Its faculty is of the highest quality and the intent is to have the structure of its top administration similar to MIT"s.
2010.04.13 View 14766
A KAIST graduate to become a professor at a prestigious university in UAE
A KAIST graduate to become a professor at a prestigious university in UAE
Dr. Jerald Yoo, a KAIST graduate, has been appointed as an assistant professor at the Masdar Institute of Science and Technology (MIST) in Abu Dhabi, United Arab Emirates (UAE), by the recommendation of the Massachusetts Institute of Technology (MIT) since April 1, 2010.
The MIST is a private, not-for-profit, independent, research-driven institute developed with the support and cooperation of MIT and the Abu Dhabi government, which was opened in September 2009. Currently, at the school, there are 25 professors and 100 students from 22 countries around the world. The institute has a campus in Masdar City where the Abu Dhabi government plans to nurture it as a “place for zero carbon emissions.”
According to an agreement between the MIST and MIT, Professor Yoo will teach and work on co-research projects at MIT for one year beginning in May 2010 and then working at the MIST thereafter.
Professor Yoo received all of his degrees (BS, MS, and Ph.D.) from KAIST majoring in electrical engineering and earned his doctoral degree in January 2010. His research works included developing a wearable patch to monitor bio signals with an application of wearable sensor networks and low energy electronic circuit technologies. During his doctoral study, Professor Yoo published papers at the IEEE International Solid-State Circuits Conference (ISSCC) and in journals of IEEE Solid-State Circuits Society (SSCS).
Professor Yoo said, "The wearable health care system is certainly necessary to improve the quality of our lives, and the field should receive a sustaining support for further research. I will do my best to continuously produce valuable research results and hope that my research works will be helpful for an academic exchange between South Korea and Abu Dhabi.”
About the Masdar Institute of Science and Technology (MIST) in Abu Dhabi:
http://www.masdar.ac.ae/
The Masdar Institute is the centerpiece of the Masdar Initiative, a landmark program announced in April 2006 by the government of Abu Dhabi to establish an entirely new economic sector dedicated to alternative and sustainable energy. Masdar is a highly-strategic initiative with primary objectives of: helping drive the economic diversification of Abu Dhabi; maintaining and expanding Abu Dhabi"s position in evolving global energy markets; positioning Abu Dhabi as a developer of technology; and making a meaningful contribution towards sustainable human development.
The Masdar Institute is a private, not-for-profit, independent, research-driven institute developed with the support and cooperation of the Massachusetts Institute of Technology (MIT). The Institute offers Masters and (eventually) PhD programs in science and engineering disciplines, with a focus on advanced energy and sustainable technologies. It welcomes and encourages applications from qualified local and international students and provides fellowships to talented students who meet its high admission standards. Its faculty is of the highest quality and the intent is to have the structure of its top administration similar to MIT"s.
2010.04.13 View 14766 -
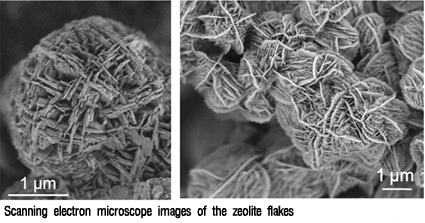 Prof. Ryoo's Team Discovers Breakthrough Method to Create New Zeolite
A group of scientists led by Prof. Ryong Ryoo of the Department of Chemistry, KAIST, has found a method to direct the growth of zeolite, a crystalline substance that is frequently used as catalyst in the chemical and petrochemical industries, the university authorities said on Thursday (Sept. 10).
Ryoo"s research team successfully created ultrathin nano-sheets, only two nano-meters thick, that are efficiently used as long-life catalysts for hydrocarbon cracking and other petrochemical applications. The breakthrough finding, which is credited with taking acidic zeolite catalysts to the limit in terms of thickness, was published in the latest edition of the peer-review journal, "Nature."
A team from the Polytechnic Univeristy of Valencia, Spain, also contributed to the research.
Zeolites are already widely used in the petrochemical industry, but making the catalysts very thin means that reactant molecules can easily diffuse into the zeolite structure and product molecules can get out quickly. This improves the efficiency of the catalyst and reduces unwanted side reactions that can produce polymeric hydrocarbon "coke" that clogs the zeolite pores and eventually kills the catalytic activity, Prof. Yoo said.
To make the thin sheets, Ryoo and his team used a surfactant as a template to direct the growth of the zeolite structure. The surfactant molecule has a polar "head" group - with two quaternary ammonium groups around which the aluminosilicate zeolite crystal grows - and a long hydrocarbon "tail," which prevents the sheets from aggregating together into larger, three dimensional crystals. When the surfactant is removed, these flakes pile up randomly with gaps in between which further aids diffusion to the catalyst sites.
"Zeolite could be used as a catalyst to convert heavy oil into gasoline. Our new zeolite could provide even more possibilities, such as being used as catalysts for transforming methanol into gasline," Ryoo said.
Prof. Ryoo, a Distinguished Professor of KAIST, has won a variety of academic awards, which included the Top Scientist Award given by the Korean government in 2005 and the 2001 KOSEF Science and Technology Award for his work on the synthesis and crystal structure of mezzoporous silica.
Ryoo obtained his bachelor"s degree from Seoul National University in 1977, master"s from KAIST in 1979, and doctorate from Stanford University in 1985.
In 2006, Ryoo and his research team announced the discovery of a form of zeolite that can catalyze petrochemical reactions much more effectively than previous zeolites. Because of the potential of this to streamline the gasoline refining process, it was greeted as a "magical substance" by the South Korean press.
2009.09.11 View 13983
Prof. Ryoo's Team Discovers Breakthrough Method to Create New Zeolite
A group of scientists led by Prof. Ryong Ryoo of the Department of Chemistry, KAIST, has found a method to direct the growth of zeolite, a crystalline substance that is frequently used as catalyst in the chemical and petrochemical industries, the university authorities said on Thursday (Sept. 10).
Ryoo"s research team successfully created ultrathin nano-sheets, only two nano-meters thick, that are efficiently used as long-life catalysts for hydrocarbon cracking and other petrochemical applications. The breakthrough finding, which is credited with taking acidic zeolite catalysts to the limit in terms of thickness, was published in the latest edition of the peer-review journal, "Nature."
A team from the Polytechnic Univeristy of Valencia, Spain, also contributed to the research.
Zeolites are already widely used in the petrochemical industry, but making the catalysts very thin means that reactant molecules can easily diffuse into the zeolite structure and product molecules can get out quickly. This improves the efficiency of the catalyst and reduces unwanted side reactions that can produce polymeric hydrocarbon "coke" that clogs the zeolite pores and eventually kills the catalytic activity, Prof. Yoo said.
To make the thin sheets, Ryoo and his team used a surfactant as a template to direct the growth of the zeolite structure. The surfactant molecule has a polar "head" group - with two quaternary ammonium groups around which the aluminosilicate zeolite crystal grows - and a long hydrocarbon "tail," which prevents the sheets from aggregating together into larger, three dimensional crystals. When the surfactant is removed, these flakes pile up randomly with gaps in between which further aids diffusion to the catalyst sites.
"Zeolite could be used as a catalyst to convert heavy oil into gasoline. Our new zeolite could provide even more possibilities, such as being used as catalysts for transforming methanol into gasline," Ryoo said.
Prof. Ryoo, a Distinguished Professor of KAIST, has won a variety of academic awards, which included the Top Scientist Award given by the Korean government in 2005 and the 2001 KOSEF Science and Technology Award for his work on the synthesis and crystal structure of mezzoporous silica.
Ryoo obtained his bachelor"s degree from Seoul National University in 1977, master"s from KAIST in 1979, and doctorate from Stanford University in 1985.
In 2006, Ryoo and his research team announced the discovery of a form of zeolite that can catalyze petrochemical reactions much more effectively than previous zeolites. Because of the potential of this to streamline the gasoline refining process, it was greeted as a "magical substance" by the South Korean press.
2009.09.11 View 13983 -
 Professor Ryong Ryoo, selected as a scientist wished to resemble and to be 2006
Professor Ryong Ryoo, selected as a scientist wished to resemble and to be 2006
Professor Ryong Ryoo (Department of Chemistry) was selected as a scientist wished to resemble and to be 2006.
Professor Ryoo developed in 2000 world’s first nanoporous carbon material in which numberless several nanometer-sized holes were drilled. The development of this nanoporous material was introduced by international scientific journal NATURE in 2000 and 2001 and expected to contribute to the progress of mankind through the development of high efficiency fuel cell or ultra-light computer. Professor Ryoo also developed a new technology that can considerably improve the catalyst activation and stability of ‘Zeolite’, a main catalyst in the petrochemical industry, which was introduced by NATURE materials. The above achievements qualified Professor Ryoo for the selection.
‘Scientists wished to resemble and to be 2006’ were selected among scientists showing vigorous activities in the science and technology circle on the basis of their recent achievements, etc. by the Ministry of Science and Technology and the Korea Science Foundation, and total 10 scientists qualified to be the model of children and the youth were announced on August 24.
2006.09.06 View 17552
Professor Ryong Ryoo, selected as a scientist wished to resemble and to be 2006
Professor Ryong Ryoo, selected as a scientist wished to resemble and to be 2006
Professor Ryong Ryoo (Department of Chemistry) was selected as a scientist wished to resemble and to be 2006.
Professor Ryoo developed in 2000 world’s first nanoporous carbon material in which numberless several nanometer-sized holes were drilled. The development of this nanoporous material was introduced by international scientific journal NATURE in 2000 and 2001 and expected to contribute to the progress of mankind through the development of high efficiency fuel cell or ultra-light computer. Professor Ryoo also developed a new technology that can considerably improve the catalyst activation and stability of ‘Zeolite’, a main catalyst in the petrochemical industry, which was introduced by NATURE materials. The above achievements qualified Professor Ryoo for the selection.
‘Scientists wished to resemble and to be 2006’ were selected among scientists showing vigorous activities in the science and technology circle on the basis of their recent achievements, etc. by the Ministry of Science and Technology and the Korea Science Foundation, and total 10 scientists qualified to be the model of children and the youth were announced on August 24.
2006.09.06 View 17552