Catalyst
-
 Spillover Phenomenon Identified Using Model Catalyst System
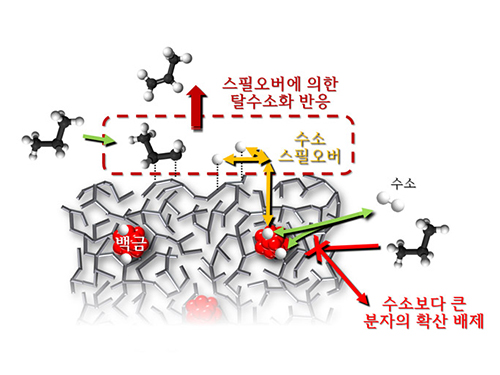
Researchers at KAIST have identified spillover phenomenon, which has remained controversial since its discovery in the early 1960s.
KAIST Department of Chemical and Biomolecular Engineering’s Professor Min-Gi Choi and his team has explained the "spillover phenomenon," using their own model catalyst system where platinum is selectively located within the amorphous aluminosilicate.
The research results were published on the 25th February online edition of Nature Communications.
Spillover refers to a phenomenon that occurs when hydrogen atoms that have been activated on the surface of metals, such as platinum, move to the surface of the catalyst. It was predicted that this phenomenon can be used to design a catalyst with high activity and stability, and thus has been actively studied over the last 50 years.
However, many cases of the known catalysts involved competing reactions on the exposed metal surface, which made it impossible to directly identify the presence and formation mechanism of spillover.
The catalysts developed by the researchers at KAIST used platinum nanoparticles covered with aluminosilicate. This only allowed the hydrogen molecules to pass through and has effectively blocked the competing reactions, enabling the research team to study the spillover phenomenon.
Through various catalyst structure and reactivity analysis, as well as computer modeling, the team has discovered that Brönsted acid sites present on the aluminosilicate plays a crucial role in spillover phenomenon.
In addition, the spillover-based hydrogenation catalyst proposed by the research team showed very high hydrogenation and dehydrogenation activity. The ability of the catalyst to significantly inhibit unwanted hydrogenolysis reaction during the petrochemical processes also suggested a large industrial potential.
Professor Min-Gi Choi said, “This particular catalyst, which can trigger the reaction only by spillover phenomenon, can be properly designed to exceed the capacity of the conventional metal catalysts. The future goal is to make a catalyst with much higher activity and selectivity.”
The research was conducted through funds subsidized by SK Innovation and Ministry of Science, ICT and Future Planning.
The senior research fellow of SK Innovation Seung-Hun Oh said, “SK Innovation will continue to develop a new commercial catalyst based on the technology from this research.”
Juh-Wan Lim and Hye-Yeong Shin led the research as joint first authors under supervision of Professor Min-Gi Choi and computer modeling works were conducted by KAIST EEWS (environment, energy, water, and sustainability) graduate school’s Professor Hyeong-Jun Kim.
2014.03.03 View 10355
Spillover Phenomenon Identified Using Model Catalyst System
Researchers at KAIST have identified spillover phenomenon, which has remained controversial since its discovery in the early 1960s.
KAIST Department of Chemical and Biomolecular Engineering’s Professor Min-Gi Choi and his team has explained the "spillover phenomenon," using their own model catalyst system where platinum is selectively located within the amorphous aluminosilicate.
The research results were published on the 25th February online edition of Nature Communications.
Spillover refers to a phenomenon that occurs when hydrogen atoms that have been activated on the surface of metals, such as platinum, move to the surface of the catalyst. It was predicted that this phenomenon can be used to design a catalyst with high activity and stability, and thus has been actively studied over the last 50 years.
However, many cases of the known catalysts involved competing reactions on the exposed metal surface, which made it impossible to directly identify the presence and formation mechanism of spillover.
The catalysts developed by the researchers at KAIST used platinum nanoparticles covered with aluminosilicate. This only allowed the hydrogen molecules to pass through and has effectively blocked the competing reactions, enabling the research team to study the spillover phenomenon.
Through various catalyst structure and reactivity analysis, as well as computer modeling, the team has discovered that Brönsted acid sites present on the aluminosilicate plays a crucial role in spillover phenomenon.
In addition, the spillover-based hydrogenation catalyst proposed by the research team showed very high hydrogenation and dehydrogenation activity. The ability of the catalyst to significantly inhibit unwanted hydrogenolysis reaction during the petrochemical processes also suggested a large industrial potential.
Professor Min-Gi Choi said, “This particular catalyst, which can trigger the reaction only by spillover phenomenon, can be properly designed to exceed the capacity of the conventional metal catalysts. The future goal is to make a catalyst with much higher activity and selectivity.”
The research was conducted through funds subsidized by SK Innovation and Ministry of Science, ICT and Future Planning.
The senior research fellow of SK Innovation Seung-Hun Oh said, “SK Innovation will continue to develop a new commercial catalyst based on the technology from this research.”
Juh-Wan Lim and Hye-Yeong Shin led the research as joint first authors under supervision of Professor Min-Gi Choi and computer modeling works were conducted by KAIST EEWS (environment, energy, water, and sustainability) graduate school’s Professor Hyeong-Jun Kim.
2014.03.03 View 10355 -
 Core Technology for Lithium Air Secondary Battery Developed
KAIST-Kyonggi University joint research team developed composite catalyst out of nano fiber and graphene
Five times improvement in capacity compared to lithium-ion secondary battery, driving 800 km at maximum
The core technology for lithium air secondary battery, the next generation high capacity battery, has been developed.
A research team formed by KAIST Department of Materials Science’s Professors Il-Doo Kim and Seokwoo Jeon, and Kyonggi University Department of Materials Science’s Professor Yong-Joon Park has created a lithium air secondary battery, with five times greater storage than the lithium-ion secondary battery, by developing a nano fiber-graphene composite catalyst. The research results are published in the August 8th online edition of Nano Letters.
A cathode of a lithium-ion battery consists of graphite and an anode of the battery consists of a lithium transition metal oxide. Lithium-ion batteries are widely used in mobile phones and laptops. However, lithium-ion batteries cannot support electric vehicles, providing energy for only 160 kilometers on one full charge. The lithium air secondary battery just developed by the research team uses lithium on the cathode and oxygen on the anode. It is earning a popular acknowledgement among the next generation secondary battery research community for having lightweight mass and high energy density.
However, lithium-ion batteries remain difficult to commercialize because of their short lifespan. Lithium and oxygen meet up to form lithium oxide (Li2O2) at discharge, and decompose again at charge. In a traditional lithium air battery, this cycle does not occur smoothly and results in high resistance, thereby reducing the lifespan of the battery. It is thus essential to develop high efficiency catalyst that facilitates the formation and decomposition of lithium oxides.
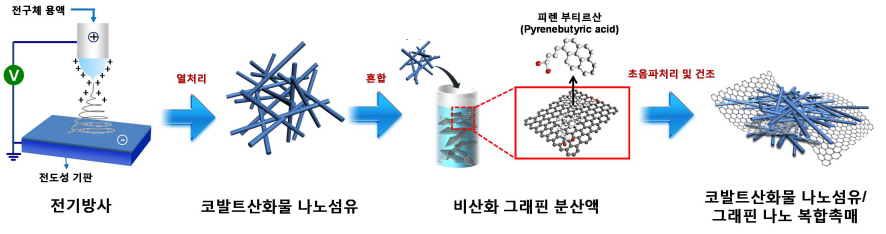
The research team used electric radiation to develop a nano composite catalyst by mixing cobalt oxide nano fiber and graphene. The performance of the battery has been maximized by settling nonoxidative graphene, which has high specific surface area and electrical conductivity, on catalyst active cobalt oxide nano fiber. Applying the nano composite catalyst on both poles of the lithium air battery resulted in an improved lifespan of over 80 recharge cycles with capacity greater than 100mAh/g, five times greater than a lithium ion battery. The newly discovered charge-discharge property is the highest among the reported performances of the lithium air battery so far.
The lithium air battery is cheap to make, as the main materials are metal oxide and graphene. “There are yet more issues to resolve such as stability, but we will collaborate with other organizations to open up the era of electronic vehicles,” said Professor Il-Doo Kim. “We hope to contribute to vitalizing the fields of next generation lithium air battery by leading nanocatalyst synthesis technology, one of the core materials in the fields of secondary battery,” Professor Kim spoke of his aspiration.
The graduate students participated in the research are Won-Hee Ryu, a postdoctorate at KAIST Department of Materials Science, Sungho Song, a PhD candidate at KAIST Department of Materials Science, and Taek-Han Yoon, a graduate student at Kyonggi University.
Picture I: Schematic Diagram of Lithium Air Battery Made of Nano Composite Catalysts
Picture II: Images of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts
Picture III: Images of Manufacturing Process of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts for Lithium Air Battery
2013.10.18 View 12127
Core Technology for Lithium Air Secondary Battery Developed
KAIST-Kyonggi University joint research team developed composite catalyst out of nano fiber and graphene
Five times improvement in capacity compared to lithium-ion secondary battery, driving 800 km at maximum
The core technology for lithium air secondary battery, the next generation high capacity battery, has been developed.
A research team formed by KAIST Department of Materials Science’s Professors Il-Doo Kim and Seokwoo Jeon, and Kyonggi University Department of Materials Science’s Professor Yong-Joon Park has created a lithium air secondary battery, with five times greater storage than the lithium-ion secondary battery, by developing a nano fiber-graphene composite catalyst. The research results are published in the August 8th online edition of Nano Letters.
A cathode of a lithium-ion battery consists of graphite and an anode of the battery consists of a lithium transition metal oxide. Lithium-ion batteries are widely used in mobile phones and laptops. However, lithium-ion batteries cannot support electric vehicles, providing energy for only 160 kilometers on one full charge. The lithium air secondary battery just developed by the research team uses lithium on the cathode and oxygen on the anode. It is earning a popular acknowledgement among the next generation secondary battery research community for having lightweight mass and high energy density.
However, lithium-ion batteries remain difficult to commercialize because of their short lifespan. Lithium and oxygen meet up to form lithium oxide (Li2O2) at discharge, and decompose again at charge. In a traditional lithium air battery, this cycle does not occur smoothly and results in high resistance, thereby reducing the lifespan of the battery. It is thus essential to develop high efficiency catalyst that facilitates the formation and decomposition of lithium oxides.
The research team used electric radiation to develop a nano composite catalyst by mixing cobalt oxide nano fiber and graphene. The performance of the battery has been maximized by settling nonoxidative graphene, which has high specific surface area and electrical conductivity, on catalyst active cobalt oxide nano fiber. Applying the nano composite catalyst on both poles of the lithium air battery resulted in an improved lifespan of over 80 recharge cycles with capacity greater than 100mAh/g, five times greater than a lithium ion battery. The newly discovered charge-discharge property is the highest among the reported performances of the lithium air battery so far.
The lithium air battery is cheap to make, as the main materials are metal oxide and graphene. “There are yet more issues to resolve such as stability, but we will collaborate with other organizations to open up the era of electronic vehicles,” said Professor Il-Doo Kim. “We hope to contribute to vitalizing the fields of next generation lithium air battery by leading nanocatalyst synthesis technology, one of the core materials in the fields of secondary battery,” Professor Kim spoke of his aspiration.
The graduate students participated in the research are Won-Hee Ryu, a postdoctorate at KAIST Department of Materials Science, Sungho Song, a PhD candidate at KAIST Department of Materials Science, and Taek-Han Yoon, a graduate student at Kyonggi University.
Picture I: Schematic Diagram of Lithium Air Battery Made of Nano Composite Catalysts
Picture II: Images of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts
Picture III: Images of Manufacturing Process of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts for Lithium Air Battery
2013.10.18 View 12127 -
 Ten Breakthroughs of the Year 2011 by Science
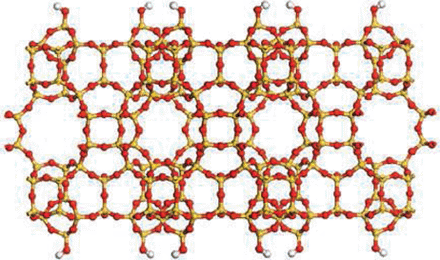
Porous Zeolite Crytals
Science, an internationally renowned scientific journal based in the US, has recently released a special issue of “Breakthrough of the Year, 2011,” dated December 23, 2011. In the issue, the journal introduces ten most important research breakthroughs made this year, and Professor Ryong Ryoo, Department of Chemistry at KAIST, was one of the scientists behind such notable advancements in 2011. Professor Ryoo has been highly regarded internationally for his research on the development of synthetic version of zeolites, a family of porous minerals that is widely used for products such as laundry detergents, cat litters, etc. Below is the article from Science, stating the zeolite research:
For Science’s “Breakthrough of the Year, 2011”, please go to:
http://www.sciencemag.org/site/special/btoy2011/
[Excerpt from the December 23, 2011 Issue of Science]
Industrial Molecules, Tailor-Made
If you ever doubt that chemistry is still a creative endeavor, just look at zeolites. This family of porous minerals was first discovered in 1756. They"re formed from different arrangements of aluminum, silicon, and oxygen atoms that crystallize into holey structures pocked with a perfect arrangement of pores. Over the past 250 years, 40 natural zeolites have been discovered, and chemists have chipped in roughly 150 more synthetic versions.
View larger version:
In this page
In a new window
Assembly required.
Porous zeolite crystals are widely used as filters and catalysts. This year, researchers found new ways to tailor the size of their pores and create thinner, cheaper membranes.
CREDIT: K. VAROON ET AL., SCIENCE334, 6052 (7 OCTOBER 2001)
This abundance isn"t just for show. Three million tons of zeolites are produced every year for use in laundry detergents, cat litter, and many other products. But zeolites really strut their stuff in two uses: as catalysts and molecular sieves. Oil refineries use zeolite catalysts to break down long hydrocarbon chains in oil into the shorter, volatile hydrocarbons in gasoline. And the minerals" small, regularly arranged pores make them ideal filters for purifying everything from the air on spaceships to the contaminated water around the nuclear reactors destroyed earlier this year in Fukushima, Japan.
Zeolites have their limitations, though. Their pores are almost universally tiny, making it tough to use them as catalysts for large molecules. And they"re difficult to form into ultrathin membranes, which researchers would like to do to enable cheaper separations. But progress by numerous teams on zeolite synthesis this year gave this “mature” area of chemistry new life.
Researchers in South Korea crafted a family of zeolites in which the usual network of small pores is surrounded by walls holed with larger voids. That combination of large and small pores should lead to catalysts for numerous large organic molecules.
Labs in Spain and China produced related large- and small-pore zeolites by using a combination of inorganic and organic materials to guide the structures as they formed.
Meanwhile, researchers in France and Germany discovered that, by carefully controlling growth conditions, they could form a large-pore zeolite without the need for the expensive organic compounds typically used to guide their architecture as they grow. The advance opens the way for cheaper catalysts. In yet another lab, researchers in Minnesota came up with a new route for making ultrathin zeolite membranes, which are likely to be useful as a wide variety of chemically selective filters.
This surge of molecular wizardry provides a vivid reminder that the creativity of chemists keeps their field ever young.
Related References and Web Sites
2011.12.23 View 12451
Ten Breakthroughs of the Year 2011 by Science
Porous Zeolite Crytals
Science, an internationally renowned scientific journal based in the US, has recently released a special issue of “Breakthrough of the Year, 2011,” dated December 23, 2011. In the issue, the journal introduces ten most important research breakthroughs made this year, and Professor Ryong Ryoo, Department of Chemistry at KAIST, was one of the scientists behind such notable advancements in 2011. Professor Ryoo has been highly regarded internationally for his research on the development of synthetic version of zeolites, a family of porous minerals that is widely used for products such as laundry detergents, cat litters, etc. Below is the article from Science, stating the zeolite research:
For Science’s “Breakthrough of the Year, 2011”, please go to:
http://www.sciencemag.org/site/special/btoy2011/
[Excerpt from the December 23, 2011 Issue of Science]
Industrial Molecules, Tailor-Made
If you ever doubt that chemistry is still a creative endeavor, just look at zeolites. This family of porous minerals was first discovered in 1756. They"re formed from different arrangements of aluminum, silicon, and oxygen atoms that crystallize into holey structures pocked with a perfect arrangement of pores. Over the past 250 years, 40 natural zeolites have been discovered, and chemists have chipped in roughly 150 more synthetic versions.
View larger version:
In this page
In a new window
Assembly required.
Porous zeolite crystals are widely used as filters and catalysts. This year, researchers found new ways to tailor the size of their pores and create thinner, cheaper membranes.
CREDIT: K. VAROON ET AL., SCIENCE334, 6052 (7 OCTOBER 2001)
This abundance isn"t just for show. Three million tons of zeolites are produced every year for use in laundry detergents, cat litter, and many other products. But zeolites really strut their stuff in two uses: as catalysts and molecular sieves. Oil refineries use zeolite catalysts to break down long hydrocarbon chains in oil into the shorter, volatile hydrocarbons in gasoline. And the minerals" small, regularly arranged pores make them ideal filters for purifying everything from the air on spaceships to the contaminated water around the nuclear reactors destroyed earlier this year in Fukushima, Japan.
Zeolites have their limitations, though. Their pores are almost universally tiny, making it tough to use them as catalysts for large molecules. And they"re difficult to form into ultrathin membranes, which researchers would like to do to enable cheaper separations. But progress by numerous teams on zeolite synthesis this year gave this “mature” area of chemistry new life.
Researchers in South Korea crafted a family of zeolites in which the usual network of small pores is surrounded by walls holed with larger voids. That combination of large and small pores should lead to catalysts for numerous large organic molecules.
Labs in Spain and China produced related large- and small-pore zeolites by using a combination of inorganic and organic materials to guide the structures as they formed.
Meanwhile, researchers in France and Germany discovered that, by carefully controlling growth conditions, they could form a large-pore zeolite without the need for the expensive organic compounds typically used to guide their architecture as they grow. The advance opens the way for cheaper catalysts. In yet another lab, researchers in Minnesota came up with a new route for making ultrathin zeolite membranes, which are likely to be useful as a wide variety of chemically selective filters.
This surge of molecular wizardry provides a vivid reminder that the creativity of chemists keeps their field ever young.
Related References and Web Sites
2011.12.23 View 12451 -
 Scientists develop highly efficient industrial catalyst
http://english.yonhapnews.co.kr/business/2011/07/14/48/0501000000AEN20110714009600320F.HTML
SEOUL, July 15 (Yonhap) -- South Korean scientists said Friday that they have developed a highly efficient nanoporous industrial catalyst that can have a considerable impact on chemical and oil-refining sectors.
The team of scientists led by Ryoo Ryong, a chemistry professor at the Korea Advanced Institute of Science and Technology (KAIST), said the solid zeolite compound developed in the laboratory has a reaction speed five to 10 times faster than that of conventional materials.
Zeolite, which is made from silica and aluminium, is frequently used as an absorbent, water purifier and in nuclear reprocessing, although it is mainly employed in the chemical industry.
The annual size of the zeolite market is estimated at US$2.5 billion with output using the material topping $30 billion. At present, 41 percent of all catalysts used in the chemical sector are nano-scale zeolite materials.
The KAIST team said that because the new zeolite is made up of different sized pores, the material can be used as a catalyst when existing materials are unable to act as a changing agent.
"Existing zeolites only have pores under 1 nanometer in diameter, but the new material has holes that range from 1 nanometer to 3.5 nanometers, which are all arranged in a regular honeycomb arrangement," Ryoo said. A nanometer is one-billionth of a meter.
He said the ability to have both micro- and meso-sized pores is key to the faster reaction speed that is an integral part of raising efficiency. The South Korean researchers used a so-called surfactant process to make the different sizes of pores.
The development is a breakthrough because researchers and companies such as Exxon Mobil Corp. have been trying to build zeolite with different sizes of pores for the past two decades without making serious headway. There are more than 200 different types of zeolites in the world.
Ryoo, who received funding from the government, has requested intellectual property rights for the discovery, which has been published in the latest issue of Science magazine. He also developed another zeolite in the past that can transform methanol to gasoline up to 10 times more efficiently than existing catalysts.
Exxon Mobil has expressed interest in the two zeolites made by Ryoo"s team. Undisclosed South Korean petrochemical companies have also made inquiries that may lead to commercial development in the future.
"There are some technical issues to resolve, mainly related with mass production and stability," the scientist said.
He said full-fledge production will be determined by how much companies are willing to spend on research to speed up development that can bring down overall production costs.
The KAIST team said it took two years to make the new zeolite, which can be custom made to meet specific needs.
(END)
2011.07.15 View 12504
Scientists develop highly efficient industrial catalyst
http://english.yonhapnews.co.kr/business/2011/07/14/48/0501000000AEN20110714009600320F.HTML
SEOUL, July 15 (Yonhap) -- South Korean scientists said Friday that they have developed a highly efficient nanoporous industrial catalyst that can have a considerable impact on chemical and oil-refining sectors.
The team of scientists led by Ryoo Ryong, a chemistry professor at the Korea Advanced Institute of Science and Technology (KAIST), said the solid zeolite compound developed in the laboratory has a reaction speed five to 10 times faster than that of conventional materials.
Zeolite, which is made from silica and aluminium, is frequently used as an absorbent, water purifier and in nuclear reprocessing, although it is mainly employed in the chemical industry.
The annual size of the zeolite market is estimated at US$2.5 billion with output using the material topping $30 billion. At present, 41 percent of all catalysts used in the chemical sector are nano-scale zeolite materials.
The KAIST team said that because the new zeolite is made up of different sized pores, the material can be used as a catalyst when existing materials are unable to act as a changing agent.
"Existing zeolites only have pores under 1 nanometer in diameter, but the new material has holes that range from 1 nanometer to 3.5 nanometers, which are all arranged in a regular honeycomb arrangement," Ryoo said. A nanometer is one-billionth of a meter.
He said the ability to have both micro- and meso-sized pores is key to the faster reaction speed that is an integral part of raising efficiency. The South Korean researchers used a so-called surfactant process to make the different sizes of pores.
The development is a breakthrough because researchers and companies such as Exxon Mobil Corp. have been trying to build zeolite with different sizes of pores for the past two decades without making serious headway. There are more than 200 different types of zeolites in the world.
Ryoo, who received funding from the government, has requested intellectual property rights for the discovery, which has been published in the latest issue of Science magazine. He also developed another zeolite in the past that can transform methanol to gasoline up to 10 times more efficiently than existing catalysts.
Exxon Mobil has expressed interest in the two zeolites made by Ryoo"s team. Undisclosed South Korean petrochemical companies have also made inquiries that may lead to commercial development in the future.
"There are some technical issues to resolve, mainly related with mass production and stability," the scientist said.
He said full-fledge production will be determined by how much companies are willing to spend on research to speed up development that can bring down overall production costs.
The KAIST team said it took two years to make the new zeolite, which can be custom made to meet specific needs.
(END)
2011.07.15 View 12504