EEG
-
 Seegene Opens Covid-19 Testing Mobile Station on Campus
Seegene donates testing reagents for 40,000 people with results available in three hours
Seegene, a molecular diagnostic testing company, donated enough testing reagents for 40,000 COVID-19 tests for the KAIST community and set up a mobile testing station run by the Seegene Medical Foundation on October 28.
The entire COVID-19 diagnosis process, including specimen collection, PCR testing, and results analysis, can be conducted at the mobile testing unit developed by Seegene. The on-site testing station will help the campus get ready to return to normal, especially as the government is transitioning toward its ‘living with Covid-19’ policy, which eases a range of social distancing restrictions. Any KAIST community member can get a Covid-19 test on campus and receive the results within three hours. The station can conduct up to 7,500 tests per day.
This is an extension of the agreement between KAIST and Seegene made in July for research collaboration. The two institutions will work together on various research projects including ultrafast PCR testing, sample collection, and cloud-based data transmission and analysis.
Prior to this donation, according to an administrative order from Daejeon City, KAIST opened a temporary COVID-19 testing center in collaboration with Seegene and conducted COVID-19 tests at the KAIST Clinic over four days starting from September 28. All students living on campus were tested, and all 2,775 tested negative.
Seegene CEO Jong-Yoon Chun said, "KAIST and Seegene signed an agreement for collaborative research on molecular diagnosis in July prior to this donation, and we are happy to maintain a connection with KAIST.” He added, “We hope that this donation will help students return to their ordinary university lives.”
Vice President for Planning and Budget Bowon Kim said, "As KAIST is currently planning to conduct offline lectures in preparation for ‘living with COVID-19’, Seegene’s donation will be particularly helpful.” He added, “The two institutions will continue to cooperate, leading to not only the short-term stabilization of the campus, but also collaborative research for the vitalization of molecular diagnosis technology and the bio industry.”
2021.11.03 View 5968
Seegene Opens Covid-19 Testing Mobile Station on Campus
Seegene donates testing reagents for 40,000 people with results available in three hours
Seegene, a molecular diagnostic testing company, donated enough testing reagents for 40,000 COVID-19 tests for the KAIST community and set up a mobile testing station run by the Seegene Medical Foundation on October 28.
The entire COVID-19 diagnosis process, including specimen collection, PCR testing, and results analysis, can be conducted at the mobile testing unit developed by Seegene. The on-site testing station will help the campus get ready to return to normal, especially as the government is transitioning toward its ‘living with Covid-19’ policy, which eases a range of social distancing restrictions. Any KAIST community member can get a Covid-19 test on campus and receive the results within three hours. The station can conduct up to 7,500 tests per day.
This is an extension of the agreement between KAIST and Seegene made in July for research collaboration. The two institutions will work together on various research projects including ultrafast PCR testing, sample collection, and cloud-based data transmission and analysis.
Prior to this donation, according to an administrative order from Daejeon City, KAIST opened a temporary COVID-19 testing center in collaboration with Seegene and conducted COVID-19 tests at the KAIST Clinic over four days starting from September 28. All students living on campus were tested, and all 2,775 tested negative.
Seegene CEO Jong-Yoon Chun said, "KAIST and Seegene signed an agreement for collaborative research on molecular diagnosis in July prior to this donation, and we are happy to maintain a connection with KAIST.” He added, “We hope that this donation will help students return to their ordinary university lives.”
Vice President for Planning and Budget Bowon Kim said, "As KAIST is currently planning to conduct offline lectures in preparation for ‘living with COVID-19’, Seegene’s donation will be particularly helpful.” He added, “The two institutions will continue to cooperate, leading to not only the short-term stabilization of the campus, but also collaborative research for the vitalization of molecular diagnosis technology and the bio industry.”
2021.11.03 View 5968 -
 New Nanoparticle Drug Combination For Atherosclerosis
Physicochemical cargo-switching nanoparticles (CSNP) designed by KAIST can help significantly reduce cholesterol and macrophage foam cells in arteries, which are the two main triggers for atherosclerotic plaque and inflammation.
The CSNP-based combination drug delivery therapy was proved to exert cholesterol-lowering, anti-inflammatory, and anti-proliferative functions of two common medications for treating and preventing atherosclerosis that are cyclodextrin and statin. Professor Ji-Ho Park and Dr. Heegon Kim from KAIST’s Department of Bio and Brain Engineering said their study has shown great potential for future applications with reduced side effects.
Atherosclerosis is a chronic inflammatory vascular disease that is characterized by the accumulation of cholesterol and cholesterol-loaded macrophage foam cells in the intima. When this atherosclerotic plaque clogs and narrows the artery walls, they restrict blood flow and cause various cardiovascular conditions such as heart attacks and strokes. Heart attacks and strokes are the world’s first and fifth causes of death respectively.
Oral statin administration has been used in clinics as a standard care for atherosclerosis, which is prescribed to lower blood cholesterol and inhibit its accumulation within the plaque. Although statins can effectively prevent the progression of plaque growth, they have only shown modest efficacy in eliminating the already-established plaque. Therefore, patients are required to take statin drugs for the rest of their lives and will always carry the risk of plaque ruptures that can trigger a blood clot.
To address these issues, Professor Park and Dr. Kim exploited another antiatherogenic agent called cyclodextrin. In their paper published in the Journal of Controlled Release on March 10, Professor Park and Dr. Kim reported that the polymeric formulation of cyclodextrin with a diameter of approximately 10 nanometers(nm) can accumulate within the atherosclerotic plaque 14 times more and effectively reduce the plaque even at lower doses, compared to cyclodextrin in a non-polymer structure.
Moreover, although cyclodextrin is known to have a cytotoxic effect on hair cells in the cochlea, which can lead to hearing loss, cyclodextrin polymers developed by Professor Park’s research group exhibited a varying biodistribution profile and did not have this side effect.
In the follow-up study reported in ACS Nano on April 28, the researchers exploited both cyclodextrin and statin and form the cyclodextrin-statin self-assembly drug complex, based on previous findings that each drug can exert local anti-atherosclerosis effect within the plaque. The complex formation processes were optimized to obtain homogeneous and stable nanoparticles with a diameter of about 100 nm for systematic injection.
The therapeutic synergy of cyclodextrin and statin could reportedly enhance plaque-targeted drug delivery and anti-inflammation. Cyclodextrin led to the regression of cholesterol in the established plaque, and the statins were shown to inhibit the proliferation of macrophage foam cells. The study suggested that combination therapy is required to resolve the complex inflammatory cholesterol-rich microenvironment within the plaque.
Professor Park said, “While nanomedicine has been mainly developed for the treatment of cancers, our studies show that nanomedicine can also play a significant role in treating and preventing atherosclerosis, which causes various cardiovascular diseases that are the leading causes of death worldwide.”
This work was supported by KAIST and the National Research Foundation (NRF) of Korea.
Publications:
1. Heegon Kim, Junhee Han, and Ji-Ho Park. (2020) ‘Cyclodextrin polymer improves atherosclerosis therapy and reduces ototoxicity’ Journal of Controlled Release. Volume 319. Page 77-86. Available online at https://doi.org/10.1016/j.jconrel.2019.12.021
2. Kim, H., et al. (2020) ‘Affinity-Driven Design of Cargo-Switching Nanoparticles to Leverage a Cholesterol-Rich Microenvironment for Atherosclerosis Therapy’ ACS Nano. Available online at https://doi.org/10.1021/acsnano.9b08216
Profile: Ji-Ho Park, Ph.D.
Associate Professor
jihopark@kaist.ac.kr
http://openwetware.org/wiki/Park_Lab
Biomaterials Engineering Laboratory (BEL)
Department of Bio and Brain Engineering (BIOENG)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Heegon Kim, Ph.D.
Postdoctoral Researcher
heegon@kaist.ac.kr
BEL, BIOENG, KAIST
(END)
2020.06.16 View 13430
New Nanoparticle Drug Combination For Atherosclerosis
Physicochemical cargo-switching nanoparticles (CSNP) designed by KAIST can help significantly reduce cholesterol and macrophage foam cells in arteries, which are the two main triggers for atherosclerotic plaque and inflammation.
The CSNP-based combination drug delivery therapy was proved to exert cholesterol-lowering, anti-inflammatory, and anti-proliferative functions of two common medications for treating and preventing atherosclerosis that are cyclodextrin and statin. Professor Ji-Ho Park and Dr. Heegon Kim from KAIST’s Department of Bio and Brain Engineering said their study has shown great potential for future applications with reduced side effects.
Atherosclerosis is a chronic inflammatory vascular disease that is characterized by the accumulation of cholesterol and cholesterol-loaded macrophage foam cells in the intima. When this atherosclerotic plaque clogs and narrows the artery walls, they restrict blood flow and cause various cardiovascular conditions such as heart attacks and strokes. Heart attacks and strokes are the world’s first and fifth causes of death respectively.
Oral statin administration has been used in clinics as a standard care for atherosclerosis, which is prescribed to lower blood cholesterol and inhibit its accumulation within the plaque. Although statins can effectively prevent the progression of plaque growth, they have only shown modest efficacy in eliminating the already-established plaque. Therefore, patients are required to take statin drugs for the rest of their lives and will always carry the risk of plaque ruptures that can trigger a blood clot.
To address these issues, Professor Park and Dr. Kim exploited another antiatherogenic agent called cyclodextrin. In their paper published in the Journal of Controlled Release on March 10, Professor Park and Dr. Kim reported that the polymeric formulation of cyclodextrin with a diameter of approximately 10 nanometers(nm) can accumulate within the atherosclerotic plaque 14 times more and effectively reduce the plaque even at lower doses, compared to cyclodextrin in a non-polymer structure.
Moreover, although cyclodextrin is known to have a cytotoxic effect on hair cells in the cochlea, which can lead to hearing loss, cyclodextrin polymers developed by Professor Park’s research group exhibited a varying biodistribution profile and did not have this side effect.
In the follow-up study reported in ACS Nano on April 28, the researchers exploited both cyclodextrin and statin and form the cyclodextrin-statin self-assembly drug complex, based on previous findings that each drug can exert local anti-atherosclerosis effect within the plaque. The complex formation processes were optimized to obtain homogeneous and stable nanoparticles with a diameter of about 100 nm for systematic injection.
The therapeutic synergy of cyclodextrin and statin could reportedly enhance plaque-targeted drug delivery and anti-inflammation. Cyclodextrin led to the regression of cholesterol in the established plaque, and the statins were shown to inhibit the proliferation of macrophage foam cells. The study suggested that combination therapy is required to resolve the complex inflammatory cholesterol-rich microenvironment within the plaque.
Professor Park said, “While nanomedicine has been mainly developed for the treatment of cancers, our studies show that nanomedicine can also play a significant role in treating and preventing atherosclerosis, which causes various cardiovascular diseases that are the leading causes of death worldwide.”
This work was supported by KAIST and the National Research Foundation (NRF) of Korea.
Publications:
1. Heegon Kim, Junhee Han, and Ji-Ho Park. (2020) ‘Cyclodextrin polymer improves atherosclerosis therapy and reduces ototoxicity’ Journal of Controlled Release. Volume 319. Page 77-86. Available online at https://doi.org/10.1016/j.jconrel.2019.12.021
2. Kim, H., et al. (2020) ‘Affinity-Driven Design of Cargo-Switching Nanoparticles to Leverage a Cholesterol-Rich Microenvironment for Atherosclerosis Therapy’ ACS Nano. Available online at https://doi.org/10.1021/acsnano.9b08216
Profile: Ji-Ho Park, Ph.D.
Associate Professor
jihopark@kaist.ac.kr
http://openwetware.org/wiki/Park_Lab
Biomaterials Engineering Laboratory (BEL)
Department of Bio and Brain Engineering (BIOENG)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Heegon Kim, Ph.D.
Postdoctoral Researcher
heegon@kaist.ac.kr
BEL, BIOENG, KAIST
(END)
2020.06.16 View 13430 -
 Cooperative Tumor Cell Membrane-Targeted Phototherapy
A KAIST research team led by Professor Ji-Ho Park in the Bio and Brain Engineering Department at KAIST developed a technology for the effective treatment of cancer by delivering synthetic receptors throughout tumor tissue. The study, led by Ph.D. candidate Heegon Kim, was published online in Nature Communications on June 19.
Cancer targeted therapy generally refers to therapy targeting specific molecules that are involved in the growth and generation of cancer. The targeted delivery of therapeutics using targeting agents such as antibodies or nanomaterials has improved the precision and safety of cancer therapy.
However, the paucity and heterogeneity of identified molecular targets within tumors have resulted in poor and uneven distribution of targeted agents, thus compromising treatment outcomes.
To solve this problem, the team constructed a cooperative targeting system in which synthetic and biological nanocomponents participate together in the tumor cell membrane-selective localization of synthetic receptors to amplify the subsequent targeting of therapeutics. Here, synthetic and biological nanocomponents refer to liposomes and extracellular vesicles, respectively.
The synthetic receptors are first delivered selectively to tumor cell membranes in the perivascular region using liposomes. By hitchhiking with extracellular vesicles secreted by the cells, the synthetic receptors are transferred to neighboring cells and further spread throughout the tumor tissues where the molecular targets are limited.
Hitchhiking extracellular vesicles for delivery of synthetic receptors was possible since extracellular vesicles, such as exosomes, mediate intercellular communications by transferring various biological components such as lipids, cytosolic proteins, and RNA through a membrane fusion process. They also play a supportive role in promoting tumor progression in that tumor-derived extracellular vesicles deliver oncogenic signals to normal host cells.
The team showed that this tumor cell membrane-targeted delivery of synthetic receptors led to a uniform distribution of synthetic receptors throughout a tumor and subsequently led to enhanced phototherapeutic efficacy of the targeted photosensitizer.
Professor Park said, “The cooperative tumor targeting system is expected to be applied in treating various diseases that are hard to target.”
The research was funded by the Basic Science Research Program through the National Research Foundation funded by the Ministry of Science, ICT & Future Planning, and the National R&D Program for Cancer Control funded by the Ministry for Health and Welfare.
(Ph.D. candidates Hee Gon Kim (left) and Chanhee Oh)
Figure 1. A schematic of a cooperative tumor targeting system via delivery of synthetic receptors.
Figure 2. A confocal microscopic image of a tumor section after cooperative targeting by synthetic receptor delivery. Green and magenta represent vessels and therapeutic agents inside a tumor respectively.
2017.07.07 View 10825
Cooperative Tumor Cell Membrane-Targeted Phototherapy
A KAIST research team led by Professor Ji-Ho Park in the Bio and Brain Engineering Department at KAIST developed a technology for the effective treatment of cancer by delivering synthetic receptors throughout tumor tissue. The study, led by Ph.D. candidate Heegon Kim, was published online in Nature Communications on June 19.
Cancer targeted therapy generally refers to therapy targeting specific molecules that are involved in the growth and generation of cancer. The targeted delivery of therapeutics using targeting agents such as antibodies or nanomaterials has improved the precision and safety of cancer therapy.
However, the paucity and heterogeneity of identified molecular targets within tumors have resulted in poor and uneven distribution of targeted agents, thus compromising treatment outcomes.
To solve this problem, the team constructed a cooperative targeting system in which synthetic and biological nanocomponents participate together in the tumor cell membrane-selective localization of synthetic receptors to amplify the subsequent targeting of therapeutics. Here, synthetic and biological nanocomponents refer to liposomes and extracellular vesicles, respectively.
The synthetic receptors are first delivered selectively to tumor cell membranes in the perivascular region using liposomes. By hitchhiking with extracellular vesicles secreted by the cells, the synthetic receptors are transferred to neighboring cells and further spread throughout the tumor tissues where the molecular targets are limited.
Hitchhiking extracellular vesicles for delivery of synthetic receptors was possible since extracellular vesicles, such as exosomes, mediate intercellular communications by transferring various biological components such as lipids, cytosolic proteins, and RNA through a membrane fusion process. They also play a supportive role in promoting tumor progression in that tumor-derived extracellular vesicles deliver oncogenic signals to normal host cells.
The team showed that this tumor cell membrane-targeted delivery of synthetic receptors led to a uniform distribution of synthetic receptors throughout a tumor and subsequently led to enhanced phototherapeutic efficacy of the targeted photosensitizer.
Professor Park said, “The cooperative tumor targeting system is expected to be applied in treating various diseases that are hard to target.”
The research was funded by the Basic Science Research Program through the National Research Foundation funded by the Ministry of Science, ICT & Future Planning, and the National R&D Program for Cancer Control funded by the Ministry for Health and Welfare.
(Ph.D. candidates Hee Gon Kim (left) and Chanhee Oh)
Figure 1. A schematic of a cooperative tumor targeting system via delivery of synthetic receptors.
Figure 2. A confocal microscopic image of a tumor section after cooperative targeting by synthetic receptor delivery. Green and magenta represent vessels and therapeutic agents inside a tumor respectively.
2017.07.07 View 10825 -
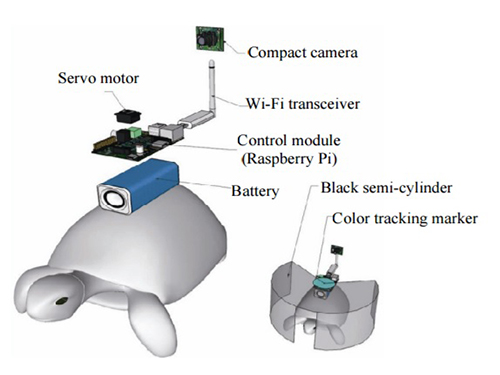 Controlling Turtle Motion with Human Thought
KAIST researchers have developed a technology that can remotely control an animal’s movement with human thought.
In the 2009 blockbuster “Avatar,” a human remotely controls the body of an alien. It does so by injecting human intelligence into a remotely located, biological body. Although still in the realm of science fiction, researchers are nevertheless developing so-called ‘brain-computer interfaces’ (BCIs) following recent advances in electronics and computing. These technologies can ‘read’ and use human thought to control machines, for example, humanoid robots.
New research has demonstrated the possibility of combining a BCI with a device that transmits information from a computer to a brain, or known as a ‘computer-to-brain interface’ (CBI). The combination of these devices could be used to establish a functional link between the brains of different species. Now, researchers from the Korea Advanced Institute of Science and Technology (KAIST) have developed a human-turtle interaction system in which a signal originating from a human brain can affect where a turtle moves.
Unlike previous research that has tried to control animal movement by applying invasive methods, most notably in insects, Professors Phill-Seung Lee of the Mechanical Engineering Department and Sungho Jo of the Computing School propose a conceptual system that can guide an animal’s moving path by controlling its instinctive escape behavior. They chose a turtle because of its cognitive abilities as well as its ability to distinguish different wavelengths of light. Specifically, turtles can recognize a white light source as an open space and so move toward it. They also show specific avoidance behavior to things that might obstruct their view. Turtles also move toward and away from obstacles in their environment in a predictable manner. It was this instinctive, predictable behavior that the researchers induced using the BCI.
The entire human-turtle setup is as follows: A head-mounted display (HMD) is combined with a BCI to immerse the human user in the turtle’s environment. The human operator wears the BCI-HMD system, while the turtle has a 'cyborg system'—consisting of a camera, Wi-Fi transceiver, computer control module, and battery—all mounted on the turtle’s upper shell. Also included on the turtle’s shell is a black semi-cylinder with a slit, which forms the ‘stimulation device.’ This can be turned ±36 degrees via the BCI.
The entire process works like this: the human operator receives images from the camera mounted on the turtle. These real-time video images allow the human operator to decide where the turtle should move. The human provides thought commands that are recognized by the wearable BCI system as electroencephalography (EEG) signals. The BCI can distinguish between three mental states: left, right, and idle. The left and right commands activate the turtle’s stimulation device via Wi-Fi, turning it so that it obstructs the turtle’s view. This invokes its natural instinct to move toward light and change its direction. Finally, the human acquires updated visual feedback from the camera mounted on the shell and in this way continues to remotely navigate the turtle’s trajectory.
The research demonstrates that the animal guiding scheme via BCI can be used in a variety of environments with turtles moving indoors and outdoors on many different surfaces, like gravel and grass, and tackling a range of obstacles, such as shallow water and trees. This technology could be developed to integrate positioning systems and improved augmented and virtual reality techniques, enabling various applications, including devices for military reconnaissance and surveillance.
***
Reference: “Remote Navigation of Turtle by Controlling Instinct Behavior via Human Brain-computer Interface,” Journal of Bionic Engineering, July 2016 (DOI: 10.1016/S1672-6529(16)60322-0)
Depiction of Cyborg System
A human controller influences the turtle’s escape behavior by sending left and right signals via Wi-Fi to a control system on the back of the turtle.
2017.02.21 View 14906
Controlling Turtle Motion with Human Thought
KAIST researchers have developed a technology that can remotely control an animal’s movement with human thought.
In the 2009 blockbuster “Avatar,” a human remotely controls the body of an alien. It does so by injecting human intelligence into a remotely located, biological body. Although still in the realm of science fiction, researchers are nevertheless developing so-called ‘brain-computer interfaces’ (BCIs) following recent advances in electronics and computing. These technologies can ‘read’ and use human thought to control machines, for example, humanoid robots.
New research has demonstrated the possibility of combining a BCI with a device that transmits information from a computer to a brain, or known as a ‘computer-to-brain interface’ (CBI). The combination of these devices could be used to establish a functional link between the brains of different species. Now, researchers from the Korea Advanced Institute of Science and Technology (KAIST) have developed a human-turtle interaction system in which a signal originating from a human brain can affect where a turtle moves.
Unlike previous research that has tried to control animal movement by applying invasive methods, most notably in insects, Professors Phill-Seung Lee of the Mechanical Engineering Department and Sungho Jo of the Computing School propose a conceptual system that can guide an animal’s moving path by controlling its instinctive escape behavior. They chose a turtle because of its cognitive abilities as well as its ability to distinguish different wavelengths of light. Specifically, turtles can recognize a white light source as an open space and so move toward it. They also show specific avoidance behavior to things that might obstruct their view. Turtles also move toward and away from obstacles in their environment in a predictable manner. It was this instinctive, predictable behavior that the researchers induced using the BCI.
The entire human-turtle setup is as follows: A head-mounted display (HMD) is combined with a BCI to immerse the human user in the turtle’s environment. The human operator wears the BCI-HMD system, while the turtle has a 'cyborg system'—consisting of a camera, Wi-Fi transceiver, computer control module, and battery—all mounted on the turtle’s upper shell. Also included on the turtle’s shell is a black semi-cylinder with a slit, which forms the ‘stimulation device.’ This can be turned ±36 degrees via the BCI.
The entire process works like this: the human operator receives images from the camera mounted on the turtle. These real-time video images allow the human operator to decide where the turtle should move. The human provides thought commands that are recognized by the wearable BCI system as electroencephalography (EEG) signals. The BCI can distinguish between three mental states: left, right, and idle. The left and right commands activate the turtle’s stimulation device via Wi-Fi, turning it so that it obstructs the turtle’s view. This invokes its natural instinct to move toward light and change its direction. Finally, the human acquires updated visual feedback from the camera mounted on the shell and in this way continues to remotely navigate the turtle’s trajectory.
The research demonstrates that the animal guiding scheme via BCI can be used in a variety of environments with turtles moving indoors and outdoors on many different surfaces, like gravel and grass, and tackling a range of obstacles, such as shallow water and trees. This technology could be developed to integrate positioning systems and improved augmented and virtual reality techniques, enabling various applications, including devices for military reconnaissance and surveillance.
***
Reference: “Remote Navigation of Turtle by Controlling Instinct Behavior via Human Brain-computer Interface,” Journal of Bionic Engineering, July 2016 (DOI: 10.1016/S1672-6529(16)60322-0)
Depiction of Cyborg System
A human controller influences the turtle’s escape behavior by sending left and right signals via Wi-Fi to a control system on the back of the turtle.
2017.02.21 View 14906