Chem
-
 KAIST to establish Ombudsperson system
KAIST has recently undergone a massive reorganization to achieve a streamlined system and highly efficient administration; and it will now implement the new “Ombudsperson” system to hear the opinions of the members of the university.
On September 9th, President Sungmo Kang held a ceremony to appoint Professors Sang-Young Shin and Hong-Gu Shim as the new “Ombudspersons”.
The previous Shinmungo system raised complaints and recommendations for improvements by members of the university, but this is the first time that KAIST has assigned a direct department for handling such matters.
The newly appointed Ombudspersons will review for the possibility of any unjust, irrational systems, violations of research ethics and such. It is their role to take a neutral stance and advise on the correction and improvement.
The merit of the Ombudsperson system is that diverse opinions can be reflected on the policy. The Ombudsperson guarantees the security of the contents of discussion so that anyone can share his or her opinion without fear of being recorded in documents.
It is expected that the Ombudsperson system will protect the interests of the individuals and thus contribute to making a “happy campus”.
President [Sungmo] Kang has said that the reason establishing the office of the Ombudsperson is “In order for KAIST to take a new leap toward the world, it is crucial to bring the minds of the members together…. Even the smallest voices must be heard to present solutions to make the university where everyone’s happy.”
In 1809, the Swedish Parliament appointed the first “Ombudsperson” to investigate and resolve civil complaints. Now, it is widely used in public institutions, corporations and universities to improve the communication and work efficiency of the members.
The new Ombudsmen: Prof. Sang-Young Shin (left) and Prof. Hong-Gu Shim (right)
2013.09.27 View 12834
KAIST to establish Ombudsperson system
KAIST has recently undergone a massive reorganization to achieve a streamlined system and highly efficient administration; and it will now implement the new “Ombudsperson” system to hear the opinions of the members of the university.
On September 9th, President Sungmo Kang held a ceremony to appoint Professors Sang-Young Shin and Hong-Gu Shim as the new “Ombudspersons”.
The previous Shinmungo system raised complaints and recommendations for improvements by members of the university, but this is the first time that KAIST has assigned a direct department for handling such matters.
The newly appointed Ombudspersons will review for the possibility of any unjust, irrational systems, violations of research ethics and such. It is their role to take a neutral stance and advise on the correction and improvement.
The merit of the Ombudsperson system is that diverse opinions can be reflected on the policy. The Ombudsperson guarantees the security of the contents of discussion so that anyone can share his or her opinion without fear of being recorded in documents.
It is expected that the Ombudsperson system will protect the interests of the individuals and thus contribute to making a “happy campus”.
President [Sungmo] Kang has said that the reason establishing the office of the Ombudsperson is “In order for KAIST to take a new leap toward the world, it is crucial to bring the minds of the members together…. Even the smallest voices must be heard to present solutions to make the university where everyone’s happy.”
In 1809, the Swedish Parliament appointed the first “Ombudsperson” to investigate and resolve civil complaints. Now, it is widely used in public institutions, corporations and universities to improve the communication and work efficiency of the members.
The new Ombudsmen: Prof. Sang-Young Shin (left) and Prof. Hong-Gu Shim (right)
2013.09.27 View 12834 -
 Chemistry World: Interview with Professor Cafer Yavuz of EEWS Graduate School
Professor Cafer Yavuz of the Graduate School of EEWS (energy, environment, water, and sustainability) at KAIST had an interview with the Chemistry World, the print and online magazine issued by the Royal Society of Chemistry, the largest organization in Europe for advancing the chemical sciences. The link below is the article published by the magazine:
http://www.rsc.org/chemistryworld/2013/08/interview-cafer-yavuz-carbon-dioxide-capture
2013.08.07 View 8789
Chemistry World: Interview with Professor Cafer Yavuz of EEWS Graduate School
Professor Cafer Yavuz of the Graduate School of EEWS (energy, environment, water, and sustainability) at KAIST had an interview with the Chemistry World, the print and online magazine issued by the Royal Society of Chemistry, the largest organization in Europe for advancing the chemical sciences. The link below is the article published by the magazine:
http://www.rsc.org/chemistryworld/2013/08/interview-cafer-yavuz-carbon-dioxide-capture
2013.08.07 View 8789 -
 Professor Jay H. Lee to receive the 2013 AIChE CAST Computing in Chemical Engineering Award
Professor Jay H. Lee of Chemical and Biomolecular Engineering Department at KAIST has won the 2013 Computing in Chemical Engineering Award of AIChE"s CAST Division (AIChE, American Institute of Chemical Engineers and CAST, Computing & Systems Technology Division).
The CAST Computing in Chemical Engineering Award, sponsored by The Dow Chemical Company, is annually given to an individual who has made outstanding contributions in the application of computing and systems technology to chemical engineering.Professor Lee has been recognized for his pioneering research contributions for “novel paradigms for much improved and robust model predictive control in industrial processes.” He is currently the Head of Chemical and Biomolecular Engineering Department and Director of Brain Korea (BK) 21 Program at the department. BK21 is the Korean government’s initiative to support the growth of research universities in the nation and foster highly trained master’s and doctoral students as well as researchers.
The CAST Computing in Chemical Engineering Award will be presented to Professor Jay H. Lee at the CAST Division dinner to be held at the AIChE Annual Meeting this November in San Francisco, where he will also deliver the after dinner lecture associated with this award.
2013.06.12 View 11790
Professor Jay H. Lee to receive the 2013 AIChE CAST Computing in Chemical Engineering Award
Professor Jay H. Lee of Chemical and Biomolecular Engineering Department at KAIST has won the 2013 Computing in Chemical Engineering Award of AIChE"s CAST Division (AIChE, American Institute of Chemical Engineers and CAST, Computing & Systems Technology Division).
The CAST Computing in Chemical Engineering Award, sponsored by The Dow Chemical Company, is annually given to an individual who has made outstanding contributions in the application of computing and systems technology to chemical engineering.Professor Lee has been recognized for his pioneering research contributions for “novel paradigms for much improved and robust model predictive control in industrial processes.” He is currently the Head of Chemical and Biomolecular Engineering Department and Director of Brain Korea (BK) 21 Program at the department. BK21 is the Korean government’s initiative to support the growth of research universities in the nation and foster highly trained master’s and doctoral students as well as researchers.
The CAST Computing in Chemical Engineering Award will be presented to Professor Jay H. Lee at the CAST Division dinner to be held at the AIChE Annual Meeting this November in San Francisco, where he will also deliver the after dinner lecture associated with this award.
2013.06.12 View 11790 -
 A KAIST research team developed in vivo flexible large scale integrated circuits
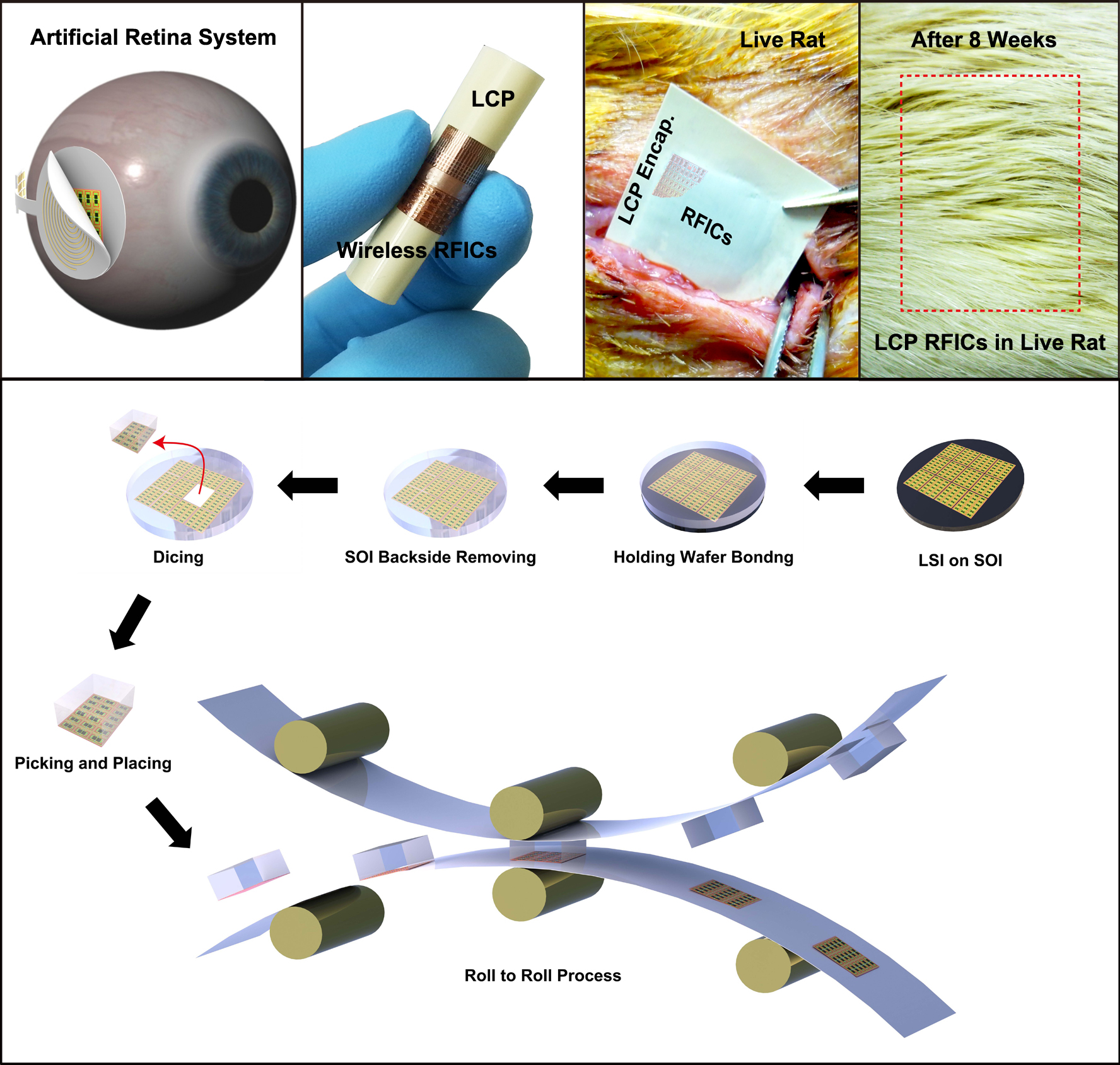
Daejeon, Republic of Korea, May 6th, 2013–-A team led by Professor Keon Jae Lee from the Department of Materials Science and Engineering at KAIST has developed in vivo silicon-based flexible large scale integrated circuits (LSI) for bio-medical wireless communication.
Silicon-based semiconductors have played significant roles in signal processing, nerve stimulation, memory storage, and wireless communication in implantable electronics. However, the rigid and bulky LSI chips have limited uses in in vivo devices due to incongruent contact with the curvilinear surfaces of human organs. Especially, artificial retinas recently approved by the Food and Drug Administration (refer to the press release of FDA"s artificial retina approval) require extremely flexible and slim LSI to incorporate it within the cramped area of the human eye.
Although several research teams have fabricated flexible integrated circuits (ICs, tens of interconnected transistors) on plastics, their inaccurate nano-scale alignment on plastics has restricted the demonstration of flexible nano-transistors and their large scale interconnection for in vivo LSI applications such as main process unit (MPU), high density memory and wireless communication. Professor Lee"s team previously demonstrated fully functional flexible memory using ultrathin silicon membranes (Nano Letters, Flexible Memristive Memory Array on Plastic Substrates), however, its integration level and transistor size (over micron scale) have limited functional applications for flexible consumer electronics.
Professor Keon Jae Lee"s team fabricated radio frequency integrated circuits (RFICs) interconnected with thousand nano-transistors on silicon wafer by state-of-the-art CMOS process, and then they removed the entire bottom substrate except top 100 nm active circuit layer by wet chemical etching. The flexible RF switches for wireless communication were monolithically encapsulated with biocompatible liquid crystal polymers (LCPs) for in vivo bio-medical applications. Finally, they implanted the LCP encapsulated RFICs into live rats to demonstrate the stable operation of flexible devices under in vivo circumstances.
Professor Lee said, "This work could provide an approach to flexible LSI for an ideal artificial retina system and other bio-medical devices. Moreover, the result represents an exciting technology with the strong potential to realize fully flexible consumer electronics such as application processor (AP) for mobile operating system, high-capacity memory, and wireless communication in the near future."
This result was published in the May online issue of the American Chemical Society"s journal, ACS Nano (In vivo Flexible RFICs Monolithically Encapsulated with LCP). They are currently engaged in commercializing efforts of roll-to-roll printing of flexible LSI on large area plastic substrates.
Movie at Youtube Link: Fabrication process for flexible LSI for flexible display, wearable computer and artificial retina for in vivo biomedical application
http://www.youtube.com/watch?v=5PpbM7m2PPs&feature=youtu.be
Applications of in Vivo Flexible Large Scale Integrated Circuits
Top: In vivo flexible large scale integrated circuits (LSI); Bottom: Schematic of roll-to-roll printing of flexible LSI on large area plastics.
2013.06.09 View 15235
A KAIST research team developed in vivo flexible large scale integrated circuits
Daejeon, Republic of Korea, May 6th, 2013–-A team led by Professor Keon Jae Lee from the Department of Materials Science and Engineering at KAIST has developed in vivo silicon-based flexible large scale integrated circuits (LSI) for bio-medical wireless communication.
Silicon-based semiconductors have played significant roles in signal processing, nerve stimulation, memory storage, and wireless communication in implantable electronics. However, the rigid and bulky LSI chips have limited uses in in vivo devices due to incongruent contact with the curvilinear surfaces of human organs. Especially, artificial retinas recently approved by the Food and Drug Administration (refer to the press release of FDA"s artificial retina approval) require extremely flexible and slim LSI to incorporate it within the cramped area of the human eye.
Although several research teams have fabricated flexible integrated circuits (ICs, tens of interconnected transistors) on plastics, their inaccurate nano-scale alignment on plastics has restricted the demonstration of flexible nano-transistors and their large scale interconnection for in vivo LSI applications such as main process unit (MPU), high density memory and wireless communication. Professor Lee"s team previously demonstrated fully functional flexible memory using ultrathin silicon membranes (Nano Letters, Flexible Memristive Memory Array on Plastic Substrates), however, its integration level and transistor size (over micron scale) have limited functional applications for flexible consumer electronics.
Professor Keon Jae Lee"s team fabricated radio frequency integrated circuits (RFICs) interconnected with thousand nano-transistors on silicon wafer by state-of-the-art CMOS process, and then they removed the entire bottom substrate except top 100 nm active circuit layer by wet chemical etching. The flexible RF switches for wireless communication were monolithically encapsulated with biocompatible liquid crystal polymers (LCPs) for in vivo bio-medical applications. Finally, they implanted the LCP encapsulated RFICs into live rats to demonstrate the stable operation of flexible devices under in vivo circumstances.
Professor Lee said, "This work could provide an approach to flexible LSI for an ideal artificial retina system and other bio-medical devices. Moreover, the result represents an exciting technology with the strong potential to realize fully flexible consumer electronics such as application processor (AP) for mobile operating system, high-capacity memory, and wireless communication in the near future."
This result was published in the May online issue of the American Chemical Society"s journal, ACS Nano (In vivo Flexible RFICs Monolithically Encapsulated with LCP). They are currently engaged in commercializing efforts of roll-to-roll printing of flexible LSI on large area plastic substrates.
Movie at Youtube Link: Fabrication process for flexible LSI for flexible display, wearable computer and artificial retina for in vivo biomedical application
http://www.youtube.com/watch?v=5PpbM7m2PPs&feature=youtu.be
Applications of in Vivo Flexible Large Scale Integrated Circuits
Top: In vivo flexible large scale integrated circuits (LSI); Bottom: Schematic of roll-to-roll printing of flexible LSI on large area plastics.
2013.06.09 View 15235 -
 Distinguished Professor Sang-Yup Lee received 2013 Amgen Biochemical Engineering Award
- Previous award winners are world-renowned scholars of biochemical engineering including James Bailey, Michael Shuler and Daniel Wang
KAIST Chemical and Biomolecular Engineering Department’s Professor Sang-Yup Lee has been selected to receive the 2013 Amgen Biochemical Engineering Award. The award ceremony will take place this June at the International Biochemical and Molecular Engineering conference in Beijing, China.
The Amgen Biochemical Engineering Award was established by Amgen, a world renowned American pharmaceutical company, in 1993. Amgen awards leading biochemical engineers every two years. The first Amgen award recipient was James Bailey of the California Institute of Technology (Caltech) in 1993. Since then leading engineers that are sometimes called “founding fathers of biochemical engineering” have received the award including MIT Professor Daniel Wang and Michael Shuler of Cornell University.
The first nine award winners were Americans and in 2011 Jens Nielson of Chalmers University of Technology, Sweden, received the Amgen award as a non-American. Professor Sang-Yup Lee is the first Asian to receive the award.
The Amgen award panel said, “Professor Lee made an incredible contribution to the fields of synthetic biology and industrial bioengineering by finding chemical material, fuel, protein and drug production and system bioengineering through metabolic engineering of microorganisms.”
Professor Lee is an expert in metabolic engineering of microorganisms and contributed to the development of system metabolic engineering and system bioengineering. Furthermore, he developed various medical and chemical products and processes which were then applied to synthesise strains of succinate, plastics, butanol and nylon.
Professor Lee is a fellow of the Korean Academy of Science and Technology and National Academy Engineering of Korea; an international member of National Academy of Engineering (US); a former fellow of the American Association for the Advancement of Science; a member of the American Institute of Chemical Engineers, the American Industrial Microbiology Society and American Academy of Microbiology. He is currently Head of Global Agenda Council on Biotechnology and is world renowned for his work in biotechnology field.
2013.04.30 View 9874
Distinguished Professor Sang-Yup Lee received 2013 Amgen Biochemical Engineering Award
- Previous award winners are world-renowned scholars of biochemical engineering including James Bailey, Michael Shuler and Daniel Wang
KAIST Chemical and Biomolecular Engineering Department’s Professor Sang-Yup Lee has been selected to receive the 2013 Amgen Biochemical Engineering Award. The award ceremony will take place this June at the International Biochemical and Molecular Engineering conference in Beijing, China.
The Amgen Biochemical Engineering Award was established by Amgen, a world renowned American pharmaceutical company, in 1993. Amgen awards leading biochemical engineers every two years. The first Amgen award recipient was James Bailey of the California Institute of Technology (Caltech) in 1993. Since then leading engineers that are sometimes called “founding fathers of biochemical engineering” have received the award including MIT Professor Daniel Wang and Michael Shuler of Cornell University.
The first nine award winners were Americans and in 2011 Jens Nielson of Chalmers University of Technology, Sweden, received the Amgen award as a non-American. Professor Sang-Yup Lee is the first Asian to receive the award.
The Amgen award panel said, “Professor Lee made an incredible contribution to the fields of synthetic biology and industrial bioengineering by finding chemical material, fuel, protein and drug production and system bioengineering through metabolic engineering of microorganisms.”
Professor Lee is an expert in metabolic engineering of microorganisms and contributed to the development of system metabolic engineering and system bioengineering. Furthermore, he developed various medical and chemical products and processes which were then applied to synthesise strains of succinate, plastics, butanol and nylon.
Professor Lee is a fellow of the Korean Academy of Science and Technology and National Academy Engineering of Korea; an international member of National Academy of Engineering (US); a former fellow of the American Association for the Advancement of Science; a member of the American Institute of Chemical Engineers, the American Industrial Microbiology Society and American Academy of Microbiology. He is currently Head of Global Agenda Council on Biotechnology and is world renowned for his work in biotechnology field.
2013.04.30 View 9874 -
 Ligand Recognition Mechanism of Protein Identified
Professor Hak-Sung Kim
-“Solved the 50 year old mystery of how protein recognises and binds to ligands”
- Exciting potential for understanding life phenomena and the further development of highly effective therapeutic agent development
KAIST’s Biological Science Department’s Professor Hak-Sung Kim, working in collaboration with Professor Sung-Chul Hong of Department of Physics, Seoul National University, has identified the mechanism of how the protein recognizes and binds to ligands within the human body.
The research findings were published in the online edition of Nature Chemical Biology (March 18), which is the most prestigious journal in the field of life science.
Since the research identified the mechanism, of which protein recognises and binds to ligands, it will take an essential role in understanding complex life phenomenon by understanding regulatory function of protein.
Also, ligand recognition of proteins is closely related to the cause of various diseases. Therefore the research team hopes to contribute to the development of highly effective treatments.
Ligands, well-known examples include nucleic acid and proteins, form the structure of an organism or are essential constituents with special functions such as information signalling.
In particular, the most important role of protein is recognising and binding to a particular ligand and hence regulating and maintaining life phenomena. The abnormal occurrence of an error in recognition of ligands may lead to various diseases.
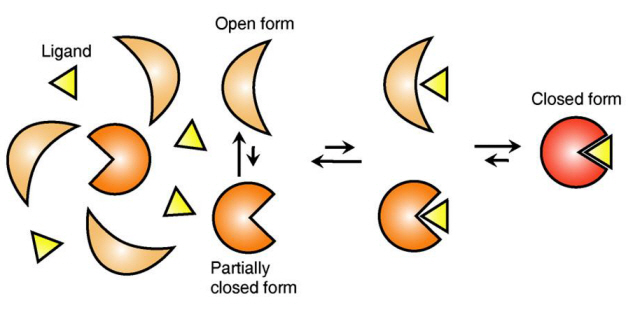
The research team focused on the repetition of change in protein structure from the most stable “open form” to a relatively unstable “partially closed form”.
Professor Kim’s team analysed the change in protein structure when binding to a ligand on a molecular level in real time to explain the ligand recognition mechanism.
The research findings showed that ligands prefer the most stable protein structure. The team was the first in the world to identify that ligands alter protein structure to the most stable, the lowest energy level, when it binds to the protein.
In addition, the team found that ligands bind to unstable partially-closed forms to change protein structure.
The existing models to explain ligand recognition mechanism of protein are “Induced Custom Model”, which involves change in protein structure in binding to ligands, and the “Structure Selection Model”, which argues that ligands select and recognise only the best protein structure out of many. The academic world considers that the team’s research findings have perfectly proved the models through experiments for the first time in the world.
Professor Kim explained, “In the presence of ligands, there exists a phenomenon where the speed of altering protein structure is changed. This phenomenon is analysed on a molecular level to prove ligand recognition mechanism of protein for the first time”. He also said, “The 50-year old mystery, that existed only as a hypothesis on biology textbooks and was thought never to be solved, has been confirmed through experiments for the first time.”
Figure 1: Proteins, with open and partially open form, recognising and binding to ligands.
Figure 2: Ligands temporarily bind to a stable protein structure, open form, which changes into the most stable structure, closed form. In addition, binding to partially closed form also changes protein structure to closed form.
2013.04.01 View 13372
Ligand Recognition Mechanism of Protein Identified
Professor Hak-Sung Kim
-“Solved the 50 year old mystery of how protein recognises and binds to ligands”
- Exciting potential for understanding life phenomena and the further development of highly effective therapeutic agent development
KAIST’s Biological Science Department’s Professor Hak-Sung Kim, working in collaboration with Professor Sung-Chul Hong of Department of Physics, Seoul National University, has identified the mechanism of how the protein recognizes and binds to ligands within the human body.
The research findings were published in the online edition of Nature Chemical Biology (March 18), which is the most prestigious journal in the field of life science.
Since the research identified the mechanism, of which protein recognises and binds to ligands, it will take an essential role in understanding complex life phenomenon by understanding regulatory function of protein.
Also, ligand recognition of proteins is closely related to the cause of various diseases. Therefore the research team hopes to contribute to the development of highly effective treatments.
Ligands, well-known examples include nucleic acid and proteins, form the structure of an organism or are essential constituents with special functions such as information signalling.
In particular, the most important role of protein is recognising and binding to a particular ligand and hence regulating and maintaining life phenomena. The abnormal occurrence of an error in recognition of ligands may lead to various diseases.
The research team focused on the repetition of change in protein structure from the most stable “open form” to a relatively unstable “partially closed form”.
Professor Kim’s team analysed the change in protein structure when binding to a ligand on a molecular level in real time to explain the ligand recognition mechanism.
The research findings showed that ligands prefer the most stable protein structure. The team was the first in the world to identify that ligands alter protein structure to the most stable, the lowest energy level, when it binds to the protein.
In addition, the team found that ligands bind to unstable partially-closed forms to change protein structure.
The existing models to explain ligand recognition mechanism of protein are “Induced Custom Model”, which involves change in protein structure in binding to ligands, and the “Structure Selection Model”, which argues that ligands select and recognise only the best protein structure out of many. The academic world considers that the team’s research findings have perfectly proved the models through experiments for the first time in the world.
Professor Kim explained, “In the presence of ligands, there exists a phenomenon where the speed of altering protein structure is changed. This phenomenon is analysed on a molecular level to prove ligand recognition mechanism of protein for the first time”. He also said, “The 50-year old mystery, that existed only as a hypothesis on biology textbooks and was thought never to be solved, has been confirmed through experiments for the first time.”
Figure 1: Proteins, with open and partially open form, recognising and binding to ligands.
Figure 2: Ligands temporarily bind to a stable protein structure, open form, which changes into the most stable structure, closed form. In addition, binding to partially closed form also changes protein structure to closed form.
2013.04.01 View 13372 -
 KAIST and Saudi Aramco agreed to establish a joint CO2 research center in Korea
The Korea Advanced Institute of Science and Technology (KAIST) and Saudi Aramco, a global energy and petrochemicals enterprise, signed a memorandum of understanding (MOU) on January 6, 2013 in Dhahran, Saudi Arabia and pledged to jointly collaborate in research and development of innovative technologies and solutions to address the world"s energy challenges.
Under the MOU, the two entities agreed to establish a research center, Saudi Aramco-KAIST CO2 Research Center, near KAIST"s main campus in Daejeon, Korea. The research center, to be jointly managed by KAIST and Saudi Aramco, will foster and facilitate research collaborations in areas such as tackling carbon dioxide (CO2) emissions by removal or capture of CO2, conversing CO2 into useful products, developing efficiency improvements in energy production, sharing carbon management technologies, establishing exchange programs, and conducting joint projects.
According to Saudi Aramco, the company"s collaboration with KAIST is the first partnership established in Asia. Khalid A. Al-Falih, President and CEO of Saudi Aramco, said,
"The CO2 Research Center represents a major step in Saudi Aramco"s research and technology strategy to partner with top global institutions to help address and find sustainable solutions to the world’s energy challenge both domestically and internationally."
2013.03.19 View 11532
KAIST and Saudi Aramco agreed to establish a joint CO2 research center in Korea
The Korea Advanced Institute of Science and Technology (KAIST) and Saudi Aramco, a global energy and petrochemicals enterprise, signed a memorandum of understanding (MOU) on January 6, 2013 in Dhahran, Saudi Arabia and pledged to jointly collaborate in research and development of innovative technologies and solutions to address the world"s energy challenges.
Under the MOU, the two entities agreed to establish a research center, Saudi Aramco-KAIST CO2 Research Center, near KAIST"s main campus in Daejeon, Korea. The research center, to be jointly managed by KAIST and Saudi Aramco, will foster and facilitate research collaborations in areas such as tackling carbon dioxide (CO2) emissions by removal or capture of CO2, conversing CO2 into useful products, developing efficiency improvements in energy production, sharing carbon management technologies, establishing exchange programs, and conducting joint projects.
According to Saudi Aramco, the company"s collaboration with KAIST is the first partnership established in Asia. Khalid A. Al-Falih, President and CEO of Saudi Aramco, said,
"The CO2 Research Center represents a major step in Saudi Aramco"s research and technology strategy to partner with top global institutions to help address and find sustainable solutions to the world’s energy challenge both domestically and internationally."
2013.03.19 View 11532 -
 High Efficiency Bio-butanol production technology developed
KAIST and Korean Company cooperative research team has developed the technology that increases the productivity of bio-butanol to equal that of bio-ethanol and decreases the cost of production.
Professor Lee Sang Yeop (Department of Biological-Chemical Engineering) collaborated with GS Caltex and BioFuelChem Ltd. to develop a bio-butanol production process using the system metabolism engineering method that increased the productivity and decreased the production cost.
Bio-butanol is being widely regarded as the environmentally friendly next generation energy source that surpasses bio-ethanol.
The energy density of bio-butanol is 29.9MJ (mega Joule) per Liter, 48% larger than bio-ethanol (19.6MJ) and comparable to gasoline (32MJ). Bio-butanol is advantageous in that it can be processed from inedible biomass and is therefore unrelated to food crises.
Especially because bio-butanol shows similar characteristics especially in its octane rating, enthalpy of vaporization, and air-fuel ratio, it can be used in a gasoline engine.
However barriers such as difficulty in gene manipulation of producer bacterium and insufficient information prevented the mass production of bio-butanol.
Professor Lee’s team applied the system metabolism engineering method that he had invented to shift the focus to the production pathway of bio-butanol and made a new metabolism model.
In the new model the bio-butanol production pathway is divided into the hot channel and the cold channel.
The research team focused on improving the efficiency of the hot channel and succeeded in improving the product yield of 49% (compared to theoretical yield) to 87%.
The team furthered their research and developed a live bio-butanol collection and removal system with GS Caltex. The collaboration succeeded in producing 585g of butanol using 1.8kg of glucose at a rate of 1.3g per hour, boasting world’s highest concentration, productivity, and rate and improving productivity of fermentation by three fold and decreasing costs by 30%.
The result of the research was published in world renowned ‘mBio’ microbiology journal.
2012.12.21 View 10136
High Efficiency Bio-butanol production technology developed
KAIST and Korean Company cooperative research team has developed the technology that increases the productivity of bio-butanol to equal that of bio-ethanol and decreases the cost of production.
Professor Lee Sang Yeop (Department of Biological-Chemical Engineering) collaborated with GS Caltex and BioFuelChem Ltd. to develop a bio-butanol production process using the system metabolism engineering method that increased the productivity and decreased the production cost.
Bio-butanol is being widely regarded as the environmentally friendly next generation energy source that surpasses bio-ethanol.
The energy density of bio-butanol is 29.9MJ (mega Joule) per Liter, 48% larger than bio-ethanol (19.6MJ) and comparable to gasoline (32MJ). Bio-butanol is advantageous in that it can be processed from inedible biomass and is therefore unrelated to food crises.
Especially because bio-butanol shows similar characteristics especially in its octane rating, enthalpy of vaporization, and air-fuel ratio, it can be used in a gasoline engine.
However barriers such as difficulty in gene manipulation of producer bacterium and insufficient information prevented the mass production of bio-butanol.
Professor Lee’s team applied the system metabolism engineering method that he had invented to shift the focus to the production pathway of bio-butanol and made a new metabolism model.
In the new model the bio-butanol production pathway is divided into the hot channel and the cold channel.
The research team focused on improving the efficiency of the hot channel and succeeded in improving the product yield of 49% (compared to theoretical yield) to 87%.
The team furthered their research and developed a live bio-butanol collection and removal system with GS Caltex. The collaboration succeeded in producing 585g of butanol using 1.8kg of glucose at a rate of 1.3g per hour, boasting world’s highest concentration, productivity, and rate and improving productivity of fermentation by three fold and decreasing costs by 30%.
The result of the research was published in world renowned ‘mBio’ microbiology journal.
2012.12.21 View 10136 -
 Distinguished Professor Lee Sang Yeop Appointed as Fellow of the American Institute of Chemical Engineers
Professor Lee Sang Yeop (Dean of the Department of Biological Sciences) has become the first Korea Scientist to be appointed as the Fellow of the American Institute of Chemical Engineers.
The American Institute of Chemical Engineers was founded in 1908 and boasts a 100 year history. It is composed of 43,000 members over 90 countries and is the largest international Academic Institute in the field of Chemical Engineering.
The Institute appoints Fellows after a rigorous procedure of recommendation and evaluation and Professor Lee is the first Korean to become a Fellow.
Professor Lee’s expertise is the field of Metabolic Engineering and successfully applied the system design method and optimization strategy of chemical engineering to biological systems thereby developing numerous core technologies for the biology based chemical industries.
Professor Lee is the founder of the System Metabolic Engineering and enabled the medical application of microorganisms by manipulating the metabolic pathways on a systems level in addition to making great progress in synthesizing various oil originated chemical materials using biology based, environmentally friends methods.
Professor Lee received the Marvin J. Johnson Award, Charles Thom Award, and has been appointed by the first Chairman of the Biotech Global Agenda Counsel of the World Economic Forum.
2012.09.22 View 10569
Distinguished Professor Lee Sang Yeop Appointed as Fellow of the American Institute of Chemical Engineers
Professor Lee Sang Yeop (Dean of the Department of Biological Sciences) has become the first Korea Scientist to be appointed as the Fellow of the American Institute of Chemical Engineers.
The American Institute of Chemical Engineers was founded in 1908 and boasts a 100 year history. It is composed of 43,000 members over 90 countries and is the largest international Academic Institute in the field of Chemical Engineering.
The Institute appoints Fellows after a rigorous procedure of recommendation and evaluation and Professor Lee is the first Korean to become a Fellow.
Professor Lee’s expertise is the field of Metabolic Engineering and successfully applied the system design method and optimization strategy of chemical engineering to biological systems thereby developing numerous core technologies for the biology based chemical industries.
Professor Lee is the founder of the System Metabolic Engineering and enabled the medical application of microorganisms by manipulating the metabolic pathways on a systems level in addition to making great progress in synthesizing various oil originated chemical materials using biology based, environmentally friends methods.
Professor Lee received the Marvin J. Johnson Award, Charles Thom Award, and has been appointed by the first Chairman of the Biotech Global Agenda Counsel of the World Economic Forum.
2012.09.22 View 10569 -
 Production of chemicals without petroleum
Systems metabolic engineering of microorganisms allows efficient production of natural and non-natural chemicals from renewable non-food biomass
In our everyday life, we use gasoline, diesel, plastics, rubbers, and numerous chemicals that are derived from fossil oil through petrochemical refinery processes. However, this is not sustainable due to the limited nature of fossil resources. Furthermore, our world is facing problems associated with climate change and other environmental problems due to the increasing use of fossil resources. One solution to address above problems is the use of renewable non-food biomass for the production of chemicals, fuels and materials through biorefineries. Microorganisms are used as biocatalysts for converting biomass to the products of interest. However, when microorganisms are isolated from nature, their efficiencies of producing our desired chemicals and materials are rather low. Metabolic engineering is thus performed to improve cellular characteristics to desired levels. Over the last decade, much advances have been made in systems biology that allows system-wide characterization of cellular networks, both qualitatively and quantitatively, followed by whole-cell level engineering based on these findings. Furthermore, rapid advances in synthetic biology allow design and synthesis of fine controlled metabolic and gene regulatory circuits. The strategies and methods of systems biology and synthetic biology are rapidly integrated with metabolic engineering, thus resulting in "systems metabolic engineering".
In the paper published online in Nature Chemical Biology on May 17, Professor Sang Yup Lee and his colleagues at the Department of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea present new general strategies of systems metabolic engineering for developing microorganisms for the production of natural and non-natural chemicals from renewable biomass. They first classified the chemicals to be produced into four categories based on whether they have thus far been identified to exist in nature (natural vs. nonnatural) and whether they can be produced by inherent pathways of microorganisms (inherent, noninherent, or created): natural-inherent, natural-noninherent, non-natural-noninherent, and non-natural-created ones. General strategies for systems metabolic engineering of microorganisms for the production of these chemicals using various tools and methods based on omics, genome-scale metabolic modeling and simulation, evolutionary engineering, synthetic biology are suggested with relevant examples. For the production of non-natural chemicals, strategies for the construction of synthetic metabolic pathways are also suggested. Having collected diverse tools and methods for systems metabolic engineering, authors also suggest how to use them and their possible limitations.
Professor Sang Yup Lee said "It is expected that increasing number of chemicals and materials will be produced through biorefineries. We are now equipped with new strategies for developing microbial strains that can produce our desired products at very high efficiencies, thus allowing cost competitiveness to those produced by petrochemical refineries."
Editor of Nature Chemical Biology, Dr. Catherine Goodman, said "It is exciting to see how quickly science is progressing in this field – ideas that used to be science fiction are taking shape in research labs and biorefineries. The article by Professor Lee and his colleagues not only highlights the most advanced techniques and strategies available, but offers critical advice to progress the field as a whole."
The works of Professor Lee have been supported by the Advanced Biomass Center and Intelligent Synthetic Biology Center of Global Frontier Program from the Korean Ministry of Education, Science and Technology through National Research Foundation.
Contact: Dr. Sang Yup Lee, Distinguished Professor and Dean, KAIST, Daejeon, Korea (leesy@kaist.ac.kr, +82-42-350-3930)
2012.05.23 View 15412
Production of chemicals without petroleum
Systems metabolic engineering of microorganisms allows efficient production of natural and non-natural chemicals from renewable non-food biomass
In our everyday life, we use gasoline, diesel, plastics, rubbers, and numerous chemicals that are derived from fossil oil through petrochemical refinery processes. However, this is not sustainable due to the limited nature of fossil resources. Furthermore, our world is facing problems associated with climate change and other environmental problems due to the increasing use of fossil resources. One solution to address above problems is the use of renewable non-food biomass for the production of chemicals, fuels and materials through biorefineries. Microorganisms are used as biocatalysts for converting biomass to the products of interest. However, when microorganisms are isolated from nature, their efficiencies of producing our desired chemicals and materials are rather low. Metabolic engineering is thus performed to improve cellular characteristics to desired levels. Over the last decade, much advances have been made in systems biology that allows system-wide characterization of cellular networks, both qualitatively and quantitatively, followed by whole-cell level engineering based on these findings. Furthermore, rapid advances in synthetic biology allow design and synthesis of fine controlled metabolic and gene regulatory circuits. The strategies and methods of systems biology and synthetic biology are rapidly integrated with metabolic engineering, thus resulting in "systems metabolic engineering".
In the paper published online in Nature Chemical Biology on May 17, Professor Sang Yup Lee and his colleagues at the Department of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea present new general strategies of systems metabolic engineering for developing microorganisms for the production of natural and non-natural chemicals from renewable biomass. They first classified the chemicals to be produced into four categories based on whether they have thus far been identified to exist in nature (natural vs. nonnatural) and whether they can be produced by inherent pathways of microorganisms (inherent, noninherent, or created): natural-inherent, natural-noninherent, non-natural-noninherent, and non-natural-created ones. General strategies for systems metabolic engineering of microorganisms for the production of these chemicals using various tools and methods based on omics, genome-scale metabolic modeling and simulation, evolutionary engineering, synthetic biology are suggested with relevant examples. For the production of non-natural chemicals, strategies for the construction of synthetic metabolic pathways are also suggested. Having collected diverse tools and methods for systems metabolic engineering, authors also suggest how to use them and their possible limitations.
Professor Sang Yup Lee said "It is expected that increasing number of chemicals and materials will be produced through biorefineries. We are now equipped with new strategies for developing microbial strains that can produce our desired products at very high efficiencies, thus allowing cost competitiveness to those produced by petrochemical refineries."
Editor of Nature Chemical Biology, Dr. Catherine Goodman, said "It is exciting to see how quickly science is progressing in this field – ideas that used to be science fiction are taking shape in research labs and biorefineries. The article by Professor Lee and his colleagues not only highlights the most advanced techniques and strategies available, but offers critical advice to progress the field as a whole."
The works of Professor Lee have been supported by the Advanced Biomass Center and Intelligent Synthetic Biology Center of Global Frontier Program from the Korean Ministry of Education, Science and Technology through National Research Foundation.
Contact: Dr. Sang Yup Lee, Distinguished Professor and Dean, KAIST, Daejeon, Korea (leesy@kaist.ac.kr, +82-42-350-3930)
2012.05.23 View 15412 -
 Distinguished Professor Sang-Yeop Lee gave keynote speech in '2011 China Bio-Refinery Summit'
Distinguished Professor Sang-Yeop Lee gave keynote speech in ‘2011 China Bio-Refinery Summit’ held in Chang’an, Beijing
Professor Lee gave a lecture on the vitalization strategy of ‘Bio-Refinery’, which is ‘A bio-based chemical industry to replace fossil fuel-based petro chemistry.
Professor Lee, insisted that for the successful construction of ‘Bio-Refinery’, there should be innovation in all value chain of biomass; biomass producer, bio-refinery business, consumer, government, etc. ▲Securement and distribution of Biomass ▲Development of strain and process for fermentation separation to effectively change biomass into chemical substance and fuel ▲Optimization of transportation and marketing.
During this summit, high-ranking government officials in politics and economics, executives of multicultural and Chinese business participated. From Korea, Do-Young Seung of Manager of technology research of GS and Hang-Deok Roh of laboratory chief of SK Chemical participated as panelist.
World Economy Forum, the gathering of leaders and experts in politics, economics, and policy created a ‘Global Agenda Council’ to find solutions on the issue of ‘sustainable growth of environment of the Earth and humanity’. Professor Lee is the chairperson of ‘Emerging Technologies Global Agenda Council (GAC)’ of Word Economy Forum.
Professor Lee, founder of ‘Systems Metabolic Engineering’, has made remarkable achievements world-wide, including a technology that manipulates metabolic circuit of microorganisms to purify various crude-originated chemical substances into environmentally friendly substances.
Currently, he is working on Systems biology research business in Ministry of Education, Science and Technology, Global Frontier Biomass business, Global Frontier Intelligent Bio-system construction and composition, to make progress in metabolic engineering which is essential for the bio-chemical industry.
2012.03.06 View 13886
Distinguished Professor Sang-Yeop Lee gave keynote speech in '2011 China Bio-Refinery Summit'
Distinguished Professor Sang-Yeop Lee gave keynote speech in ‘2011 China Bio-Refinery Summit’ held in Chang’an, Beijing
Professor Lee gave a lecture on the vitalization strategy of ‘Bio-Refinery’, which is ‘A bio-based chemical industry to replace fossil fuel-based petro chemistry.
Professor Lee, insisted that for the successful construction of ‘Bio-Refinery’, there should be innovation in all value chain of biomass; biomass producer, bio-refinery business, consumer, government, etc. ▲Securement and distribution of Biomass ▲Development of strain and process for fermentation separation to effectively change biomass into chemical substance and fuel ▲Optimization of transportation and marketing.
During this summit, high-ranking government officials in politics and economics, executives of multicultural and Chinese business participated. From Korea, Do-Young Seung of Manager of technology research of GS and Hang-Deok Roh of laboratory chief of SK Chemical participated as panelist.
World Economy Forum, the gathering of leaders and experts in politics, economics, and policy created a ‘Global Agenda Council’ to find solutions on the issue of ‘sustainable growth of environment of the Earth and humanity’. Professor Lee is the chairperson of ‘Emerging Technologies Global Agenda Council (GAC)’ of Word Economy Forum.
Professor Lee, founder of ‘Systems Metabolic Engineering’, has made remarkable achievements world-wide, including a technology that manipulates metabolic circuit of microorganisms to purify various crude-originated chemical substances into environmentally friendly substances.
Currently, he is working on Systems biology research business in Ministry of Education, Science and Technology, Global Frontier Biomass business, Global Frontier Intelligent Bio-system construction and composition, to make progress in metabolic engineering which is essential for the bio-chemical industry.
2012.03.06 View 13886 -
 'Scientist-Engineer of the Month' for December: Professor Choi Joon Ho
Professor Choi Joon Ho (department of Biological Sciences) was made ‘Scientist-Engineer of December’ for his discovery of new gene (twenty-four) that helps biorhythm and proving that this gene helps control biorhythm.
Professor Choi published 100 dissertations over the past 25 years and made significant advancements in the field of molecular virus and neurobiology.
In 1995 Professor Choi uncovered the fact that the NS3 protein in C type hepatitis function as RNA helicase thereby opening the path to developing a cure for C type hepatitis; this is an international patent with Chiron corporation. The result was published in Biochemical and Biophysical Research Communications Journal and was the most domestically referred to dissertation in biological sciences in 1999.
In addition Professor Choi published in Nature magazine in 1999, a dissertation that uncovered the fact that the DNA of papillomar virus has another protein (hSNF5) that direct it apart from ordinary proteins.
In 2000~2005 Professor Choi published many dissertations in journals like Immunity, Cancer Research, Molecular and Cellular Biology, Oncogene, Journal of Virology, and etc.
Professor Choi screened over 10,000 species of pomace fly mutations and discovered the twenty-four gene that affects the biorhythm of pomace flies. He analyzed this gene further and found a new function that was different from known biorhythm mechanisms.
This research allowed a better understanding of biological clock of pomace flies and therefore was another step towards better understanding the control mechanism of human biological clock.
2012.01.31 View 10960
'Scientist-Engineer of the Month' for December: Professor Choi Joon Ho
Professor Choi Joon Ho (department of Biological Sciences) was made ‘Scientist-Engineer of December’ for his discovery of new gene (twenty-four) that helps biorhythm and proving that this gene helps control biorhythm.
Professor Choi published 100 dissertations over the past 25 years and made significant advancements in the field of molecular virus and neurobiology.
In 1995 Professor Choi uncovered the fact that the NS3 protein in C type hepatitis function as RNA helicase thereby opening the path to developing a cure for C type hepatitis; this is an international patent with Chiron corporation. The result was published in Biochemical and Biophysical Research Communications Journal and was the most domestically referred to dissertation in biological sciences in 1999.
In addition Professor Choi published in Nature magazine in 1999, a dissertation that uncovered the fact that the DNA of papillomar virus has another protein (hSNF5) that direct it apart from ordinary proteins.
In 2000~2005 Professor Choi published many dissertations in journals like Immunity, Cancer Research, Molecular and Cellular Biology, Oncogene, Journal of Virology, and etc.
Professor Choi screened over 10,000 species of pomace fly mutations and discovered the twenty-four gene that affects the biorhythm of pomace flies. He analyzed this gene further and found a new function that was different from known biorhythm mechanisms.
This research allowed a better understanding of biological clock of pomace flies and therefore was another step towards better understanding the control mechanism of human biological clock.
2012.01.31 View 10960