somatic+mutation
-
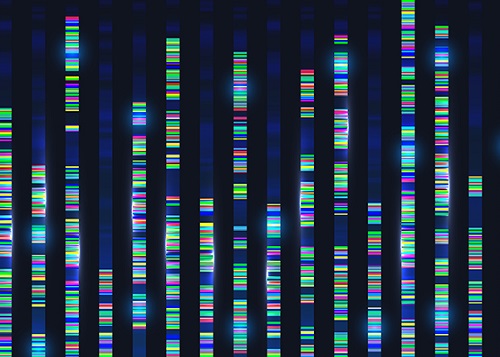 Genomic Data Reveals New Insights into Human Embryonic Development
KAIST researchers have used whole-genome sequencing to track the development from a single fertilized-egg to a human body
Genomic scientists at KAIST have revealed new insights into the process of human embryonic development using large-scale, whole-genome sequencing of cells and tissues from adult humans. The study, published in Nature on Aug.25, is the first to analyse somatic mutations in normal tissue across multiple organs within and between humans.
An adult human body comprises trillions of cells of more than 200 types. How a human develops from a single fertilized egg to a fully grown adult is a fundamental question in biomedical science. Due to the ethical challenges of performing studies on human embryos, however, the details of this process remain largely unknown.
To overcome these issues, the research team took a different approach. They analysed genetic mutations in cells taken from adult human post-mortem tissue. Specifically, they identified mutations that occur spontaneously in early developmental cell divisions. These mutations, also called genomic scars, act like unique genetic fingerprints that can be used to trace the embryonic development process.
The study, which looked at 334 single-cell colonies and 379 tissue samples from seven recently deceased human body donors, is the largest single-cell, whole-genome analysis carried out to date. The researchers examined the genomic scars of each individual in order to reconstruct their early embryonic cellular dynamics.
The result revealed several key characteristics of the human embryonic development process. Firstly, mutation rates are higher in the first cell division, but then decrease to approximately one mutation per cell during later cell division. Secondly, early cells contributed unequally to the development of the embryo in all informative donors, for example, at the two-cell stage, one of the cells always left more progeny cells than the other. The ratio of this was different from person to person, implying that the process varies between individuals and is not fully deterministic.
The researchers were also able to deduce the timing of when cells begin to differentiate into individual organ-specific cells. They found that within three days of fertilization, embryonic cells began to be distributed asymmetrically into tissues for the left and right sides of the body, followed by differentiation into three germ layers, and then differentiation into specific tissues and organs.
“It is an impressive scientific achievement that, within 20 years of the completion of human genome project, genomic technology has advanced to the extent that we are now able to accurately identify mutations in a single-cell genome,” said Professor Young Seok Ju from the Graduate School of Medical Science and Engineering at KAIST. “This technology will enable us to track human embryogenesis at even higher resolutions in the future.”
The techniques used in this study could be used to improve our understanding of rare diseases caused by abnormalities in embryonic development, and to design new precision diagnostics and treatments for patients.
The research was completed in collaboration with Kyungpook National University Hospital, the Korea Institute of Science and Technology Information, Catholic University of Korea School of Medicine, Genome Insights Inc, and Immune Square Inc. This work was supported by the Suh Kyungbae Foundation, the Ministry of Health and Welfare of Korea, the National Research Foundastion of Korea.
-PublicationSeongyeol Park, Nanda Mali, Ryul Kim et al. ‘Clonal dynamics in early human embryogenesis inferred from somatic mutation’ Nature Online ahead of print, Aug. 25, 2021 (https://doi.org/10.1038/s41586-021-03786-8)
-ProfileProfessor Young Seok JuLab of Cancer Genomics (https://www.julab.kaist.ac.kr/)Graduate School of Medical Science and EngineeringKAIST
2021.08.31 View 10266
Genomic Data Reveals New Insights into Human Embryonic Development
KAIST researchers have used whole-genome sequencing to track the development from a single fertilized-egg to a human body
Genomic scientists at KAIST have revealed new insights into the process of human embryonic development using large-scale, whole-genome sequencing of cells and tissues from adult humans. The study, published in Nature on Aug.25, is the first to analyse somatic mutations in normal tissue across multiple organs within and between humans.
An adult human body comprises trillions of cells of more than 200 types. How a human develops from a single fertilized egg to a fully grown adult is a fundamental question in biomedical science. Due to the ethical challenges of performing studies on human embryos, however, the details of this process remain largely unknown.
To overcome these issues, the research team took a different approach. They analysed genetic mutations in cells taken from adult human post-mortem tissue. Specifically, they identified mutations that occur spontaneously in early developmental cell divisions. These mutations, also called genomic scars, act like unique genetic fingerprints that can be used to trace the embryonic development process.
The study, which looked at 334 single-cell colonies and 379 tissue samples from seven recently deceased human body donors, is the largest single-cell, whole-genome analysis carried out to date. The researchers examined the genomic scars of each individual in order to reconstruct their early embryonic cellular dynamics.
The result revealed several key characteristics of the human embryonic development process. Firstly, mutation rates are higher in the first cell division, but then decrease to approximately one mutation per cell during later cell division. Secondly, early cells contributed unequally to the development of the embryo in all informative donors, for example, at the two-cell stage, one of the cells always left more progeny cells than the other. The ratio of this was different from person to person, implying that the process varies between individuals and is not fully deterministic.
The researchers were also able to deduce the timing of when cells begin to differentiate into individual organ-specific cells. They found that within three days of fertilization, embryonic cells began to be distributed asymmetrically into tissues for the left and right sides of the body, followed by differentiation into three germ layers, and then differentiation into specific tissues and organs.
“It is an impressive scientific achievement that, within 20 years of the completion of human genome project, genomic technology has advanced to the extent that we are now able to accurately identify mutations in a single-cell genome,” said Professor Young Seok Ju from the Graduate School of Medical Science and Engineering at KAIST. “This technology will enable us to track human embryogenesis at even higher resolutions in the future.”
The techniques used in this study could be used to improve our understanding of rare diseases caused by abnormalities in embryonic development, and to design new precision diagnostics and treatments for patients.
The research was completed in collaboration with Kyungpook National University Hospital, the Korea Institute of Science and Technology Information, Catholic University of Korea School of Medicine, Genome Insights Inc, and Immune Square Inc. This work was supported by the Suh Kyungbae Foundation, the Ministry of Health and Welfare of Korea, the National Research Foundastion of Korea.
-PublicationSeongyeol Park, Nanda Mali, Ryul Kim et al. ‘Clonal dynamics in early human embryogenesis inferred from somatic mutation’ Nature Online ahead of print, Aug. 25, 2021 (https://doi.org/10.1038/s41586-021-03786-8)
-ProfileProfessor Young Seok JuLab of Cancer Genomics (https://www.julab.kaist.ac.kr/)Graduate School of Medical Science and EngineeringKAIST
2021.08.31 View 10266 -
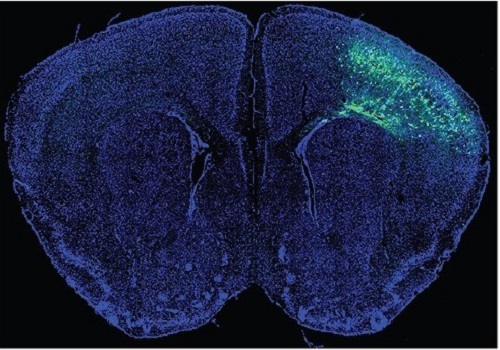 A Mechanism Underlying Most Common Cause of Epileptic Seizures Revealed
An interdisciplinary study shows that neurons carrying somatic mutations in MTOR can lead to focal epileptogenesis via non-cell-autonomous hyperexcitability of nearby nonmutated neurons
During fetal development, cells should migrate to the outer edge of the brain to form critical connections for information transfer and regulation in the body. When even a few cells fail to move to the correct location, the neurons become disorganized and this results in focal cortical dysplasia. This condition is the most common cause of seizures that cannot be controlled with medication in children and the second most common cause in adults.
Now, an interdisciplinary team studying neurogenetics, neural networks, and neurophysiology at KAIST has revealed how dysfunctions in even a small percentage of cells can cause disorder across the entire brain. They published their results on June 28 in Annals of Neurology.
The work builds on a previous finding, also by a KAIST scientists, who found that focal cortical dysplasia was caused by mutations in the cells involved in mTOR, a pathway that regulates signaling between neurons in the brain.
“Only 1 to 2% of neurons carrying mutations in the mTOR signaling pathway that regulates cell signaling in the brain have been found to include seizures in animal models of focal cortical dysplasia,” said Professor Jong-Woo Sohn from the Department of Biological Sciences. “The main challenge of this study was to explain how nearby non-mutated neurons are hyperexcitable.”
Initially, the researchers hypothesized that the mutated cells affected the number of excitatory and inhibitory synapses in all neurons, mutated or not. These neural gates can trigger or halt activity, respectively, in other neurons. Seizures are a result of extreme activity, called hyperexcitability. If the mutated cells upend the balance and result in more excitatory cells, the researchers thought, it made sense that the cells would be more susceptible to hyperexcitability and, as a result, seizures.
“Contrary to our expectations, the synaptic input balance was not changed in either the mutated or non-mutated neurons,” said Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering. “We turned our attention to a protein overproduced by mutated neurons.”
The protein is adenosine kinase, which lowers the concentration of adenosine. This naturally occurring compound is an anticonvulsant and works to relax vessels. In mice engineered to have focal cortical dysplasia, the researchers injected adenosine to replace the levels lowered by the protein. It worked and the neurons became less excitable.
“We demonstrated that augmentation of adenosine signaling could attenuate the excitability of non-mutated neurons,” said Professor Se-Bum Paik from the Department of Bio and Brain Engineering.
The effect on the non-mutated neurons was the surprising part, according to Paik. “The seizure-triggering hyperexcitability originated not in the mutation-carrying neurons, but instead in the nearby non-mutated neurons,” he said.
The mutated neurons excreted more adenosine kinase, reducing the adenosine levels in the local environment of all the cells. With less adenosine, the non-mutated neurons became hyperexcitable, leading to seizures.
“While we need further investigate into the relationship between the concentration of adenosine and the increased excitation of nearby neurons, our results support the medical use of drugs to activate adenosine signaling as a possible treatment pathway for focal cortical dysplasia,” Professor Lee said.
The Suh Kyungbae Foundation, the Korea Health Technology Research and Development Project, the Ministry of Health & Welfare, and the National Research Foundation in Korea funded this work.
-Publication:Koh, H.Y., Jang, J., Ju, S.H., Kim, R., Cho, G.-B., Kim, D.S., Sohn, J.-W., Paik, S.-B. and Lee, J.H. (2021), ‘Non–Cell Autonomous Epileptogenesis in Focal Cortical Dysplasia’ Annals of Neurology, 90: 285 299. (https://doi.org/10.1002/ana.26149)
-ProfileProfessor Jeong Ho Lee Translational Neurogenetics Labhttps://tnl.kaist.ac.kr/ Graduate School of Medical Science and Engineering KAIST
Professor Se-Bum Paik Visual System and Neural Network Laboratory http://vs.kaist.ac.kr/ Department of Bio and Brain EngineeringKAIST
Professor Jong-Woo Sohn Laboratory for Neurophysiology, https://sites.google.com/site/sohnlab2014/home Department of Biological SciencesKAIST
Dr. Hyun Yong Koh Translational Neurogenetics LabGraduate School of Medical Science and EngineeringKAIST
Dr. Jaeson Jang Ph.D.Visual System and Neural Network LaboratoryDepartment of Bio and Brain Engineering KAIST
Sang Hyeon Ju M.D.Laboratory for NeurophysiologyDepartment of Biological SciencesKAIST
2021.08.26 View 15005
A Mechanism Underlying Most Common Cause of Epileptic Seizures Revealed
An interdisciplinary study shows that neurons carrying somatic mutations in MTOR can lead to focal epileptogenesis via non-cell-autonomous hyperexcitability of nearby nonmutated neurons
During fetal development, cells should migrate to the outer edge of the brain to form critical connections for information transfer and regulation in the body. When even a few cells fail to move to the correct location, the neurons become disorganized and this results in focal cortical dysplasia. This condition is the most common cause of seizures that cannot be controlled with medication in children and the second most common cause in adults.
Now, an interdisciplinary team studying neurogenetics, neural networks, and neurophysiology at KAIST has revealed how dysfunctions in even a small percentage of cells can cause disorder across the entire brain. They published their results on June 28 in Annals of Neurology.
The work builds on a previous finding, also by a KAIST scientists, who found that focal cortical dysplasia was caused by mutations in the cells involved in mTOR, a pathway that regulates signaling between neurons in the brain.
“Only 1 to 2% of neurons carrying mutations in the mTOR signaling pathway that regulates cell signaling in the brain have been found to include seizures in animal models of focal cortical dysplasia,” said Professor Jong-Woo Sohn from the Department of Biological Sciences. “The main challenge of this study was to explain how nearby non-mutated neurons are hyperexcitable.”
Initially, the researchers hypothesized that the mutated cells affected the number of excitatory and inhibitory synapses in all neurons, mutated or not. These neural gates can trigger or halt activity, respectively, in other neurons. Seizures are a result of extreme activity, called hyperexcitability. If the mutated cells upend the balance and result in more excitatory cells, the researchers thought, it made sense that the cells would be more susceptible to hyperexcitability and, as a result, seizures.
“Contrary to our expectations, the synaptic input balance was not changed in either the mutated or non-mutated neurons,” said Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering. “We turned our attention to a protein overproduced by mutated neurons.”
The protein is adenosine kinase, which lowers the concentration of adenosine. This naturally occurring compound is an anticonvulsant and works to relax vessels. In mice engineered to have focal cortical dysplasia, the researchers injected adenosine to replace the levels lowered by the protein. It worked and the neurons became less excitable.
“We demonstrated that augmentation of adenosine signaling could attenuate the excitability of non-mutated neurons,” said Professor Se-Bum Paik from the Department of Bio and Brain Engineering.
The effect on the non-mutated neurons was the surprising part, according to Paik. “The seizure-triggering hyperexcitability originated not in the mutation-carrying neurons, but instead in the nearby non-mutated neurons,” he said.
The mutated neurons excreted more adenosine kinase, reducing the adenosine levels in the local environment of all the cells. With less adenosine, the non-mutated neurons became hyperexcitable, leading to seizures.
“While we need further investigate into the relationship between the concentration of adenosine and the increased excitation of nearby neurons, our results support the medical use of drugs to activate adenosine signaling as a possible treatment pathway for focal cortical dysplasia,” Professor Lee said.
The Suh Kyungbae Foundation, the Korea Health Technology Research and Development Project, the Ministry of Health & Welfare, and the National Research Foundation in Korea funded this work.
-Publication:Koh, H.Y., Jang, J., Ju, S.H., Kim, R., Cho, G.-B., Kim, D.S., Sohn, J.-W., Paik, S.-B. and Lee, J.H. (2021), ‘Non–Cell Autonomous Epileptogenesis in Focal Cortical Dysplasia’ Annals of Neurology, 90: 285 299. (https://doi.org/10.1002/ana.26149)
-ProfileProfessor Jeong Ho Lee Translational Neurogenetics Labhttps://tnl.kaist.ac.kr/ Graduate School of Medical Science and Engineering KAIST
Professor Se-Bum Paik Visual System and Neural Network Laboratory http://vs.kaist.ac.kr/ Department of Bio and Brain EngineeringKAIST
Professor Jong-Woo Sohn Laboratory for Neurophysiology, https://sites.google.com/site/sohnlab2014/home Department of Biological SciencesKAIST
Dr. Hyun Yong Koh Translational Neurogenetics LabGraduate School of Medical Science and EngineeringKAIST
Dr. Jaeson Jang Ph.D.Visual System and Neural Network LaboratoryDepartment of Bio and Brain Engineering KAIST
Sang Hyeon Ju M.D.Laboratory for NeurophysiologyDepartment of Biological SciencesKAIST
2021.08.26 View 15005 -
 Rare Mutations May Have Big Impact on Schizophrenia Pathology
- Somatic mutations found only in brain cells disrupt synaptic function. -
Schizophrenia is a neurodevelopmental disorder that disrupts brain activity, producing hallucinations, delusions, and other cognitive disturbances. Researchers have long searched for genetic influences in the disease, but genetic mutations have been identified in only a small fraction—fewer than a quarter—of sequenced patients. Now a study shows that “somatic” gene mutations in brain cells could account for some of the disease’s neuropathology.
The results of the study, led by Professor Jeong Ho Lee at the Graduate School of Medical Science and Engineering in collaboration with the Stanley Medical Research Institute in the US, appeared in Biological Psychiatry.
Traditional genetic mutations, called germline mutations, occur in sperm or egg cells and are passed on to offspring by their parents. Somatic mutations, in contrast, occur in an embryo after fertilization, and they can show up throughout the body or in isolated pockets of tissues, making them much harder to detect from blood or saliva samples, which are typically used for such sequencing studies.
Recently, more-advanced genetic sequencing techniques have allowed researchers to detect somatic mutations and studies have shown that even mutations present at very low levels can have functional consequences. A previous study hinted that brain somatic mutations were associated with schizophrenia, but it was not powerful enough to cement an association between brain somatic mutations and schizophrenia.
In the current study, the researchers used deep whole-exome sequencing to determine the genetic code of all exomes, the parts of genes that encode proteins. The scientists sequenced postmortem samples from brain, liver, spleen, or heart tissue of 27 people with schizophrenia and 31 control participants allowing them to compare the sequences in the two tissues. Using a powerful analytic technique, the team identified an average of 4.9 somatic single-nucleotide variants, or mutations, in brain samples from people with schizophrenia, and 5.6 somatic single-nucleotide variants in brain samples from control subjects.
Although there were no significant quantitative differences in somatic single-nucleotide variants between schizophrenia and control tissue samples, the researchers found that the mutations in schizophrenia patients were found in genes already associated with schizophrenia. Of the germline mutations that had previously been associated with schizophrenia, the genes affected encode proteins associated with synaptic neural communication, particularly in a brain region called the dorsolateral prefrontal cortex.
In the new analysis, the researchers determined which proteins might be affected by the newly identified somatic mutations. Remarkably, a protein called GRIN2B emerged as highly affected and two patients with schizophrenia carried somatic mutations on the GRIN2B gene itself. GRIN2B is a protein component of NMDA-type glutamate receptors, which are critical for neural signaling. Faulty glutamate receptors have long been suspected of contributing to schizophrenia pathology; GRIN2B ranks among the most-studied genes in schizophrenia. The somatic mutations identified in the study had a variant allele frequency of only ~1%, indicating that the mutations were rare among brain cells as a whole. Nevertheless, they have the potential to create widespread cortical dysfunction.
Professor Lee said, “Besides the comprehensive genetic analysis of brain-only mutations in postmortem tissues from schizophrenia patients, this study experimentally showed the biological consequence of identified somatic mutations, which led to neuronal abnormalities associated with schizophrenia. Thus, this study suggests that brain somatic mutations can be a hidden major contributor to schizophrenia and provides new insights into the molecular genetic architecture of schizophrenia.
John Krystal, MD, editor of Biological Psychiatry, said of the work, "The genetics of schizophrenia has received intensive study for several decades. Now a new possibility emerges, that in some cases, mutations in the DNA of brain cells contributes to the biology of schizophrenia. Remarkably this new biology points to an old schizophrenia story: NMDA glutamate receptor dysfunction. Perhaps the path through which somatic mutations contribute to schizophrenia converges with other sources of abnormalities in glutamate signaling in this disorder."
Professor Lee and the team next want to assess the functional consequences of the somatic mutations. Because of the location of the GRIN2B mutations found in schizophrenia patients, the researchers hypothesized that they might interfere with the receptors’ localization on neurons. Experiments on the cortical neurons of mice showed that the mutations indeed disrupted the receptors’ usual localization to dendrites, the “listening” ends of neurons, which in turn prevented the formation of normal synapses in the neurons. This finding suggests that the somatic mutations could disrupt neural communication, contributing to schizophrenia pathology.
- Profile:
Professor Jeong Ho Lee
Translational Neurogenetics Laboratory ( https://tnl.kaist.ac.kr/)
The Graduate School of Medical Science and Engineering
KAIST
(END)
2021.03.11 View 8851
Rare Mutations May Have Big Impact on Schizophrenia Pathology
- Somatic mutations found only in brain cells disrupt synaptic function. -
Schizophrenia is a neurodevelopmental disorder that disrupts brain activity, producing hallucinations, delusions, and other cognitive disturbances. Researchers have long searched for genetic influences in the disease, but genetic mutations have been identified in only a small fraction—fewer than a quarter—of sequenced patients. Now a study shows that “somatic” gene mutations in brain cells could account for some of the disease’s neuropathology.
The results of the study, led by Professor Jeong Ho Lee at the Graduate School of Medical Science and Engineering in collaboration with the Stanley Medical Research Institute in the US, appeared in Biological Psychiatry.
Traditional genetic mutations, called germline mutations, occur in sperm or egg cells and are passed on to offspring by their parents. Somatic mutations, in contrast, occur in an embryo after fertilization, and they can show up throughout the body or in isolated pockets of tissues, making them much harder to detect from blood or saliva samples, which are typically used for such sequencing studies.
Recently, more-advanced genetic sequencing techniques have allowed researchers to detect somatic mutations and studies have shown that even mutations present at very low levels can have functional consequences. A previous study hinted that brain somatic mutations were associated with schizophrenia, but it was not powerful enough to cement an association between brain somatic mutations and schizophrenia.
In the current study, the researchers used deep whole-exome sequencing to determine the genetic code of all exomes, the parts of genes that encode proteins. The scientists sequenced postmortem samples from brain, liver, spleen, or heart tissue of 27 people with schizophrenia and 31 control participants allowing them to compare the sequences in the two tissues. Using a powerful analytic technique, the team identified an average of 4.9 somatic single-nucleotide variants, or mutations, in brain samples from people with schizophrenia, and 5.6 somatic single-nucleotide variants in brain samples from control subjects.
Although there were no significant quantitative differences in somatic single-nucleotide variants between schizophrenia and control tissue samples, the researchers found that the mutations in schizophrenia patients were found in genes already associated with schizophrenia. Of the germline mutations that had previously been associated with schizophrenia, the genes affected encode proteins associated with synaptic neural communication, particularly in a brain region called the dorsolateral prefrontal cortex.
In the new analysis, the researchers determined which proteins might be affected by the newly identified somatic mutations. Remarkably, a protein called GRIN2B emerged as highly affected and two patients with schizophrenia carried somatic mutations on the GRIN2B gene itself. GRIN2B is a protein component of NMDA-type glutamate receptors, which are critical for neural signaling. Faulty glutamate receptors have long been suspected of contributing to schizophrenia pathology; GRIN2B ranks among the most-studied genes in schizophrenia. The somatic mutations identified in the study had a variant allele frequency of only ~1%, indicating that the mutations were rare among brain cells as a whole. Nevertheless, they have the potential to create widespread cortical dysfunction.
Professor Lee said, “Besides the comprehensive genetic analysis of brain-only mutations in postmortem tissues from schizophrenia patients, this study experimentally showed the biological consequence of identified somatic mutations, which led to neuronal abnormalities associated with schizophrenia. Thus, this study suggests that brain somatic mutations can be a hidden major contributor to schizophrenia and provides new insights into the molecular genetic architecture of schizophrenia.
John Krystal, MD, editor of Biological Psychiatry, said of the work, "The genetics of schizophrenia has received intensive study for several decades. Now a new possibility emerges, that in some cases, mutations in the DNA of brain cells contributes to the biology of schizophrenia. Remarkably this new biology points to an old schizophrenia story: NMDA glutamate receptor dysfunction. Perhaps the path through which somatic mutations contribute to schizophrenia converges with other sources of abnormalities in glutamate signaling in this disorder."
Professor Lee and the team next want to assess the functional consequences of the somatic mutations. Because of the location of the GRIN2B mutations found in schizophrenia patients, the researchers hypothesized that they might interfere with the receptors’ localization on neurons. Experiments on the cortical neurons of mice showed that the mutations indeed disrupted the receptors’ usual localization to dendrites, the “listening” ends of neurons, which in turn prevented the formation of normal synapses in the neurons. This finding suggests that the somatic mutations could disrupt neural communication, contributing to schizophrenia pathology.
- Profile:
Professor Jeong Ho Lee
Translational Neurogenetics Laboratory ( https://tnl.kaist.ac.kr/)
The Graduate School of Medical Science and Engineering
KAIST
(END)
2021.03.11 View 8851 -
 Professor J.H. Lee Wins the Innovators in Science Award
Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering won the Early-Career Scientist Award of the 2020 Innovators in Science Award. The New York Academy of Sciences administers the award in partnership with Takeda Pharmaceutical Company.
The Innovators in Science Award grants two prizes of US $200,000 each year: one to an Early-Career Scientist and the other to a well-established Senior Scientist who have distinguished themselves for the creative thinking and impact of their rare disease research. The Senior Scientist Awardee is Dr. Adrian R. Krainer, at Cold Spring Harbor Laboratory whose research focused on the mechanisms and control of RNA splicing.
Prof. Lee is recognized for his research investigating genetic mutations in stem cells in the brain that result in rare developmental brain disorders. He was the first to identify the causes of intractable epilepsies and has identified the genes responsible for several developmental brain disorders, including focal cortical dysplasia, Joubert syndrome—a disorder characterized by an underdevelopment of the brainstem—and hemimegaloencephaly, which is the abnormal enlargement of one side of the brain.
“It is a great honor to be recognized by a jury of such globally respected scientists whom I greatly admire,” said Prof. Lee. “More importantly, this award validates research into brain somatic mutations as an important area of exploration to help patients suffering from devastating and untreatable neurological disorders.”
Prof. Lee also is the Director of the National Creative Research Initiative Center for Brain Somatic Mutations, and Co-founder and Chief Technology Officer of SoVarGen, a biopharmaceutical company aiming to discover novel therapeutics and diagnosis for intractable central nervous system (CNS) diseases caused by low-level somatic mutation.
The Innovators in Science Award is a limited submission competition in which research universities, academic institutions, government or non-profit institutions, or equivalent from around the globe with a well-established record of scientific excellence are invited to nominate their most promising Early-Career Scientists and their most outstanding Senior Scientists working in one of four selected therapeutic fields of neuroscience, gastroenterology, oncology, and regenerative medicine. The 2020 Winners will be honored at the virtual Innovators in Science Award Ceremony and Symposium in October 2020.
2020.07.09 View 11195
Professor J.H. Lee Wins the Innovators in Science Award
Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering won the Early-Career Scientist Award of the 2020 Innovators in Science Award. The New York Academy of Sciences administers the award in partnership with Takeda Pharmaceutical Company.
The Innovators in Science Award grants two prizes of US $200,000 each year: one to an Early-Career Scientist and the other to a well-established Senior Scientist who have distinguished themselves for the creative thinking and impact of their rare disease research. The Senior Scientist Awardee is Dr. Adrian R. Krainer, at Cold Spring Harbor Laboratory whose research focused on the mechanisms and control of RNA splicing.
Prof. Lee is recognized for his research investigating genetic mutations in stem cells in the brain that result in rare developmental brain disorders. He was the first to identify the causes of intractable epilepsies and has identified the genes responsible for several developmental brain disorders, including focal cortical dysplasia, Joubert syndrome—a disorder characterized by an underdevelopment of the brainstem—and hemimegaloencephaly, which is the abnormal enlargement of one side of the brain.
“It is a great honor to be recognized by a jury of such globally respected scientists whom I greatly admire,” said Prof. Lee. “More importantly, this award validates research into brain somatic mutations as an important area of exploration to help patients suffering from devastating and untreatable neurological disorders.”
Prof. Lee also is the Director of the National Creative Research Initiative Center for Brain Somatic Mutations, and Co-founder and Chief Technology Officer of SoVarGen, a biopharmaceutical company aiming to discover novel therapeutics and diagnosis for intractable central nervous system (CNS) diseases caused by low-level somatic mutation.
The Innovators in Science Award is a limited submission competition in which research universities, academic institutions, government or non-profit institutions, or equivalent from around the globe with a well-established record of scientific excellence are invited to nominate their most promising Early-Career Scientists and their most outstanding Senior Scientists working in one of four selected therapeutic fields of neuroscience, gastroenterology, oncology, and regenerative medicine. The 2020 Winners will be honored at the virtual Innovators in Science Award Ceremony and Symposium in October 2020.
2020.07.09 View 11195 -
 Two Professors Recognized for the National R&D Excellence 100
< Professor Haeng-Ki Lee (left) and Professor Jeong-Ho Lee (right) >
Two KAIST professors were listed among the 2019 National R&D Excellence 100 announced by the Ministry of Science and ICT and the Korea Institute of S&T Evaluation and Planning.
Professor Haeng-Ki Lee from the Department of Civil and Environmental Engineering was recognized in the field of mechanics and materials for his research on developing new construction materials through the convergence of nano- and biotechnologies.
In the field of life and marine science, Professor Jeong-Ho Lee from the Graduate School of Medical Science and Engineering was lauded for his research of diagnostic tools and therapies for glioblastoma and pediatric brain tumors.
A certificate from the Minister of Ministry of Science and ICT will be conferred to these two professors, and their names will be inscribed on a special 2019 National R&D Excellence 100 plaque to celebrate their achievements. The professors will also be given privileges during the process of new R&D project selection.
(END)
2019.10.15 View 13848
Two Professors Recognized for the National R&D Excellence 100
< Professor Haeng-Ki Lee (left) and Professor Jeong-Ho Lee (right) >
Two KAIST professors were listed among the 2019 National R&D Excellence 100 announced by the Ministry of Science and ICT and the Korea Institute of S&T Evaluation and Planning.
Professor Haeng-Ki Lee from the Department of Civil and Environmental Engineering was recognized in the field of mechanics and materials for his research on developing new construction materials through the convergence of nano- and biotechnologies.
In the field of life and marine science, Professor Jeong-Ho Lee from the Graduate School of Medical Science and Engineering was lauded for his research of diagnostic tools and therapies for glioblastoma and pediatric brain tumors.
A certificate from the Minister of Ministry of Science and ICT will be conferred to these two professors, and their names will be inscribed on a special 2019 National R&D Excellence 100 plaque to celebrate their achievements. The professors will also be given privileges during the process of new R&D project selection.
(END)
2019.10.15 View 13848 -
 Deciphering Brain Somatic Mutations Associated with Alzheimer's Disease
Researchers have found a potential link between non-inherited somatic mutations in the brain and the progression of Alzheimer’s disease
Researchers have identified somatic mutations in the brain that could contribute to the development of Alzheimer’s disease (AD). Their findings were published in the journal Nature Communications last week.
Decades worth of research has identified inherited mutations that lead to early-onset familial AD. Inherited mutations, however, are behind at most half the cases of late onset sporadic AD, in which there is no family history of the disease. But the genetic factors causing the other half of these sporadic cases have been unclear.
Professor Jeong Ho Lee at the Graduate School of Medical Science and Engineering and colleagues analysed the DNA present in post-mortem hippocampal formations and in blood samples from people aged 70 to 96 with AD and age-matched controls. They specifically looked for non-inherited somatic mutations in their brains using high-depth whole exome sequencing.
The team developed a bioinformatics pipeline that enabled them to detect low-level brain somatic single nucleotide variations (SNVs) – mutations that involve the substitution of a single nucleotide with another nucleotide. Brain somatic SNVs have been reported on and accumulate throughout our lives and can sometimes be associated with a range of neurological diseases.
The number of somatic SNVs did not differ between individuals with AD and non-demented controls. Interestingly, somatic SNVs in AD brains arise about 4.8 times more slowly than in blood. When the team performed gene-set enrichment tests, 26.9 percent of the AD brain samples had pathogenic brain somatic SNVs known to be linked to hyperphosphorylation of tau proteins, which is one of major hallmarks of AD.
Then, they pinpointed a pathogenic SNV in the PIN1 gene, a cis/trans isomerase that balances phosphorylation in tau proteins, found in one AD patient’s brain. They found the mutation was 4.9 time more abundant in AT8-positive – a marker for hyper-phosphorylated tau proteins– neurons in the entorhinal cortex than the bulk hippocampal tissue. Furthermore, in a series of functional assays, they observed the mutation causing a loss of function in PIN1 and such haploinsufficiency increased the phosphorylation and aggregation of tau proteins.
“Our study provides new insights into the molecular genetic factors behind Alzheimer’s disease and other neurodegenerative diseases potentially linked to somatic mutations in the brain,” said Professor Lee.
The team is planning to expand their study to a larger cohort in order to establish stronger links between these brain somatic mutations and the pathogenesis of Alzheimer’s disease.
(Figure 1. Bioinformatic pipeline for detecting low-level brain somatic mutations in AD and non-AD.)
(Figure 2. Pathogenic brain somatic mutations associated with tau phosphorylation are significantly enriched in AD brains.)
(Figure 3. A pathogenic brain somatic mutation in PIN1 (c. 477 C>T) is a loss-of-function and related functional assays show its haploinsufficiency increases phosphorylation and aggregation of tau.)
2019.07.19 View 37389
Deciphering Brain Somatic Mutations Associated with Alzheimer's Disease
Researchers have found a potential link between non-inherited somatic mutations in the brain and the progression of Alzheimer’s disease
Researchers have identified somatic mutations in the brain that could contribute to the development of Alzheimer’s disease (AD). Their findings were published in the journal Nature Communications last week.
Decades worth of research has identified inherited mutations that lead to early-onset familial AD. Inherited mutations, however, are behind at most half the cases of late onset sporadic AD, in which there is no family history of the disease. But the genetic factors causing the other half of these sporadic cases have been unclear.
Professor Jeong Ho Lee at the Graduate School of Medical Science and Engineering and colleagues analysed the DNA present in post-mortem hippocampal formations and in blood samples from people aged 70 to 96 with AD and age-matched controls. They specifically looked for non-inherited somatic mutations in their brains using high-depth whole exome sequencing.
The team developed a bioinformatics pipeline that enabled them to detect low-level brain somatic single nucleotide variations (SNVs) – mutations that involve the substitution of a single nucleotide with another nucleotide. Brain somatic SNVs have been reported on and accumulate throughout our lives and can sometimes be associated with a range of neurological diseases.
The number of somatic SNVs did not differ between individuals with AD and non-demented controls. Interestingly, somatic SNVs in AD brains arise about 4.8 times more slowly than in blood. When the team performed gene-set enrichment tests, 26.9 percent of the AD brain samples had pathogenic brain somatic SNVs known to be linked to hyperphosphorylation of tau proteins, which is one of major hallmarks of AD.
Then, they pinpointed a pathogenic SNV in the PIN1 gene, a cis/trans isomerase that balances phosphorylation in tau proteins, found in one AD patient’s brain. They found the mutation was 4.9 time more abundant in AT8-positive – a marker for hyper-phosphorylated tau proteins– neurons in the entorhinal cortex than the bulk hippocampal tissue. Furthermore, in a series of functional assays, they observed the mutation causing a loss of function in PIN1 and such haploinsufficiency increased the phosphorylation and aggregation of tau proteins.
“Our study provides new insights into the molecular genetic factors behind Alzheimer’s disease and other neurodegenerative diseases potentially linked to somatic mutations in the brain,” said Professor Lee.
The team is planning to expand their study to a larger cohort in order to establish stronger links between these brain somatic mutations and the pathogenesis of Alzheimer’s disease.
(Figure 1. Bioinformatic pipeline for detecting low-level brain somatic mutations in AD and non-AD.)
(Figure 2. Pathogenic brain somatic mutations associated with tau phosphorylation are significantly enriched in AD brains.)
(Figure 3. A pathogenic brain somatic mutation in PIN1 (c. 477 C>T) is a loss-of-function and related functional assays show its haploinsufficiency increases phosphorylation and aggregation of tau.)
2019.07.19 View 37389 -
 Professor Ju, to Receive Grants from HFSP
(Professor Young Seok Ju)
Professor Young Seok Ju from the Graduate School of Medical Science and Engineering was selected as a young investigator to receive research funds from the Human Frontiers Science Program.
The Human Frontiers Science Program (HFSP) was founded in 1989 with members of the G7 and European Union to stimulate innovative research in the field of life sciences.
Professor Ju placed third out of the eight teams that were selected from 158 applicants representing 60 countries. He is now the fourth Korean to receive a research grant as a young investigator. Professor Jae Kyoung Kim from the Department of Mathematical Sciences also received this prize last year, hence KAIST has produced grant recipients for two consecutive years.
Professor Ju is a medical doctor specializing in cancer genomics and computer biology. He has been studying somatic mutations and their functional consequences in human cancer in a bioinformatics way. He has published papers in international journals including Nature, Science, Genome Research, and Journal of Clinical Oncology.
With a title ‘Tracing AID/APOBEC- and MSI-mediated hyper-mutagenesis in the clonal evolution of gastric cancer,’ Professor Ju will receive 1.05 million dollars for three years along with Professor Bon-Kyoung Koo from the Institute of Molecular Biotechnology at Austrian Academy of Sciences, and Sinppert Hugo from University Medical Center Utrecht.
Professor Ju said, “As a young investigator, it is my great honor to receive this research fund from this organization. Through this internationally collaborative research, I will carry out groundbreaking research to understand the pathophysiology of cancers at a molecular level.”
2018.04.24 View 10021
Professor Ju, to Receive Grants from HFSP
(Professor Young Seok Ju)
Professor Young Seok Ju from the Graduate School of Medical Science and Engineering was selected as a young investigator to receive research funds from the Human Frontiers Science Program.
The Human Frontiers Science Program (HFSP) was founded in 1989 with members of the G7 and European Union to stimulate innovative research in the field of life sciences.
Professor Ju placed third out of the eight teams that were selected from 158 applicants representing 60 countries. He is now the fourth Korean to receive a research grant as a young investigator. Professor Jae Kyoung Kim from the Department of Mathematical Sciences also received this prize last year, hence KAIST has produced grant recipients for two consecutive years.
Professor Ju is a medical doctor specializing in cancer genomics and computer biology. He has been studying somatic mutations and their functional consequences in human cancer in a bioinformatics way. He has published papers in international journals including Nature, Science, Genome Research, and Journal of Clinical Oncology.
With a title ‘Tracing AID/APOBEC- and MSI-mediated hyper-mutagenesis in the clonal evolution of gastric cancer,’ Professor Ju will receive 1.05 million dollars for three years along with Professor Bon-Kyoung Koo from the Institute of Molecular Biotechnology at Austrian Academy of Sciences, and Sinppert Hugo from University Medical Center Utrecht.
Professor Ju said, “As a young investigator, it is my great honor to receive this research fund from this organization. Through this internationally collaborative research, I will carry out groundbreaking research to understand the pathophysiology of cancers at a molecular level.”
2018.04.24 View 10021