BIO
-
 A KAIST startup, YBrain, builds a wearable device to cure Alzheimer's
A group of KAIST graduates from the Departments of Bio and Brain Engineering, Computer Science, Materials Science Engineering, and Industrial Design created a startup called YBrain (http://ybrain.com/). YBrain develops a wearable neuroscience technology to treat or reduce the symptoms of degenerative brain diseases such as dementia and Alzheimer’s. Their recent technological developments were covered in e27, one of the leading blogs based in Singapore. The blog covers topics like the latest technology innovation, startups, and entrepreneurship in Asia. A news article follows below:
e27, June 24, 2014
“This wearable tech may be able to combat effects of Alzheimer’s”
http://e27.co/this-wearable-tech-may-be-able-combat-effects-of-alzheimers-20140624/
2014.06.25 View 13896
A KAIST startup, YBrain, builds a wearable device to cure Alzheimer's
A group of KAIST graduates from the Departments of Bio and Brain Engineering, Computer Science, Materials Science Engineering, and Industrial Design created a startup called YBrain (http://ybrain.com/). YBrain develops a wearable neuroscience technology to treat or reduce the symptoms of degenerative brain diseases such as dementia and Alzheimer’s. Their recent technological developments were covered in e27, one of the leading blogs based in Singapore. The blog covers topics like the latest technology innovation, startups, and entrepreneurship in Asia. A news article follows below:
e27, June 24, 2014
“This wearable tech may be able to combat effects of Alzheimer’s”
http://e27.co/this-wearable-tech-may-be-able-combat-effects-of-alzheimers-20140624/
2014.06.25 View 13896 -
 Professor Ki Jun Jeong Selected As the Winner of the 'Young Asian Biotechnologist Prize'
Professor Ki Jun Jeong from the Department of Chemical and Biomolecular Engineering, KAIST, has been selected as the winner of this year’s Young Asian Biotechnologist Prize.
Professor Jeong was invited to the 66th Japan Biotechnology and Bioengineering Society Conference scheduled in September 9th-11th, 2014, in Sapporo, Japan, where his award ceremony will be held.
The award is presented to Professor Jeong in recognition of his outstanding research on microbial-based production of antibodies and efficiency improvement.
The Young Asian Biotechnologist Prize is awarded annually by the Japan Biotechnology and Bioengineering Society to the researchers in Asia under the age of 45, who have achieved excellent research results in the field of bioengineering.
2014.06.14 View 10937
Professor Ki Jun Jeong Selected As the Winner of the 'Young Asian Biotechnologist Prize'
Professor Ki Jun Jeong from the Department of Chemical and Biomolecular Engineering, KAIST, has been selected as the winner of this year’s Young Asian Biotechnologist Prize.
Professor Jeong was invited to the 66th Japan Biotechnology and Bioengineering Society Conference scheduled in September 9th-11th, 2014, in Sapporo, Japan, where his award ceremony will be held.
The award is presented to Professor Jeong in recognition of his outstanding research on microbial-based production of antibodies and efficiency improvement.
The Young Asian Biotechnologist Prize is awarded annually by the Japan Biotechnology and Bioengineering Society to the researchers in Asia under the age of 45, who have achieved excellent research results in the field of bioengineering.
2014.06.14 View 10937 -
 Professor YongKeun Park Produces Undergraduate Students with International Achievements
Three undergraduate students under the supervision of Professor YongKeun Park from the Department of Physics, KAIST, have published papers in globally renowned academic journals.
The most recent publication was made by YoungJu Jo, a senior in physics. Jo’s paper entitled “Angle-resolved light scattering of individual rod-shaped bacteria based on Fourier transform light scattering” was published in the May 28th edition of Scientific Reports.
Analyzing bacteria is a very important task in the field of health and food hygiene, but using the conventional biochemical methods of analysis takes days. However, observation with Jo’s newly developed method using light scattering analyzes bacteria within a matter of seconds.
SangYeon Cho from the Department of Chemistry also published papers in Cell (2012) and Nature (2013), respectively, under the guidance of Professor Park. SangYeon Cho’s outstanding research achievements were recognized by Harvard and MIT. He was accepted with a full scholarship to Harvard-MIT Health Sciences and Technology Graduate School. He will begin his graduate studies at Harvard-MIT this September.
Last March, SeoEun Lee from the Department of Biology was the recipient of the Best Paper Award by the Optical Society of Korea. She plans to pursue a doctoral degree at the College of Physicians and Surgeons, Columbia University in New York.
Professor Park said, “Undergraduate students, who are learning a variety of subjects concurrently, are at the most creative time of their lives. KAIST has offered many opportunities to undergraduate students to partake in various research programs.”
- Picture (a) and (b): Rod-shaped bacteria’s phase image and light-scattering patterns
- Picture (c): Quantitative analysis to illustrate the extraction of information from bacteria
2014.06.03 View 14980
Professor YongKeun Park Produces Undergraduate Students with International Achievements
Three undergraduate students under the supervision of Professor YongKeun Park from the Department of Physics, KAIST, have published papers in globally renowned academic journals.
The most recent publication was made by YoungJu Jo, a senior in physics. Jo’s paper entitled “Angle-resolved light scattering of individual rod-shaped bacteria based on Fourier transform light scattering” was published in the May 28th edition of Scientific Reports.
Analyzing bacteria is a very important task in the field of health and food hygiene, but using the conventional biochemical methods of analysis takes days. However, observation with Jo’s newly developed method using light scattering analyzes bacteria within a matter of seconds.
SangYeon Cho from the Department of Chemistry also published papers in Cell (2012) and Nature (2013), respectively, under the guidance of Professor Park. SangYeon Cho’s outstanding research achievements were recognized by Harvard and MIT. He was accepted with a full scholarship to Harvard-MIT Health Sciences and Technology Graduate School. He will begin his graduate studies at Harvard-MIT this September.
Last March, SeoEun Lee from the Department of Biology was the recipient of the Best Paper Award by the Optical Society of Korea. She plans to pursue a doctoral degree at the College of Physicians and Surgeons, Columbia University in New York.
Professor Park said, “Undergraduate students, who are learning a variety of subjects concurrently, are at the most creative time of their lives. KAIST has offered many opportunities to undergraduate students to partake in various research programs.”
- Picture (a) and (b): Rod-shaped bacteria’s phase image and light-scattering patterns
- Picture (c): Quantitative analysis to illustrate the extraction of information from bacteria
2014.06.03 View 14980 -
 Newsletter: KAIST Breakthroughs in Engineering and Information Science & Technology
The
College of Engineering and the College of Information Science & Technology
at KAIST jointly published a bi-annual online newsletter, KAIST Breakthroughs in Engineering and Information Science &
Technology. The newsletter highlights major research achievements of the
two colleges while updating readers on any news or developments in their educational
programs.
For
the spring issue of the newsletter, please go to:
http://kaist.e-eyagi.com/newsletter/2014/01/
2014.03.28 View 7689
Newsletter: KAIST Breakthroughs in Engineering and Information Science & Technology
The
College of Engineering and the College of Information Science & Technology
at KAIST jointly published a bi-annual online newsletter, KAIST Breakthroughs in Engineering and Information Science &
Technology. The newsletter highlights major research achievements of the
two colleges while updating readers on any news or developments in their educational
programs.
For
the spring issue of the newsletter, please go to:
http://kaist.e-eyagi.com/newsletter/2014/01/
2014.03.28 View 7689 -
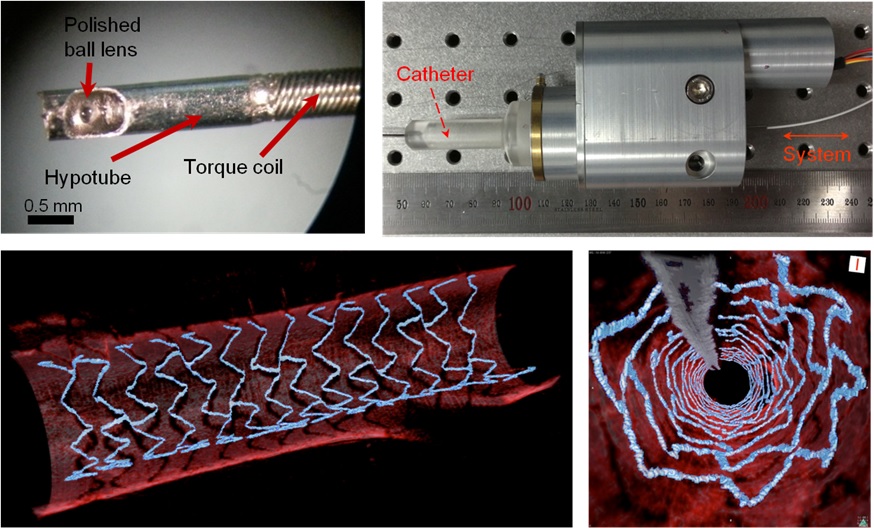 High Resolution 3D Blood Vessel Endoscope System Developed
Professor Wangyeol Oh of KAIST’s Mechanical Engineering Department has succeeded in developing an optical imaging endoscope system that employs an imaging velocity, which is up to 3.5 times faster than the previous systems. Furthermore, he has utilized this endoscope to acquire the world’s first high-resolution 3D images of the insides of in vivo blood vessel.
Professor Oh’s work is Korea’s first development of blood vessel endoscope system, possessing an imaging speed, resolution, imaging quality, and image-capture area. The system can also simultaneously perform a functional imaging, such as polarized imaging, which is advantageous for identifying the vulnerability of the blood vessel walls.
The Endoscopic Optical Coherence Tomography (OCT) System provides the highest resolution that is used to diagnose cardiovascular diseases, represented mainly by myocardial infarction.
However, the previous system was not fast enough to take images inside of the vessels, and therefore it was often impossible to accurately identify and analyze the vessel condition. To achieve an in vivo blood vessel optical imaging in clinical trials, the endoscope needed to be inserted, after which a clear liquid flows instantly, and pictures can be taken in only a few seconds.
The KAIST research team proposed a solution for such problem by developing a high-speed, high-resolution optical tomographic imaging system, a flexible endoscope with a diameter of 0.8 mm, as well as a device that can scan the imaging light within the blood vessels at high speed. Then, these devices were combined to visualize the internal structure of the vessel wall.
Using the developed system, the researchers were able to obtain high-resolution images of about 7 cm blood vessels of a rabbit’s aorta, which is similar size to human’s coronary arteries. The tomography scan took only 5.8 seconds, at a speed of 350 scans per second in all three directions with a resolution of 10~35㎛.
If the images are taken every 200 ㎛, like the currently available commercial vascular imaging endoscopes, a 7cm length vessel can be imaged in only one second.
Professor Wangyeol Oh said, “Our newly developed blood vessel endoscope system was tested by imaging a live animal’s blood vessels, which is similar to human blood vessels. The result was very successful.”
“Collaborating closely with hospitals, we are preparing to produce the imaging of an animal’s coronary arteries, which is similar in size to the human heart,” commented Professor Oh on the future clinical application and commercialization of the endoscope system. He added, “After such procedures, the technique can be applied in clinical patients within a few years.”
Professor Oh’s research was supported by the National Research Foundation of Korea and the Global Frontier Project by the Korean government. The research results were published in the 2014 January’s edition of Biomedical Optics Express.
Figure 1: End portion of optical endoscope (upper left)
Figure 2: High-speed optical scanning unit of the endoscope (top right)
Figure 3: High-resolution images of the inside of in vivo animal blood vessels (in the direction of vascular circumference and length)
Figure 4: High-resolution images of the inside of in vivo animal blood vessels (in the direction of the vein depth)
2014.03.25 View 13189
High Resolution 3D Blood Vessel Endoscope System Developed
Professor Wangyeol Oh of KAIST’s Mechanical Engineering Department has succeeded in developing an optical imaging endoscope system that employs an imaging velocity, which is up to 3.5 times faster than the previous systems. Furthermore, he has utilized this endoscope to acquire the world’s first high-resolution 3D images of the insides of in vivo blood vessel.
Professor Oh’s work is Korea’s first development of blood vessel endoscope system, possessing an imaging speed, resolution, imaging quality, and image-capture area. The system can also simultaneously perform a functional imaging, such as polarized imaging, which is advantageous for identifying the vulnerability of the blood vessel walls.
The Endoscopic Optical Coherence Tomography (OCT) System provides the highest resolution that is used to diagnose cardiovascular diseases, represented mainly by myocardial infarction.
However, the previous system was not fast enough to take images inside of the vessels, and therefore it was often impossible to accurately identify and analyze the vessel condition. To achieve an in vivo blood vessel optical imaging in clinical trials, the endoscope needed to be inserted, after which a clear liquid flows instantly, and pictures can be taken in only a few seconds.
The KAIST research team proposed a solution for such problem by developing a high-speed, high-resolution optical tomographic imaging system, a flexible endoscope with a diameter of 0.8 mm, as well as a device that can scan the imaging light within the blood vessels at high speed. Then, these devices were combined to visualize the internal structure of the vessel wall.
Using the developed system, the researchers were able to obtain high-resolution images of about 7 cm blood vessels of a rabbit’s aorta, which is similar size to human’s coronary arteries. The tomography scan took only 5.8 seconds, at a speed of 350 scans per second in all three directions with a resolution of 10~35㎛.
If the images are taken every 200 ㎛, like the currently available commercial vascular imaging endoscopes, a 7cm length vessel can be imaged in only one second.
Professor Wangyeol Oh said, “Our newly developed blood vessel endoscope system was tested by imaging a live animal’s blood vessels, which is similar to human blood vessels. The result was very successful.”
“Collaborating closely with hospitals, we are preparing to produce the imaging of an animal’s coronary arteries, which is similar in size to the human heart,” commented Professor Oh on the future clinical application and commercialization of the endoscope system. He added, “After such procedures, the technique can be applied in clinical patients within a few years.”
Professor Oh’s research was supported by the National Research Foundation of Korea and the Global Frontier Project by the Korean government. The research results were published in the 2014 January’s edition of Biomedical Optics Express.
Figure 1: End portion of optical endoscope (upper left)
Figure 2: High-speed optical scanning unit of the endoscope (top right)
Figure 3: High-resolution images of the inside of in vivo animal blood vessels (in the direction of vascular circumference and length)
Figure 4: High-resolution images of the inside of in vivo animal blood vessels (in the direction of the vein depth)
2014.03.25 View 13189 -
 Visit by Sir Paul Maxime Nurse, President of the Royal Society
Sir Paul Maxime
Nurse, who is an English geneticist and cell biologist, visited KAIST and gave
a lecture entitled The Great Ideas of
Biology on March 11, 2014.
Sir Paul was awarded the 2001 Nobel Prize in
Physiology or Medicine with Leland H. Hartwell and R. Timothy Hunt for their discoveries
of protein molecules that control the division of cells in the cell cycle.
He was Professor of Microbiology at the University
of Oxford, CEO of the Imperial Cancer Research Fund and Cancer Research UK, and
President of Rockefeller University in New York. Sir Paul is currently the President of
the Royal Society as well as Director and Chief Executive of the Francis Crick
Institute.
Founded in London in 1660, the Royal Society is composed of the world’s most distinguished scientists drawn from all areas of
science, engineering, and medicine.
Below is a summary of his lecture, The Great Ideas of Biology:
Four major ideas of biology
are the theory of genes, evolution by natural selection, the proposal that the
cell is the fundamental unit of all life, and the chemical composition of a cell.
When considering the
question “what is life?” these ideas come together. The special way cells reproduce
provides the conditions by which natural selection takes place, allowing living
organisms to evolve. The organization of chemistry within the cell provides
explanations for life’s phenomena.
In addition, an emerging idea
is the nature of biological self-organization with which living cells and organisms
process information and acquire specific forms. These great ideas have
influenced one another and changed the way we perceive biology and science
today.
2014.03.11 View 12084
Visit by Sir Paul Maxime Nurse, President of the Royal Society
Sir Paul Maxime
Nurse, who is an English geneticist and cell biologist, visited KAIST and gave
a lecture entitled The Great Ideas of
Biology on March 11, 2014.
Sir Paul was awarded the 2001 Nobel Prize in
Physiology or Medicine with Leland H. Hartwell and R. Timothy Hunt for their discoveries
of protein molecules that control the division of cells in the cell cycle.
He was Professor of Microbiology at the University
of Oxford, CEO of the Imperial Cancer Research Fund and Cancer Research UK, and
President of Rockefeller University in New York. Sir Paul is currently the President of
the Royal Society as well as Director and Chief Executive of the Francis Crick
Institute.
Founded in London in 1660, the Royal Society is composed of the world’s most distinguished scientists drawn from all areas of
science, engineering, and medicine.
Below is a summary of his lecture, The Great Ideas of Biology:
Four major ideas of biology
are the theory of genes, evolution by natural selection, the proposal that the
cell is the fundamental unit of all life, and the chemical composition of a cell.
When considering the
question “what is life?” these ideas come together. The special way cells reproduce
provides the conditions by which natural selection takes place, allowing living
organisms to evolve. The organization of chemistry within the cell provides
explanations for life’s phenomena.
In addition, an emerging idea
is the nature of biological self-organization with which living cells and organisms
process information and acquire specific forms. These great ideas have
influenced one another and changed the way we perceive biology and science
today.
2014.03.11 View 12084 -
 Professor Yoon-Key Nam Received the 2013 Emerging Scholars Award
Professor Yoon-Key Nam, the Department of Bio and Brain Engineering at KAIST, received the 2013 Emerging Scholars Award from the Korean BioChip Society (KBCS), an organization consisted of professionals and researchers in the biochip field such as proteomics, functional genomics, Bio-MEMS, nanotechnology, biosensors, and bioinformatics, at the fall annual conference of KBCS held on November 13th, 2013 at Kangwon National University in Korea.
Professor Nam was recognized for his development of neuron-on-a-chip technology through the convergence research of neuroscience and biochip.
Since 2008, the KBCS has been giving an award to one or two scholars under 40 years of age who have made a great stride in biochip research.
2014.01.27 View 9316
Professor Yoon-Key Nam Received the 2013 Emerging Scholars Award
Professor Yoon-Key Nam, the Department of Bio and Brain Engineering at KAIST, received the 2013 Emerging Scholars Award from the Korean BioChip Society (KBCS), an organization consisted of professionals and researchers in the biochip field such as proteomics, functional genomics, Bio-MEMS, nanotechnology, biosensors, and bioinformatics, at the fall annual conference of KBCS held on November 13th, 2013 at Kangwon National University in Korea.
Professor Nam was recognized for his development of neuron-on-a-chip technology through the convergence research of neuroscience and biochip.
Since 2008, the KBCS has been giving an award to one or two scholars under 40 years of age who have made a great stride in biochip research.
2014.01.27 View 9316 -
 First International Conference on Science and Technology for Society
KAIST co-organized the 2013 International Conference on Science and Technology for Society which was held on November 28 at the Grace Hall in Seoul EL-Tower. More than 300 people, including members of the Global Social Technology Advisory Board, domestic social technology experts, private companies, government officials, private citizens, and students joined the conference to discuss the roles and responsibilities of science and technology for society.
R&D policies and technologies for solving social issues were introduced, and discussions were held on desirable directions for technological development.
The first speaker, Yasushi Watanabe, Director of RISTEX (Research Institute of Science and Technology for Society) in Japan, introduced the importance of science and technology for society under the title “Change of R&D Paradigm for Society.”
Robert Wimmer, GrAT (Center for Appropriate Technology), Vienna University of Technology in Austria, presented “Need-oriented Design & Solutions for Development.” Kiyoaki Murakami, MRI, Japan, presented “Introduction of Platinum Vision” and Robert Ries, University of Florida, U.S.A., presented “Evaluating the Social Impacts of the Built Environment Using Life Cycle Assessment.”
Case studies on social enterprises and presentations on R&D for solving social problems were introduced by ICISTS (International Conference for the Integration of Science, Technology and Society), which is a student group at KAIST, National Research Foundation of Korea (NRF), Korea Institute of Machinery and Materials (KIMM), Korea Research Institute of Bioscience and Biotechnology (KRIBB), Korea Institute of Industrial Technology (KITECH), Electronics and Telecommunication Research Institute (ETRI), and Korea Research Institute of Chemical Technology (KRICT).The conference was hosted by the Ministry of Science, ICT, and Future Planning and co-organized by NRF, KIMM, KRIBB, KITECH, ETRI and KRICT.
2013.12.11 View 13467
First International Conference on Science and Technology for Society
KAIST co-organized the 2013 International Conference on Science and Technology for Society which was held on November 28 at the Grace Hall in Seoul EL-Tower. More than 300 people, including members of the Global Social Technology Advisory Board, domestic social technology experts, private companies, government officials, private citizens, and students joined the conference to discuss the roles and responsibilities of science and technology for society.
R&D policies and technologies for solving social issues were introduced, and discussions were held on desirable directions for technological development.
The first speaker, Yasushi Watanabe, Director of RISTEX (Research Institute of Science and Technology for Society) in Japan, introduced the importance of science and technology for society under the title “Change of R&D Paradigm for Society.”
Robert Wimmer, GrAT (Center for Appropriate Technology), Vienna University of Technology in Austria, presented “Need-oriented Design & Solutions for Development.” Kiyoaki Murakami, MRI, Japan, presented “Introduction of Platinum Vision” and Robert Ries, University of Florida, U.S.A., presented “Evaluating the Social Impacts of the Built Environment Using Life Cycle Assessment.”
Case studies on social enterprises and presentations on R&D for solving social problems were introduced by ICISTS (International Conference for the Integration of Science, Technology and Society), which is a student group at KAIST, National Research Foundation of Korea (NRF), Korea Institute of Machinery and Materials (KIMM), Korea Research Institute of Bioscience and Biotechnology (KRIBB), Korea Institute of Industrial Technology (KITECH), Electronics and Telecommunication Research Institute (ETRI), and Korea Research Institute of Chemical Technology (KRICT).The conference was hosted by the Ministry of Science, ICT, and Future Planning and co-organized by NRF, KIMM, KRIBB, KITECH, ETRI and KRICT.
2013.12.11 View 13467 -
 UN biological weapons expert gives lecture at KAIST
KAIST’s student organization, the ICISTS Organizing Committee, invited United Nations Security Council expert Terence Taylor to deliver a speech under the topic of ‘Terrorists and Scientists: Biological Weapons and its impact on Global Society’. The lecture took place on November 19 on the Daejeon campus.
Taylor shared his experiences as a biochemical weapons expert at Iraq and discussed the fast-approaching future of the world with biochemical weapons.
Terence Taylor is a former British military officer, who served various governmental and non-governmental organizations around the world, including UK and U.S. agencies, as well as the UN. His current work involves the non-proliferation and disarmament of nuclear or biological weapons, toxic substances and other weapons of mass destruction.
ICISTS Organizing Committee is a student organization run by of KAIST students. Since 2005, it has actively held one of the largest student conferences in Asia, ICISTS-KAIST, at KAIST every year. "ICISTS" stands for “International Conference for the Integration of Science, Technology, and Society”, which conveys its vision in achieving a harmony between science and society.
UN Security Council expert Terence Taylor
2013.11.28 View 10403
UN biological weapons expert gives lecture at KAIST
KAIST’s student organization, the ICISTS Organizing Committee, invited United Nations Security Council expert Terence Taylor to deliver a speech under the topic of ‘Terrorists and Scientists: Biological Weapons and its impact on Global Society’. The lecture took place on November 19 on the Daejeon campus.
Taylor shared his experiences as a biochemical weapons expert at Iraq and discussed the fast-approaching future of the world with biochemical weapons.
Terence Taylor is a former British military officer, who served various governmental and non-governmental organizations around the world, including UK and U.S. agencies, as well as the UN. His current work involves the non-proliferation and disarmament of nuclear or biological weapons, toxic substances and other weapons of mass destruction.
ICISTS Organizing Committee is a student organization run by of KAIST students. Since 2005, it has actively held one of the largest student conferences in Asia, ICISTS-KAIST, at KAIST every year. "ICISTS" stands for “International Conference for the Integration of Science, Technology, and Society”, which conveys its vision in achieving a harmony between science and society.
UN Security Council expert Terence Taylor
2013.11.28 View 10403 -
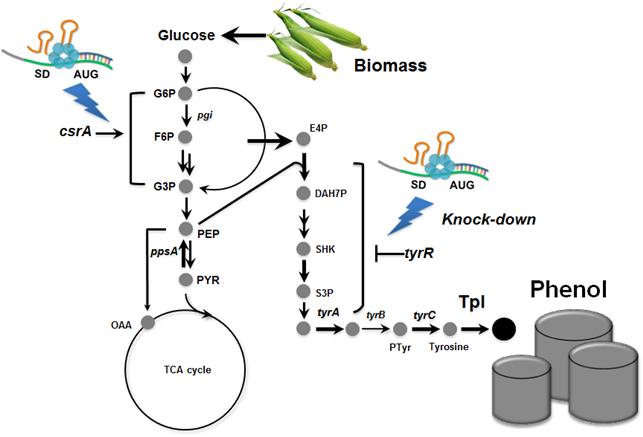 Metabolically engineered E. coli producing phenol
Many chemicals we use in everyday life are derived from fossil resources. Due to the increasing concerns on the use of fossil resources, there has been much interest in producing chemicals from renewable resources through biotechnology.
Phenol is an important commodity chemical, and is a starting material for the production of numerous industrial chemicals and polymers, including bisphenol A and phenolic resins, and others. At present, the production of phenol entirely depends on the chemical synthesis from benzene, and its annual production exceeds 8 million tons worldwide. Microbial production of phenol seems to be a non-viable process considering the high toxicity of phenol to the cell.
In the paper published online in Biotechnology Journal, a Korean research team led by Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering from the Korea Advanced Institute of Science and Technology (KAIST) reported the successful development of an engineered Escherichia coli (E. coli) strain which can produce phenol from glucose. E. coli has been a workhorse for biological production of various value-added compounds such as succinic acid and 1,4-butanediol in industrial scale. However, due to its low tolerance to phenol, E. coli was not considered a viable host strain for the biological production of phenol.
Professor Lee"s team, a leading research group in metabolic engineering, noted the genetic and physiological differences of various E. coli strains and investigated 18 different E. coli strains with respect to phenol tolerance and engineered all of the 18 strains simultaneously. If the traditional genetic engineering methods were used, this work would have taken years to do. To overcome this challenge, the research team used synthetic small RNA (sRNA) technology they recently developed (Nature Biotechnology, vol 31, pp 170-174, 2013). The sRNA technology allowed the team to screen 18 E. coli strains with respect to the phenol tolerance, and the activities of the metabolic pathway and enzyme involved in the production of phenol. The research team also metabolically engineered the E. coli strains to increase carbon flux toward phenol and finally generated an engineered E. coli strain which can produce phenol from glucose.
Furthermore, the team developed a biphasic extractive fermentation process to minimize the toxicity of phenol to E. coli cells. Glycerol tributyrate was found to have low toxicity to E. coli and allowed efficient extraction of phenol from the culture broth. Through the biphasic fed-batch fermentation using glycerol tributyrate as an in situ extractant, the final engineered E. coli strain produced phenol to the highest titer and productivity reported (3.8 g/L and 0.18 g/L/h, respectively). The strategy used for the strain development and the fermentation process will serve as a framework for metabolic engineering of microorganisms for the production of toxic chemicals from renewable resources.
This work was supported by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) of the Ministry of Science, ICT & Future Planning through the National Research Foundation of Korea.
Process of Phenol Production
2013.11.05 View 11547
Metabolically engineered E. coli producing phenol
Many chemicals we use in everyday life are derived from fossil resources. Due to the increasing concerns on the use of fossil resources, there has been much interest in producing chemicals from renewable resources through biotechnology.
Phenol is an important commodity chemical, and is a starting material for the production of numerous industrial chemicals and polymers, including bisphenol A and phenolic resins, and others. At present, the production of phenol entirely depends on the chemical synthesis from benzene, and its annual production exceeds 8 million tons worldwide. Microbial production of phenol seems to be a non-viable process considering the high toxicity of phenol to the cell.
In the paper published online in Biotechnology Journal, a Korean research team led by Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering from the Korea Advanced Institute of Science and Technology (KAIST) reported the successful development of an engineered Escherichia coli (E. coli) strain which can produce phenol from glucose. E. coli has been a workhorse for biological production of various value-added compounds such as succinic acid and 1,4-butanediol in industrial scale. However, due to its low tolerance to phenol, E. coli was not considered a viable host strain for the biological production of phenol.
Professor Lee"s team, a leading research group in metabolic engineering, noted the genetic and physiological differences of various E. coli strains and investigated 18 different E. coli strains with respect to phenol tolerance and engineered all of the 18 strains simultaneously. If the traditional genetic engineering methods were used, this work would have taken years to do. To overcome this challenge, the research team used synthetic small RNA (sRNA) technology they recently developed (Nature Biotechnology, vol 31, pp 170-174, 2013). The sRNA technology allowed the team to screen 18 E. coli strains with respect to the phenol tolerance, and the activities of the metabolic pathway and enzyme involved in the production of phenol. The research team also metabolically engineered the E. coli strains to increase carbon flux toward phenol and finally generated an engineered E. coli strain which can produce phenol from glucose.
Furthermore, the team developed a biphasic extractive fermentation process to minimize the toxicity of phenol to E. coli cells. Glycerol tributyrate was found to have low toxicity to E. coli and allowed efficient extraction of phenol from the culture broth. Through the biphasic fed-batch fermentation using glycerol tributyrate as an in situ extractant, the final engineered E. coli strain produced phenol to the highest titer and productivity reported (3.8 g/L and 0.18 g/L/h, respectively). The strategy used for the strain development and the fermentation process will serve as a framework for metabolic engineering of microorganisms for the production of toxic chemicals from renewable resources.
This work was supported by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) of the Ministry of Science, ICT & Future Planning through the National Research Foundation of Korea.
Process of Phenol Production
2013.11.05 View 11547 -
 KAIST announced a novel technology to produce gasoline by a metabolically engineered microorganism
A major scientific breakthrough in the development of renewable energy sources and other important chemicals; The research team succeeded in producing 580 mg of gasoline per liter of cultured broth by converting in vivo generated fatty acids
For many decades, we have been relying on fossil resources to produce liquid fuels such as gasoline, diesel, and many industrial and consumer chemicals for daily use. However, increasing strains on natural resources as well as environmental issues including global warming have triggered a strong interest in developing sustainable ways to obtain fuels and chemicals.
Gasoline, the petroleum-derived product that is most widely used as a fuel for transportation, is a mixture of hydrocarbons, additives, and blending agents. The hydrocarbons, called alkanes, consist only of carbon and hydrogen atoms. Gasoline has a combination of straight-chain and branched-chain alkanes (hydrocarbons) consisted of 4-12 carbon atoms linked by direct carbon-carbon bonds.
Previously, through metabolic engineering of Escherichia coli (E. coli), there have been a few research results on the production of long-chain alkanes, which consist of 13-17 carbon atoms, suitable for replacing diesel. However, there has been no report on the microbial production of short-chain alkanes, a possible substitute for gasoline.
In the paper (entitled "Microbial Production of Short-chain Alkanes") published online in Nature on September 29, a Korean research team led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at the Korea Advanced Institute of Science and Technology (KAIST) reported, for the first time, the development of a novel strategy for microbial gasoline production through metabolic engineering of E. coli.
The research team engineered the fatty acid metabolism to provide the fatty acid derivatives that are shorter than normal intracellular fatty acid metabolites, and introduced a novel synthetic pathway for the biosynthesis of short-chain alkanes. This allowed the development of platform E. coli strain capable of producing gasoline for the first time. Furthermore, this platform strain, if desired, can be modified to produce other products such as short-chain fatty esters and short-chain fatty alcohols.
In this paper, the Korean researchers described detailed strategies for 1) screening of enzymes associated with the production of fatty acids, 2) engineering of enzymes and fatty acid biosynthetic pathways to concentrate carbon flux towards the short-chain fatty acid production, and 3) converting short-chain fatty acids to their corresponding alkanes (gasoline) by introducing a novel synthetic pathway and optimization of culture conditions. Furthermore, the research team showed the possibility of producing fatty esters and alcohols by introducing responsible enzymes into the same platform strain.
Professor Sang Yup Lee said, "It is only the beginning of the work towards sustainable production of gasoline. The titer is rather low due to the low metabolic flux towards the formation of short-chain fatty acids and their derivatives. We are currently working on increasing the titer, yield and productivity of bio-gasoline. Nonetheless, we are pleased to report, for the first time, the production of gasoline through the metabolic engineering of E. coli, which we hope will serve as a basis for the metabolic engineering of microorganisms to produce fuels and chemicals from renewable resources."
This research was supported by the Advanced Biomass Research and Development Center of Korea (ABC-2010-0029799) through the Global Frontier Research Program of the Ministry of Science, ICT and Future Planning (MSIP) through the National Research Foundation (NRF), Republic of Korea. Systems metabolic engineering work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012-C1AAA001-2012M1A2A2026556) by MSIP through NRF.
Short-Chain Alkanes Generated from Renewable Biomass
This diagram shows the metabolic engineering of Escherichia coli for the production of short-chain alkanes (gasoline) from renewable biomass.
Nature Cover Page (September 29th, 2013)
2013.11.04 View 13573
KAIST announced a novel technology to produce gasoline by a metabolically engineered microorganism
A major scientific breakthrough in the development of renewable energy sources and other important chemicals; The research team succeeded in producing 580 mg of gasoline per liter of cultured broth by converting in vivo generated fatty acids
For many decades, we have been relying on fossil resources to produce liquid fuels such as gasoline, diesel, and many industrial and consumer chemicals for daily use. However, increasing strains on natural resources as well as environmental issues including global warming have triggered a strong interest in developing sustainable ways to obtain fuels and chemicals.
Gasoline, the petroleum-derived product that is most widely used as a fuel for transportation, is a mixture of hydrocarbons, additives, and blending agents. The hydrocarbons, called alkanes, consist only of carbon and hydrogen atoms. Gasoline has a combination of straight-chain and branched-chain alkanes (hydrocarbons) consisted of 4-12 carbon atoms linked by direct carbon-carbon bonds.
Previously, through metabolic engineering of Escherichia coli (E. coli), there have been a few research results on the production of long-chain alkanes, which consist of 13-17 carbon atoms, suitable for replacing diesel. However, there has been no report on the microbial production of short-chain alkanes, a possible substitute for gasoline.
In the paper (entitled "Microbial Production of Short-chain Alkanes") published online in Nature on September 29, a Korean research team led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at the Korea Advanced Institute of Science and Technology (KAIST) reported, for the first time, the development of a novel strategy for microbial gasoline production through metabolic engineering of E. coli.
The research team engineered the fatty acid metabolism to provide the fatty acid derivatives that are shorter than normal intracellular fatty acid metabolites, and introduced a novel synthetic pathway for the biosynthesis of short-chain alkanes. This allowed the development of platform E. coli strain capable of producing gasoline for the first time. Furthermore, this platform strain, if desired, can be modified to produce other products such as short-chain fatty esters and short-chain fatty alcohols.
In this paper, the Korean researchers described detailed strategies for 1) screening of enzymes associated with the production of fatty acids, 2) engineering of enzymes and fatty acid biosynthetic pathways to concentrate carbon flux towards the short-chain fatty acid production, and 3) converting short-chain fatty acids to their corresponding alkanes (gasoline) by introducing a novel synthetic pathway and optimization of culture conditions. Furthermore, the research team showed the possibility of producing fatty esters and alcohols by introducing responsible enzymes into the same platform strain.
Professor Sang Yup Lee said, "It is only the beginning of the work towards sustainable production of gasoline. The titer is rather low due to the low metabolic flux towards the formation of short-chain fatty acids and their derivatives. We are currently working on increasing the titer, yield and productivity of bio-gasoline. Nonetheless, we are pleased to report, for the first time, the production of gasoline through the metabolic engineering of E. coli, which we hope will serve as a basis for the metabolic engineering of microorganisms to produce fuels and chemicals from renewable resources."
This research was supported by the Advanced Biomass Research and Development Center of Korea (ABC-2010-0029799) through the Global Frontier Research Program of the Ministry of Science, ICT and Future Planning (MSIP) through the National Research Foundation (NRF), Republic of Korea. Systems metabolic engineering work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012-C1AAA001-2012M1A2A2026556) by MSIP through NRF.
Short-Chain Alkanes Generated from Renewable Biomass
This diagram shows the metabolic engineering of Escherichia coli for the production of short-chain alkanes (gasoline) from renewable biomass.
Nature Cover Page (September 29th, 2013)
2013.11.04 View 13573 -
 A powerful strategy for developing microbial cell factories by employing synthetic small RNAs
The current systems for the production of chemicals, fuels and materials heavily rely on the use of fossil resources. Due to the increasing concerns on climate change and other environmental problems, however, there has been much interest in developing biorefineries for the production of such chemicals, fuels and materials from renewable resources. For the biorefineries to be competitive with the traditional fossil resource-based refineries, development of high performance microorganisms is the most important as it will affect the overall economics of the process most significantly. Metabolic engineering, which can be defined as purposeful modification of cellular metabolic and regulatory networks with an aim to improve the production of a desired product, has been successfully employed to improve the performance of the cell. However, it is not trivial to engineer the cellular metabolism and regulatory circuits in the cell due to their high complexity.
In metabolic engineering, it is important to find the genes that need to be amplified and attenuated in order to increase the product formation rate while minimizing the production of undesirable byproducts. Gene knock-out experiments are often performed to delete those metabolic fluxes that will consequently result in the increase of the desired product formation. However, gene knock-out experiments require much effort and time to perform, and are difficult to do for a large number of genes. Furthermore, the gene knock-out experiments performed in one strain cannot be transferred to another organism and thus the whole experimental process has to be repeated. This is a big problem in developing a high performance microbial cell factory because it is required to find the best platform strain among many different strains. Therefore, researchers have been eager to develop a strategy that allows rapid identification of multiple genes to be attenuated in multiple strains at the same time.
A Korean research team led by Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering from the Korea Advanced Institute of Science and Technology (KAIST) reported the development of a strategy for efficiently developing microbial cell factories by employing synthetic small RNAs (sRNAs). They first reported the development of such system in Nature Biotechnology last February. This strategy of employing synthetic sRNAs in metabolic engineering has been receiving great interest worldwide as it allows easy, rapid, high-throughput, tunable, and un-doable knock-down of multiple genes in multiple strains at the same time. The research team published a paper online on August 8 as a cover page (September issue) in Nature Protocols, describing the detailed strategy and protocol to employ synthetic sRNAs for metabolic engineering.
In this paper, researchers described the detailed step-by-step protocol for synthetic sRNA-based gene expression control, including the sRNA design principles. Tailor-made synthetic sRNAs can be easily manipulated by using conventional gene cloning method. The use of synthetic sRNAs for gene expression regulation provides several advantages such as portability, conditionality, and tunability in high-throughput experiments. Plasmid-based synthetic sRNA expression system does not leave any scar on the chromosome, and can be easily transferred to many other host strains to be examined. Thus, the construction of libraries and examination of different host strains are much easier than the conventional hard-coded gene manipulation systems. Also, the expression of genes can be conditionally repressed by controlling the production of synthetic sRNAs. Synthetic sRNAs possessing different repression efficiencies make it possible to finely tune the gene expression levels as well. Furthermore, synthetic sRNAs allow knock-down of the expression of essential genes, which was not possible by conventional gene knock-out experiments.
Synthetic sRNAs can be utilized for diverse experiments where gene expression regulation is needed. One of promising applications is high-throughput screening of the target genes to be manipulated and multiple strains simultaneously to enhance the production of chemicals and materials of interest. Such simultaneous optimization of gene targets and strains has been one of the big challenges in metabolic engineering. Another application is to fine tune the expression of the screened genes for flux optimization, which would enhance chemical production further by balancing the flux between biomass formation and target chemical production. Synthetic sRNAs can also be applied to finely regulating genetic interactions in a circuit or network, which is essential in synthetic biology. Once a sRNA scaffold-harboring plasmid is constructed, tailor-made, synthetic sRNAs can be made within 3-4 days, followed by the desired application experiments.
Dr. Eytan Zlotorynski, an editor at Nature Protocols, said "This paper describes the detailed protocol for the design and applications of synthetic sRNA. The method, which has many advantages, is likely to become common practice, and prove useful for metabolic engineering and synthetic biology studies."
This paper published in Nature Protocols will be useful for all researchers in academia and industry who are interested in the use of synthetic sRNAs for fundamental and applied biological and biotechnological studies.
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012-C1AAA001-2012M1A2A2026556) and the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) of the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea.
2013.10.31 View 11066
A powerful strategy for developing microbial cell factories by employing synthetic small RNAs
The current systems for the production of chemicals, fuels and materials heavily rely on the use of fossil resources. Due to the increasing concerns on climate change and other environmental problems, however, there has been much interest in developing biorefineries for the production of such chemicals, fuels and materials from renewable resources. For the biorefineries to be competitive with the traditional fossil resource-based refineries, development of high performance microorganisms is the most important as it will affect the overall economics of the process most significantly. Metabolic engineering, which can be defined as purposeful modification of cellular metabolic and regulatory networks with an aim to improve the production of a desired product, has been successfully employed to improve the performance of the cell. However, it is not trivial to engineer the cellular metabolism and regulatory circuits in the cell due to their high complexity.
In metabolic engineering, it is important to find the genes that need to be amplified and attenuated in order to increase the product formation rate while minimizing the production of undesirable byproducts. Gene knock-out experiments are often performed to delete those metabolic fluxes that will consequently result in the increase of the desired product formation. However, gene knock-out experiments require much effort and time to perform, and are difficult to do for a large number of genes. Furthermore, the gene knock-out experiments performed in one strain cannot be transferred to another organism and thus the whole experimental process has to be repeated. This is a big problem in developing a high performance microbial cell factory because it is required to find the best platform strain among many different strains. Therefore, researchers have been eager to develop a strategy that allows rapid identification of multiple genes to be attenuated in multiple strains at the same time.
A Korean research team led by Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering from the Korea Advanced Institute of Science and Technology (KAIST) reported the development of a strategy for efficiently developing microbial cell factories by employing synthetic small RNAs (sRNAs). They first reported the development of such system in Nature Biotechnology last February. This strategy of employing synthetic sRNAs in metabolic engineering has been receiving great interest worldwide as it allows easy, rapid, high-throughput, tunable, and un-doable knock-down of multiple genes in multiple strains at the same time. The research team published a paper online on August 8 as a cover page (September issue) in Nature Protocols, describing the detailed strategy and protocol to employ synthetic sRNAs for metabolic engineering.
In this paper, researchers described the detailed step-by-step protocol for synthetic sRNA-based gene expression control, including the sRNA design principles. Tailor-made synthetic sRNAs can be easily manipulated by using conventional gene cloning method. The use of synthetic sRNAs for gene expression regulation provides several advantages such as portability, conditionality, and tunability in high-throughput experiments. Plasmid-based synthetic sRNA expression system does not leave any scar on the chromosome, and can be easily transferred to many other host strains to be examined. Thus, the construction of libraries and examination of different host strains are much easier than the conventional hard-coded gene manipulation systems. Also, the expression of genes can be conditionally repressed by controlling the production of synthetic sRNAs. Synthetic sRNAs possessing different repression efficiencies make it possible to finely tune the gene expression levels as well. Furthermore, synthetic sRNAs allow knock-down of the expression of essential genes, which was not possible by conventional gene knock-out experiments.
Synthetic sRNAs can be utilized for diverse experiments where gene expression regulation is needed. One of promising applications is high-throughput screening of the target genes to be manipulated and multiple strains simultaneously to enhance the production of chemicals and materials of interest. Such simultaneous optimization of gene targets and strains has been one of the big challenges in metabolic engineering. Another application is to fine tune the expression of the screened genes for flux optimization, which would enhance chemical production further by balancing the flux between biomass formation and target chemical production. Synthetic sRNAs can also be applied to finely regulating genetic interactions in a circuit or network, which is essential in synthetic biology. Once a sRNA scaffold-harboring plasmid is constructed, tailor-made, synthetic sRNAs can be made within 3-4 days, followed by the desired application experiments.
Dr. Eytan Zlotorynski, an editor at Nature Protocols, said "This paper describes the detailed protocol for the design and applications of synthetic sRNA. The method, which has many advantages, is likely to become common practice, and prove useful for metabolic engineering and synthetic biology studies."
This paper published in Nature Protocols will be useful for all researchers in academia and industry who are interested in the use of synthetic sRNAs for fundamental and applied biological and biotechnological studies.
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012-C1AAA001-2012M1A2A2026556) and the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) of the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea.
2013.10.31 View 11066