Environmnet
-
 Adsorbent That Can Selectively Remove Water Contaminants
Professor Cafer T. Yavuz and his team at the Graduate School of Energy, Environment, Water, and Sustainability (EEWS) have developed an adsorbent that can selectively capture soluble organic contaminants in water.
This water treatment adsorbent is a fluorine-based nanoporous polymer that can selectively remove water-soluble micromolecules. It has the added advantage of being cheap and easily synthesized, while also being renewable.
The results of this research have been published online in Nature Communication on November 10, 2016. The research paper is titled “Charge-specific Size-dependent Separation of Water-soluble Organic Molecules by Fluorinated Nanoporous Networks.” (DOI: 10.1038/ncomms13377)
Water pollution is accelerating as a result of global industrial development and warming. As new materials are produced and applied in the agricultural and industrial sectors, the types of contaminants expelled as sewage and waste water are also becoming diverse.
Chemicals such as dyes and pesticides can be especially harmful because they are made up of small and highly soluble organic particles that cannot be completely removed during the water treatment process, ultimately ending up in our drinking water.
The current conventional water treatment systems utilize processes such as activated carbon, ozonolysis, and reverse osmosis membrane. These processes, however, are designed to remove larger organic molecules with lower solubility, thus removal of very small molecules with high solubility is difficult. In addition, these micromolecules tend to be charged, therefore are less easily separated in aqueous form.
The research team aimed to remove these small molecules using a new adsorbent technology.
In order to remove aqueous organic molecular contaminants, the team needed an adsorbent that can adsorb micro-sized molecules. It also needed to introduce a chemical function that would allow it to selectively adsorb molecules, and lastly, the adsorbent needed to be structurally stable as it would be used underwater.
The team subsequently developed an adsorbent of fluorine-based porous organic polymer that met all the conditions listed above. By controlling the size of the pores, this adsorbent is able to selectively adsorb aqueous micromolecules of less than 1-2 nm in size.
In addition, in order to separate specific contaminants, there should be a chemical functionality, such as the ability to strongly interact with the target material. Fluorine, the most electronegative atom, interacts strongly with charged soluble organic molecules.
The research team incorporated fluorine into an adsorbent, enabling it to separate charged organic molecules up to 8 times faster than neutral molecules.
The adsorbent developed by Professor Yavuz’s team has wide industrial applications. It can be used in batch-adsorption tests, as well as in column separation for size- and charge-specific adsorption.
Professor Yavuz stated that “the charge-selective properties displayed by fluorine has the potential to be applied in desalination or water treatment processes using membranes."
This paper was first-authored by Dr. Jeehye Byun, and the research was funded by KAIST’s High Risk High Return Program and the Ministry of Science, ICT and Future Planning of Korea’s Mid-Career Researcher Program, as well as its Technology Development Program to Solve Climate Change.
Figure 1. Diagram conceptualizing the process of charge- and size-specific separation by the fluorine-based porous polymer adsorbent
Figure 2. Difference in absorbance before and after using a porous fluorine polymer column to separate organic molecules
Figure 3. Adsorption properties of a fluorine polymer according to the charge and size of organic molecules
2017.01.17 View 11100
Adsorbent That Can Selectively Remove Water Contaminants
Professor Cafer T. Yavuz and his team at the Graduate School of Energy, Environment, Water, and Sustainability (EEWS) have developed an adsorbent that can selectively capture soluble organic contaminants in water.
This water treatment adsorbent is a fluorine-based nanoporous polymer that can selectively remove water-soluble micromolecules. It has the added advantage of being cheap and easily synthesized, while also being renewable.
The results of this research have been published online in Nature Communication on November 10, 2016. The research paper is titled “Charge-specific Size-dependent Separation of Water-soluble Organic Molecules by Fluorinated Nanoporous Networks.” (DOI: 10.1038/ncomms13377)
Water pollution is accelerating as a result of global industrial development and warming. As new materials are produced and applied in the agricultural and industrial sectors, the types of contaminants expelled as sewage and waste water are also becoming diverse.
Chemicals such as dyes and pesticides can be especially harmful because they are made up of small and highly soluble organic particles that cannot be completely removed during the water treatment process, ultimately ending up in our drinking water.
The current conventional water treatment systems utilize processes such as activated carbon, ozonolysis, and reverse osmosis membrane. These processes, however, are designed to remove larger organic molecules with lower solubility, thus removal of very small molecules with high solubility is difficult. In addition, these micromolecules tend to be charged, therefore are less easily separated in aqueous form.
The research team aimed to remove these small molecules using a new adsorbent technology.
In order to remove aqueous organic molecular contaminants, the team needed an adsorbent that can adsorb micro-sized molecules. It also needed to introduce a chemical function that would allow it to selectively adsorb molecules, and lastly, the adsorbent needed to be structurally stable as it would be used underwater.
The team subsequently developed an adsorbent of fluorine-based porous organic polymer that met all the conditions listed above. By controlling the size of the pores, this adsorbent is able to selectively adsorb aqueous micromolecules of less than 1-2 nm in size.
In addition, in order to separate specific contaminants, there should be a chemical functionality, such as the ability to strongly interact with the target material. Fluorine, the most electronegative atom, interacts strongly with charged soluble organic molecules.
The research team incorporated fluorine into an adsorbent, enabling it to separate charged organic molecules up to 8 times faster than neutral molecules.
The adsorbent developed by Professor Yavuz’s team has wide industrial applications. It can be used in batch-adsorption tests, as well as in column separation for size- and charge-specific adsorption.
Professor Yavuz stated that “the charge-selective properties displayed by fluorine has the potential to be applied in desalination or water treatment processes using membranes."
This paper was first-authored by Dr. Jeehye Byun, and the research was funded by KAIST’s High Risk High Return Program and the Ministry of Science, ICT and Future Planning of Korea’s Mid-Career Researcher Program, as well as its Technology Development Program to Solve Climate Change.
Figure 1. Diagram conceptualizing the process of charge- and size-specific separation by the fluorine-based porous polymer adsorbent
Figure 2. Difference in absorbance before and after using a porous fluorine polymer column to separate organic molecules
Figure 3. Adsorption properties of a fluorine polymer according to the charge and size of organic molecules
2017.01.17 View 11100 -
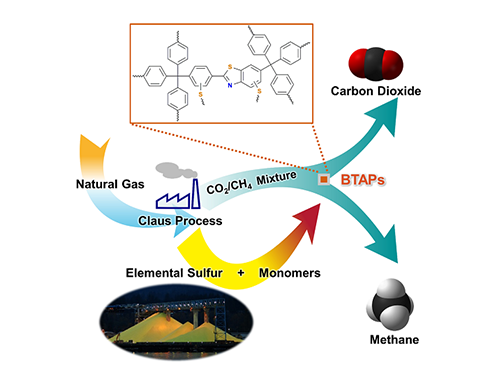 Direct Utilization of Elemental Sulfur for Microporous Polymer Synthesis
Using elemental sulfur as an alternative chemical feedstock, KAIST researchers have produced novel microporous polymers to sift CO2 from methane in natural-gas processing.
Methane, a primary component of natural gas, has emerged recently as an important energy source, largely owing to its abundance and relatively clean nature compared with other fossil fuels. In order to use natural gas as a fuel, however, it must undergo a procedure called “hydrodesulfurization” or “natural gas sweetening” to reduce sulfur-dioxide emissions from combustion of fossil fuels. This process leads to excessive and involuntary production of elemental sulfur. Although sulfur is one of the world’s most versatile and common elements, it has relatively few large-scale applications, mostly for gunpowder and sulfuric acid production.
Thus, the development of synthetic and processing methods to convert sulfur into useful chemicals remains a challenge. A research team led by Professor Ali Coskun from the Graduate School of EEWS (Energy, Environment, Water and Sustainability) at Korea Advanced Institute of Science and Technology (KAIST) has recently introduced a new approach to resolving this problem by employing elemental sulfur directly in the synthesis of microporous polymers for the process of natural-gas sweetening.
Natural gas, containing varying amounts of carbon dioxide (CO2) and hydrogen sulfide (H2S), is generally treated with amine solutions, followed by the regeneration of these solutions at increased temperatures to release captured CO2 and H2S. A two-step separation is involved in removing these gases. The amine solutions first remove H2S, and then CO2 is separated from methane (CH4) with either amine solutions or porous sorbents such as microporous polymers.
Using elemental sulfur and organic linkers, the research team developed a solvent and catalyst-free strategy for the synthesis of ultramicroporous benzothiazole polymers (BTAPs) in quantitative yields. BTAPs were found to be highly porous and showed exceptional physiochemical stability. In-situ chemical impregnation of sulfur within the micropores increased CO2 affinity of the sorbent, while limiting diffusion of CH4. BTAPs, as low-cost, scalable solid-sorbents, showed outstanding CO2 separation ability for flue gas, as well as for natural and landfill gas conditions.
The team noted that: “Each year, millions of tons of elemental sulfur are generated as a by-product of petroleum refining and natural-gas processing, but industries and businesses lacked good ideas for using it. Our research provides a solution: the direct utilization of elemental sulfur into the synthesis of ultramicroporous polymers that can be recycled back into an efficient and sustainable process for CO2 separation. Our novel polymeric materials offer new possibilities for the application of a little-used natural resource, sulfur, to provide a sustainable solution to challenging environmental issues.”
This work was published online in Chem on September 8, 2016 and also highlighted in C&EN (Chemical & Engineering News) by the American Chemical Society (ACS) on September 19, 2016. The research paper was entitled “Direct Utilization of Elemental Sulfur in the Synthesis of Microporous Polymers for Natural Gas Sweetening.” (DOI: 10.1016/j.chempr.2016.08.003)
Figure 1. A Schematic Image of Direct Utilization of Elemental Sulfur
This image shows direct utilization of elemental sulfur in the synthesis of microporous polymers and its gas separation performance.
Figure 2. BTAP’s Breakthrough Experiment under Pre-mixed Gas Conditions
This data presents the breakthrough measurements for CO2-containing binary gas-mixture streams with different feed-gas compositions to investigate the CO2 capture capacity of ultramicroporous benzothiazole polymers (BTAPs) for large-scale applications under simulated conditions of natural and landfill gases.
2016.10.05 View 9652
Direct Utilization of Elemental Sulfur for Microporous Polymer Synthesis
Using elemental sulfur as an alternative chemical feedstock, KAIST researchers have produced novel microporous polymers to sift CO2 from methane in natural-gas processing.
Methane, a primary component of natural gas, has emerged recently as an important energy source, largely owing to its abundance and relatively clean nature compared with other fossil fuels. In order to use natural gas as a fuel, however, it must undergo a procedure called “hydrodesulfurization” or “natural gas sweetening” to reduce sulfur-dioxide emissions from combustion of fossil fuels. This process leads to excessive and involuntary production of elemental sulfur. Although sulfur is one of the world’s most versatile and common elements, it has relatively few large-scale applications, mostly for gunpowder and sulfuric acid production.
Thus, the development of synthetic and processing methods to convert sulfur into useful chemicals remains a challenge. A research team led by Professor Ali Coskun from the Graduate School of EEWS (Energy, Environment, Water and Sustainability) at Korea Advanced Institute of Science and Technology (KAIST) has recently introduced a new approach to resolving this problem by employing elemental sulfur directly in the synthesis of microporous polymers for the process of natural-gas sweetening.
Natural gas, containing varying amounts of carbon dioxide (CO2) and hydrogen sulfide (H2S), is generally treated with amine solutions, followed by the regeneration of these solutions at increased temperatures to release captured CO2 and H2S. A two-step separation is involved in removing these gases. The amine solutions first remove H2S, and then CO2 is separated from methane (CH4) with either amine solutions or porous sorbents such as microporous polymers.
Using elemental sulfur and organic linkers, the research team developed a solvent and catalyst-free strategy for the synthesis of ultramicroporous benzothiazole polymers (BTAPs) in quantitative yields. BTAPs were found to be highly porous and showed exceptional physiochemical stability. In-situ chemical impregnation of sulfur within the micropores increased CO2 affinity of the sorbent, while limiting diffusion of CH4. BTAPs, as low-cost, scalable solid-sorbents, showed outstanding CO2 separation ability for flue gas, as well as for natural and landfill gas conditions.
The team noted that: “Each year, millions of tons of elemental sulfur are generated as a by-product of petroleum refining and natural-gas processing, but industries and businesses lacked good ideas for using it. Our research provides a solution: the direct utilization of elemental sulfur into the synthesis of ultramicroporous polymers that can be recycled back into an efficient and sustainable process for CO2 separation. Our novel polymeric materials offer new possibilities for the application of a little-used natural resource, sulfur, to provide a sustainable solution to challenging environmental issues.”
This work was published online in Chem on September 8, 2016 and also highlighted in C&EN (Chemical & Engineering News) by the American Chemical Society (ACS) on September 19, 2016. The research paper was entitled “Direct Utilization of Elemental Sulfur in the Synthesis of Microporous Polymers for Natural Gas Sweetening.” (DOI: 10.1016/j.chempr.2016.08.003)
Figure 1. A Schematic Image of Direct Utilization of Elemental Sulfur
This image shows direct utilization of elemental sulfur in the synthesis of microporous polymers and its gas separation performance.
Figure 2. BTAP’s Breakthrough Experiment under Pre-mixed Gas Conditions
This data presents the breakthrough measurements for CO2-containing binary gas-mixture streams with different feed-gas compositions to investigate the CO2 capture capacity of ultramicroporous benzothiazole polymers (BTAPs) for large-scale applications under simulated conditions of natural and landfill gases.
2016.10.05 View 9652 -
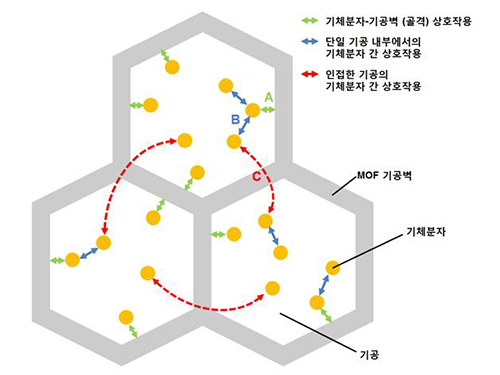 A New Way to Look at MOFs
An international research team composed of researchers from KAIST (led by Professors Osamu Terasaki and Jeung Ku Kang at the Graduate School of Energy, Environment, Water and Sustainability) and other universities, including UC Berkeley, has recently published research results on the adsorption process of metal-organic frameworks (MOFs) in Nature (November 9, 2015).
MOFs are porous three-dimensional crystals with a high internal surface area, which have a wide range of applications involving adsorption such as hydrogen, methane, or carbon dioxide storage. In the paper entitled “Extra Adsorption and Adsorbate Superlattice Formation in Metal-organic Frameworks,” the research team described their observation of a very specific interpore interaction process in MOFs.
For additional information, please see:
A New Way to Look at MOFs
International study challenges prevailing view on how metal organic frameworks store gases
EurekAlert, November 9, 2015
http://www.eurekalert.org/pub_releases/2015-11/dbnl-anw110915.php
(Courtesy of the US Department of Energy and Lawrence Berkeley National Laboratory news release)
2015.11.13 View 8963
A New Way to Look at MOFs
An international research team composed of researchers from KAIST (led by Professors Osamu Terasaki and Jeung Ku Kang at the Graduate School of Energy, Environment, Water and Sustainability) and other universities, including UC Berkeley, has recently published research results on the adsorption process of metal-organic frameworks (MOFs) in Nature (November 9, 2015).
MOFs are porous three-dimensional crystals with a high internal surface area, which have a wide range of applications involving adsorption such as hydrogen, methane, or carbon dioxide storage. In the paper entitled “Extra Adsorption and Adsorbate Superlattice Formation in Metal-organic Frameworks,” the research team described their observation of a very specific interpore interaction process in MOFs.
For additional information, please see:
A New Way to Look at MOFs
International study challenges prevailing view on how metal organic frameworks store gases
EurekAlert, November 9, 2015
http://www.eurekalert.org/pub_releases/2015-11/dbnl-anw110915.php
(Courtesy of the US Department of Energy and Lawrence Berkeley National Laboratory news release)
2015.11.13 View 8963 -
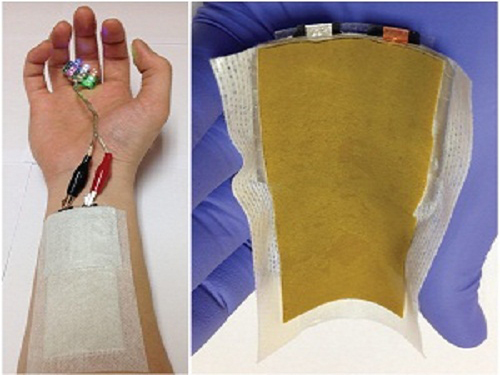 KAIST Develops a Credit-Card-Thick Flexible Lithium Ion Battery
Since the battery can be charged wirelessly, useful applications are expected including medical patches and smart cards.
Professor Jang Wook Choi at KAIST’s Graduate School of Energy, Environment, Water, and Sustainability (EEWS) and Dr. Jae Yong Song at the Korea Research Institute of Standards and Science jointly led research to invent a flexible lithium ion battery that is thinner than a credit card and can be charged wirelessly.
Their research findings were published online in Nano Letters on March 6, 2015. Lithium ion batteries are widely used today in various electronics including mobile devices and electronic cars. Researchers said that their work could help accelerate the development of flexible and wearable electronics.
Conventional lithium ion batteries are manufactured based on a layering technology, stacking up anodes, separating films, and cathodes like a sandwich, which makes it difficult to reduce their thickness. In addition, friction arises between layers, making the batteries impossible to bend. The coating films of electrodes easily come off, which contributes to the batteries’ poor performance.
The research team abandoned the existing production technology. Instead, they removed the separating films, layered the cathodes and anodes collinearly on a plane, and created a partition between electrodes to eliminate potential problems, such as short circuits and voltage dips, commonly present in lithium ion batteries.
After more than five thousand consecutive flexing experiments, the research team confirmed the possibility of a more flexible electrode structure while maintaining the battery performance comparable to the level of current lithium ion batteries.
Flexible batteries can be applied to integrated smart cards, cosmetic and medical patches, and skin adhesive sensors that can control a computer with voice commands or gesture as seen in the movie “Iron Man.”
Moreover, the team has successfully developed wireless-charging technology using electromagnetic induction and solar batteries.
They are currently developing a mass production process to combine this planar battery technology and printing, to ultimately create a new paradigm to print semiconductors and batteries using 3D printers.
Professor Choi said, “This new technology will contribute to diversifying patch functions as it is applicable to power various adhesive medical patches.”
Picture 1: Medical patch (left) and flexible secondary battery (right)
Picture 2: Diagram of flexible battery
Picture 3: Smart card embedding flexible battery
2015.03.24 View 13729
KAIST Develops a Credit-Card-Thick Flexible Lithium Ion Battery
Since the battery can be charged wirelessly, useful applications are expected including medical patches and smart cards.
Professor Jang Wook Choi at KAIST’s Graduate School of Energy, Environment, Water, and Sustainability (EEWS) and Dr. Jae Yong Song at the Korea Research Institute of Standards and Science jointly led research to invent a flexible lithium ion battery that is thinner than a credit card and can be charged wirelessly.
Their research findings were published online in Nano Letters on March 6, 2015. Lithium ion batteries are widely used today in various electronics including mobile devices and electronic cars. Researchers said that their work could help accelerate the development of flexible and wearable electronics.
Conventional lithium ion batteries are manufactured based on a layering technology, stacking up anodes, separating films, and cathodes like a sandwich, which makes it difficult to reduce their thickness. In addition, friction arises between layers, making the batteries impossible to bend. The coating films of electrodes easily come off, which contributes to the batteries’ poor performance.
The research team abandoned the existing production technology. Instead, they removed the separating films, layered the cathodes and anodes collinearly on a plane, and created a partition between electrodes to eliminate potential problems, such as short circuits and voltage dips, commonly present in lithium ion batteries.
After more than five thousand consecutive flexing experiments, the research team confirmed the possibility of a more flexible electrode structure while maintaining the battery performance comparable to the level of current lithium ion batteries.
Flexible batteries can be applied to integrated smart cards, cosmetic and medical patches, and skin adhesive sensors that can control a computer with voice commands or gesture as seen in the movie “Iron Man.”
Moreover, the team has successfully developed wireless-charging technology using electromagnetic induction and solar batteries.
They are currently developing a mass production process to combine this planar battery technology and printing, to ultimately create a new paradigm to print semiconductors and batteries using 3D printers.
Professor Choi said, “This new technology will contribute to diversifying patch functions as it is applicable to power various adhesive medical patches.”
Picture 1: Medical patch (left) and flexible secondary battery (right)
Picture 2: Diagram of flexible battery
Picture 3: Smart card embedding flexible battery
2015.03.24 View 13729