Environment
-
 Enhanced Natural Gas Storage to Help Reduce Global Warming
< Professor Atilhan (left) and Professor Yavuz (right) >
Researchers have designed plastic-based materials that can store natural gas more effectively. These new materials can not only make large-scale, cost-effective, and safe natural gas storage possible, but further hold a strong promise for combating global warming.
Natural gas (predominantly methane) is a clean energy alternative. It is stored by compression, liquefaction, or adsorption. Among these, adsorbed natural gas (ANG) storage is a more efficient, cheaper, and safer alternative to conventional compressed natural gas (CNG) and liquefied natural gas (LNG) storage approaches that have drawbacks such as low storage efficiency, high costs, and safety concerns. However, developing adsorptive materials that can more fully exploit the advantages of ANG storage has remained a challenging task.
A KAIST research team led by Professor Cafer T. Yavuz from the Graduate School of Energy, Environment, Water, and Sustainability (EEWS), in collaboration with Professor Mert Atilhan’s group from Texas A&M University, synthesized 29 unique porous polymeric structures with inherent flexibility, and tested their methane gas uptake capacity at high pressures. These porous polymers had varying synthetic complexities, porosities, and morphologies, and the researchers subjected each porous polymer to pure methane gas under various conditions to study the ANG performances.
Of these 29 distinct chemical structures, COP-150 was particularly noteworthy as it achieved a high deliverable gravimetric methane working capacity when cycled between 5 and 100 bar at 273 K, which is 98% of the total uptake capacity. This result surpassed the target set by the United States Department of Energy (US DOE).
COP-150 is the first ever structure to fulfil both the gravimetric and volumetric requirements of the US DOE for successful vehicular use, and the total cost to produce the COP-150 adsorbent was only 1 USD per kilogram.
COP-150 can be produced using freely available and easily accessible plastic materials, and moreover, its synthesis takes place at room temperature, open to the air, and no previous purification of the chemicals is required. The pressure-triggered flexible structure of COP-150 is also advantageous in terms of the total working capacity of deliverable methane for real applications.
The research team believed that the increased pressure flexes the network structure of COP-150 showing “swelling” behavior, and suggested that the flexibility provides rapid desorption and thermal management, while the hydrophobicity and the nature of the covalently bonded framework allow these promising materials to tolerate harsh conditions.
This swelling mechanism of expansion-contraction solves two other major issues, the team noted. Firstly, when using adsorbents based on such a mechanism, unsafe pressure spikes that may occur due to temperature swings can be eliminated. In addition, contamination can also be minimized, since the adsorbent remains contracted when no gas is stored.
Professor Yavuz said, “We envision a whole host of new designs and mechanisms to be developed based on our concept. Since natural gas is a much cleaner fuel than coal and petroleum, new developments in this realm will help switching to the use of less polluting fuels.”
Professor Atilhan agreed the most important impact of their research is on the environment. “Using natural gas more than coal and petroleum will significantly reduce greenhouse gas emissions. We believe, one day, we might see vehicles equipped with our materials that are run by a cleaner natural gas fuel,” he added.
This study, reported in Nature Energy on July 8, was supported by National Research Foundation of Korea (NRF) grants ( NRF-2016R1A2B4011027, NRF-2017M3A7B4042140, and NRF-2017M3A7B4042235).
< Suggested chemical structure of COP-150 >
< Initial ingredients (left) and final product (right) of COP-150 synthesis >
< Comparison of highest reported volumetric working capacities >
(END)
2019.08.09 View 26746
Enhanced Natural Gas Storage to Help Reduce Global Warming
< Professor Atilhan (left) and Professor Yavuz (right) >
Researchers have designed plastic-based materials that can store natural gas more effectively. These new materials can not only make large-scale, cost-effective, and safe natural gas storage possible, but further hold a strong promise for combating global warming.
Natural gas (predominantly methane) is a clean energy alternative. It is stored by compression, liquefaction, or adsorption. Among these, adsorbed natural gas (ANG) storage is a more efficient, cheaper, and safer alternative to conventional compressed natural gas (CNG) and liquefied natural gas (LNG) storage approaches that have drawbacks such as low storage efficiency, high costs, and safety concerns. However, developing adsorptive materials that can more fully exploit the advantages of ANG storage has remained a challenging task.
A KAIST research team led by Professor Cafer T. Yavuz from the Graduate School of Energy, Environment, Water, and Sustainability (EEWS), in collaboration with Professor Mert Atilhan’s group from Texas A&M University, synthesized 29 unique porous polymeric structures with inherent flexibility, and tested their methane gas uptake capacity at high pressures. These porous polymers had varying synthetic complexities, porosities, and morphologies, and the researchers subjected each porous polymer to pure methane gas under various conditions to study the ANG performances.
Of these 29 distinct chemical structures, COP-150 was particularly noteworthy as it achieved a high deliverable gravimetric methane working capacity when cycled between 5 and 100 bar at 273 K, which is 98% of the total uptake capacity. This result surpassed the target set by the United States Department of Energy (US DOE).
COP-150 is the first ever structure to fulfil both the gravimetric and volumetric requirements of the US DOE for successful vehicular use, and the total cost to produce the COP-150 adsorbent was only 1 USD per kilogram.
COP-150 can be produced using freely available and easily accessible plastic materials, and moreover, its synthesis takes place at room temperature, open to the air, and no previous purification of the chemicals is required. The pressure-triggered flexible structure of COP-150 is also advantageous in terms of the total working capacity of deliverable methane for real applications.
The research team believed that the increased pressure flexes the network structure of COP-150 showing “swelling” behavior, and suggested that the flexibility provides rapid desorption and thermal management, while the hydrophobicity and the nature of the covalently bonded framework allow these promising materials to tolerate harsh conditions.
This swelling mechanism of expansion-contraction solves two other major issues, the team noted. Firstly, when using adsorbents based on such a mechanism, unsafe pressure spikes that may occur due to temperature swings can be eliminated. In addition, contamination can also be minimized, since the adsorbent remains contracted when no gas is stored.
Professor Yavuz said, “We envision a whole host of new designs and mechanisms to be developed based on our concept. Since natural gas is a much cleaner fuel than coal and petroleum, new developments in this realm will help switching to the use of less polluting fuels.”
Professor Atilhan agreed the most important impact of their research is on the environment. “Using natural gas more than coal and petroleum will significantly reduce greenhouse gas emissions. We believe, one day, we might see vehicles equipped with our materials that are run by a cleaner natural gas fuel,” he added.
This study, reported in Nature Energy on July 8, was supported by National Research Foundation of Korea (NRF) grants ( NRF-2016R1A2B4011027, NRF-2017M3A7B4042140, and NRF-2017M3A7B4042235).
< Suggested chemical structure of COP-150 >
< Initial ingredients (left) and final product (right) of COP-150 synthesis >
< Comparison of highest reported volumetric working capacities >
(END)
2019.08.09 View 26746 -
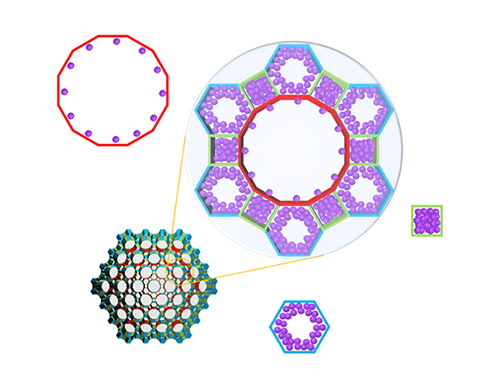 Real-Time Analysis of MOF Adsorption Behavior
Researchers have developed a technology to analyze the adsorption behavior of molecules in each individual pore of a metal organic framework (MOF). This system has large specific surface areas, allowing for the real-time observation of the adsorption process of an MOF, a new material effective for sorting carbon dioxide, hydrogen, and methane.
Accurate measurements and assessments of gas adsorption isotherms are important for characterizing porous materials and developing their applications. The existing technology is only able to measure the amount of gas molecules adsorbed to the material, without directly observing the adsorption behavior.
The research team led by Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability (EEWS) prescribed a real time gas adsorption crystallography system by integrating an existing X-ray diffraction (XRD) measurement device that can provide structural information and a gas adsorption measurement device.
Specifically, the system allowed the observation of a mesoporous MOF that has multiple pores rather than a single pore structure. The research team categorized the adsorption behaviors of MOF molecules by pore type, followed by observations and measurements, resulting in the identification of a stepwise adsorption process that was previously not possible to analyze.
Further, the team systematically and quantitatively analyzed how the pore structure and the type of adsorption molecule affect the adsorption behavior to suggest what type of MOF structure is appropriate as a storage material for each type of adsorption behavior.
Professor Kang said, “We quantitatively analyzed each pore molecule in real time to identify the effects of chemical and structural properties of pores on adsorption behavior.” He continued, “By understanding the real-time adsorption behavior of molecules at the level of the pores that form the material, rather than the whole material, we will be able to apply this technology to develop a new high-capacity storage material.”
This research was published in Nature Chemistry online on May 13, 2019 under the title ‘Isotherms of Individual Pores by Gas Adsorption Crystallography’.
(Figure. Schematic illustration of molecules adsorbed on metal organic frameworks with different pores of various structures, where the In-situ X-ray crystallography has been developed to classify each pore structure and analyze the position of the molecule to determine the amount of molecules adsorbed to each pore.)
2019.06.18 View 39031
Real-Time Analysis of MOF Adsorption Behavior
Researchers have developed a technology to analyze the adsorption behavior of molecules in each individual pore of a metal organic framework (MOF). This system has large specific surface areas, allowing for the real-time observation of the adsorption process of an MOF, a new material effective for sorting carbon dioxide, hydrogen, and methane.
Accurate measurements and assessments of gas adsorption isotherms are important for characterizing porous materials and developing their applications. The existing technology is only able to measure the amount of gas molecules adsorbed to the material, without directly observing the adsorption behavior.
The research team led by Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability (EEWS) prescribed a real time gas adsorption crystallography system by integrating an existing X-ray diffraction (XRD) measurement device that can provide structural information and a gas adsorption measurement device.
Specifically, the system allowed the observation of a mesoporous MOF that has multiple pores rather than a single pore structure. The research team categorized the adsorption behaviors of MOF molecules by pore type, followed by observations and measurements, resulting in the identification of a stepwise adsorption process that was previously not possible to analyze.
Further, the team systematically and quantitatively analyzed how the pore structure and the type of adsorption molecule affect the adsorption behavior to suggest what type of MOF structure is appropriate as a storage material for each type of adsorption behavior.
Professor Kang said, “We quantitatively analyzed each pore molecule in real time to identify the effects of chemical and structural properties of pores on adsorption behavior.” He continued, “By understanding the real-time adsorption behavior of molecules at the level of the pores that form the material, rather than the whole material, we will be able to apply this technology to develop a new high-capacity storage material.”
This research was published in Nature Chemistry online on May 13, 2019 under the title ‘Isotherms of Individual Pores by Gas Adsorption Crystallography’.
(Figure. Schematic illustration of molecules adsorbed on metal organic frameworks with different pores of various structures, where the In-situ X-ray crystallography has been developed to classify each pore structure and analyze the position of the molecule to determine the amount of molecules adsorbed to each pore.)
2019.06.18 View 39031 -
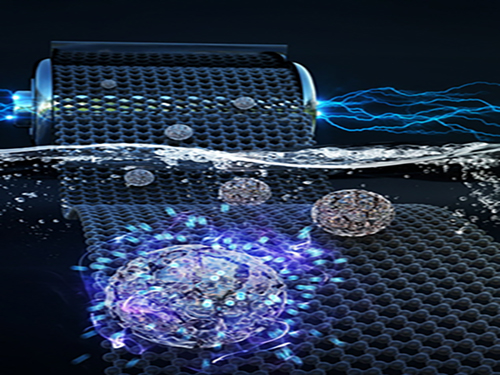 Faster and More Powerful Aqueous Hybrid Capacitor
(Professor Jeung Ku Kang from the Graduate School of EEWS)
A KAIST research team made it one step closer to realizing safe energy storage with high energy density, high power density, and a longer cycle life. This hybrid storage alternative shows power density 100 times faster than conventional batteries, allowing it to be charged within a few seconds. Hence, it is suitable for small portable electronic devices.
Conventional electrochemical energy storage systems, including lithium-ion batteries (LIBs), have a high voltage range and energy density, but are subject to safety issues raised by flammable organic electrolytes, which are used to ensure the beneficial properties. Additionally, they suffer from slow electrochemical reaction rates, which lead to a poor charging rate and low power density with a capacity that fades quickly, resulting in a short cycle life.
On the other hand, capacitors based on aqueous electrolytes are receiving a great deal of attention because they are considered to be safe and environmentally friendly alternatives. However, aqueous electrolytes lag behind energy storage systems based on organic electrolytes in terms of energy density due to their limited voltage range and low capacitance.
Hence, developing aqueous energy storage with high energy density and a long cycle life in addition to the high power density that enables fast charging is the most challenging task for advancing next-generation electrochemical energy storage devices.
Here, Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability and his team developed an aqueous hybrid capacitor (AHC) that boasts high energy density, high power, and excellent cycle stability by synthesizing two types of porous metal oxide nanoclusters on graphene to create positive and negative electrodes for AHCs.
The porous metal oxide nanoparticles are composed of nanoclusters as small as two to three nanometers and have mesopores that are smaller than five nanometers. In these porous structures, ions can be rapidly transferred to the material surfaces and a large number of ions can be stored inside the metal oxide particles very quickly due to their small particle size and large surface area.
The team applied porous manganese oxide on graphene for positive electrodes and porous iron oxide on graphene for negative electrodes to design an aqueous hybrid capacitor that can operate at an extended voltage range of 2V.
Professor Kang said, “This newly developed AHC with high capacity and power density driven from porous metal oxide electrodes will contribute to commercializing a new type of energy storage system. This technology allows ultra-fast charging within several seconds, making it suitable as a power source for mobile devices or electric vehicles where solar energy is directly stored as electricity.”
This research, co-led by Professor Hyung Mo Jeong from Kangwon National University, was published in Advanced Functional Materials on August 15, 2018.
Figure 1. Image that shows properties of porous metal oxide nanoparticles formed on graphene in the aqueous hybrid capacitor
2018.11.09 View 7486
Faster and More Powerful Aqueous Hybrid Capacitor
(Professor Jeung Ku Kang from the Graduate School of EEWS)
A KAIST research team made it one step closer to realizing safe energy storage with high energy density, high power density, and a longer cycle life. This hybrid storage alternative shows power density 100 times faster than conventional batteries, allowing it to be charged within a few seconds. Hence, it is suitable for small portable electronic devices.
Conventional electrochemical energy storage systems, including lithium-ion batteries (LIBs), have a high voltage range and energy density, but are subject to safety issues raised by flammable organic electrolytes, which are used to ensure the beneficial properties. Additionally, they suffer from slow electrochemical reaction rates, which lead to a poor charging rate and low power density with a capacity that fades quickly, resulting in a short cycle life.
On the other hand, capacitors based on aqueous electrolytes are receiving a great deal of attention because they are considered to be safe and environmentally friendly alternatives. However, aqueous electrolytes lag behind energy storage systems based on organic electrolytes in terms of energy density due to their limited voltage range and low capacitance.
Hence, developing aqueous energy storage with high energy density and a long cycle life in addition to the high power density that enables fast charging is the most challenging task for advancing next-generation electrochemical energy storage devices.
Here, Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability and his team developed an aqueous hybrid capacitor (AHC) that boasts high energy density, high power, and excellent cycle stability by synthesizing two types of porous metal oxide nanoclusters on graphene to create positive and negative electrodes for AHCs.
The porous metal oxide nanoparticles are composed of nanoclusters as small as two to three nanometers and have mesopores that are smaller than five nanometers. In these porous structures, ions can be rapidly transferred to the material surfaces and a large number of ions can be stored inside the metal oxide particles very quickly due to their small particle size and large surface area.
The team applied porous manganese oxide on graphene for positive electrodes and porous iron oxide on graphene for negative electrodes to design an aqueous hybrid capacitor that can operate at an extended voltage range of 2V.
Professor Kang said, “This newly developed AHC with high capacity and power density driven from porous metal oxide electrodes will contribute to commercializing a new type of energy storage system. This technology allows ultra-fast charging within several seconds, making it suitable as a power source for mobile devices or electric vehicles where solar energy is directly stored as electricity.”
This research, co-led by Professor Hyung Mo Jeong from Kangwon National University, was published in Advanced Functional Materials on August 15, 2018.
Figure 1. Image that shows properties of porous metal oxide nanoparticles formed on graphene in the aqueous hybrid capacitor
2018.11.09 View 7486 -
 Permanent, Wireless Self-charging System Using NIR Band
(Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability)
As wearable devices are emerging, there are numerous studies on wireless charging systems. Here, a KAIST research team has developed a permanent, wireless self-charging platform for low-power wearable electronics by converting near-infrared (NIR) band irradiation to electrical energy. This novel technology can be applied to flexible, wearable charging systems without needing any attachments.
Colloidal-quantum-dots (CQDs) are promising materials for manufacturing semiconductors; in particular, PbS-based CQDs have facile optical tunability from the visible to infrared wavelength region. Hence, they can be applied to various devices, such as lighting, photovoltaics (PVs), and photodetectors.
Continuous research on CQD-based optoelectronic devices has increased their power conversion efficiency (PCE) to 12%; however, applicable fields have not yet been found for them. Meanwhile, wearable electronic devices commonly face the problem of inconvenient charging systems because users have to constantly charge batteries attached to an energy source.
A joint team led by Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability and Jang Wok Choi from Seoul National University decided to apply CQD PVs, which have high quantum efficiency in NIR band to self-charging systems on wearable devices.
They employed a stable and efficient NIR energy conversion strategy. The system was comprised of a PbS CQD-based PV module, a flexible interdigitated lithium-ion battery, and various types of NIR-transparent films.
The team removed the existing battery from the already commercialized wearable healthcare bracelet and replaced it with the proposed self-charging system. They confirmed that the system can be applied to a low power wearable device via the NIR band.
There have been numerous platforms using solar irradiation, but the newly developed platform has more advantages because it allows conventional devices to be much more comfortable to wear and charged easily in everyday life using various irradiation sources for constant charging.
With this aspect, the proposed platform facilitates more flexible designs, which are the important component for actual commercialization. It also secures higher photostability and efficient than existing structures.
Professor Lee said, “By using the NIR band, we proposed a new approach to solve charging system issues of wearable devices. I believe that this platform will be a novel platform for energy conversion and that its application can be further extended to various fields, including mobiles, IoTs, and drones.”
This research, led by PhD Se-Woong Baek and M.S. candidate Jungmin Cho, was published in Advanced Materials on May 11.
Figure 1. a) Conceptual NIR-driven self-charging system including a flexible CQD PVs module and an interdigitatedly structured LIB. b) Photographic images of a conventional wearable healthcare bracelet and a self-charging system-integrated wearable device.
Figure 2. Illustration of the CQD PVs structure and performance of the wireless self-charging platform.
2018.10.08 View 8264
Permanent, Wireless Self-charging System Using NIR Band
(Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability)
As wearable devices are emerging, there are numerous studies on wireless charging systems. Here, a KAIST research team has developed a permanent, wireless self-charging platform for low-power wearable electronics by converting near-infrared (NIR) band irradiation to electrical energy. This novel technology can be applied to flexible, wearable charging systems without needing any attachments.
Colloidal-quantum-dots (CQDs) are promising materials for manufacturing semiconductors; in particular, PbS-based CQDs have facile optical tunability from the visible to infrared wavelength region. Hence, they can be applied to various devices, such as lighting, photovoltaics (PVs), and photodetectors.
Continuous research on CQD-based optoelectronic devices has increased their power conversion efficiency (PCE) to 12%; however, applicable fields have not yet been found for them. Meanwhile, wearable electronic devices commonly face the problem of inconvenient charging systems because users have to constantly charge batteries attached to an energy source.
A joint team led by Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability and Jang Wok Choi from Seoul National University decided to apply CQD PVs, which have high quantum efficiency in NIR band to self-charging systems on wearable devices.
They employed a stable and efficient NIR energy conversion strategy. The system was comprised of a PbS CQD-based PV module, a flexible interdigitated lithium-ion battery, and various types of NIR-transparent films.
The team removed the existing battery from the already commercialized wearable healthcare bracelet and replaced it with the proposed self-charging system. They confirmed that the system can be applied to a low power wearable device via the NIR band.
There have been numerous platforms using solar irradiation, but the newly developed platform has more advantages because it allows conventional devices to be much more comfortable to wear and charged easily in everyday life using various irradiation sources for constant charging.
With this aspect, the proposed platform facilitates more flexible designs, which are the important component for actual commercialization. It also secures higher photostability and efficient than existing structures.
Professor Lee said, “By using the NIR band, we proposed a new approach to solve charging system issues of wearable devices. I believe that this platform will be a novel platform for energy conversion and that its application can be further extended to various fields, including mobiles, IoTs, and drones.”
This research, led by PhD Se-Woong Baek and M.S. candidate Jungmin Cho, was published in Advanced Materials on May 11.
Figure 1. a) Conceptual NIR-driven self-charging system including a flexible CQD PVs module and an interdigitatedly structured LIB. b) Photographic images of a conventional wearable healthcare bracelet and a self-charging system-integrated wearable device.
Figure 2. Illustration of the CQD PVs structure and performance of the wireless self-charging platform.
2018.10.08 View 8264 -
 Improved Efficiency of CQD Solar Cells Using an Organic Thin Film
(from left: Professor Jung-Yong Lee and Dr. Se-Woong Baek)
Recently, the power conversion efficiency (PCE) of colloidal quantum dot (CQD)-based solar cells has been enhanced, paving the way for their commercialization in various fields; nevertheless, they are still a long way from being commercialized due to their efficiency not matching their stability. In this research, a KAIST team achieved highly stable and efficient CQD-based solar cells by using an amorphous organic layer to block oxygen and water permeation.
CQD-based solar cells are light-weight, flexible, and they boost light harvesting by absorbing near-infrared lights. Especially, they draw special attention for their optical properties controlled efficiently by changing the quantum dot sizes. However, they are still incompatible with existing solar cells in terms of efficiency, stability, and cost. Therefore, there is great demand for a novel technology that can simultaneously improve both PCE and stability while using an inexpensive electrode material.
Responding to this demand, Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability and his team introduced a technology to improve the efficiency and stability of CQD-based solar cells.
The team found that an amorphous organic thin film has a strong resistance to oxygen and water. Using these properties, they employed this doped organic layer as a top-hole selective layer (HSL) for the PbS CQD solar cells, and confirmed that the hydro/oxo-phobic properties of the layer efficiently protected the PbS layer. According to the molecular dynamics simulations, the layer significantly postponed the oxygen and water permeation into the PbS layer. Moreover, the efficient injection of the holes in the layer reduced interfacial resistance and improved performance.
With this technology, the team finally developed CQD-based solar cells with excellent stability. The PCE of their device stood at 11.7% and maintained over 90% of its initial performance when stored for one year under ambient conditions.
Professor Lee said, “This technology can be also applied to QD LEDs and Perovskite devices. I hope this technology can hasten the commercialization of CQD-based solar cells.”
This research, led by Dr. Se-Woong Baek and a Ph.D. student, Sang-Hoon Lee, was published in Energy & Environmental Science on May 10.
Figure 1. The schematic of the equilibrated structure of the amorphous organic film
Figure 2. Schematic illustration of CQD-based solar cells and graphs showing their performance
2018.08.27 View 8068
Improved Efficiency of CQD Solar Cells Using an Organic Thin Film
(from left: Professor Jung-Yong Lee and Dr. Se-Woong Baek)
Recently, the power conversion efficiency (PCE) of colloidal quantum dot (CQD)-based solar cells has been enhanced, paving the way for their commercialization in various fields; nevertheless, they are still a long way from being commercialized due to their efficiency not matching their stability. In this research, a KAIST team achieved highly stable and efficient CQD-based solar cells by using an amorphous organic layer to block oxygen and water permeation.
CQD-based solar cells are light-weight, flexible, and they boost light harvesting by absorbing near-infrared lights. Especially, they draw special attention for their optical properties controlled efficiently by changing the quantum dot sizes. However, they are still incompatible with existing solar cells in terms of efficiency, stability, and cost. Therefore, there is great demand for a novel technology that can simultaneously improve both PCE and stability while using an inexpensive electrode material.
Responding to this demand, Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability and his team introduced a technology to improve the efficiency and stability of CQD-based solar cells.
The team found that an amorphous organic thin film has a strong resistance to oxygen and water. Using these properties, they employed this doped organic layer as a top-hole selective layer (HSL) for the PbS CQD solar cells, and confirmed that the hydro/oxo-phobic properties of the layer efficiently protected the PbS layer. According to the molecular dynamics simulations, the layer significantly postponed the oxygen and water permeation into the PbS layer. Moreover, the efficient injection of the holes in the layer reduced interfacial resistance and improved performance.
With this technology, the team finally developed CQD-based solar cells with excellent stability. The PCE of their device stood at 11.7% and maintained over 90% of its initial performance when stored for one year under ambient conditions.
Professor Lee said, “This technology can be also applied to QD LEDs and Perovskite devices. I hope this technology can hasten the commercialization of CQD-based solar cells.”
This research, led by Dr. Se-Woong Baek and a Ph.D. student, Sang-Hoon Lee, was published in Energy & Environmental Science on May 10.
Figure 1. The schematic of the equilibrated structure of the amorphous organic film
Figure 2. Schematic illustration of CQD-based solar cells and graphs showing their performance
2018.08.27 View 8068 -
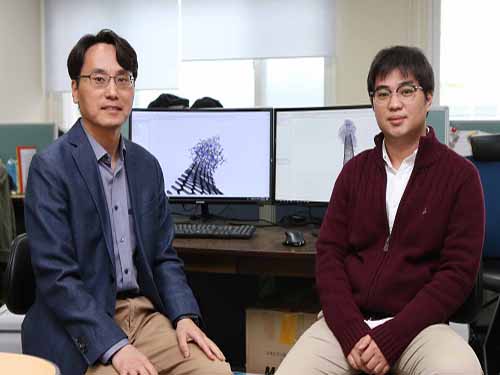 Computer Simulation Identifies a Key Principle for Next-generation Carbon Fibers
(from left: Professor Yong-Hoon Kim and PhD candidate Juho Lee)
Performing state-of-the-art computer simulations, a KAIST research team identified an atomistic design principle to produce high-quality, next-generation carbon fibers.
Carbon fibers are light-weight yet excellent in mechanical strength and thermal resistance. Boasting these properties, they can be diversely applied in high-technology sectors, including automotive, aerospace, and nuclear engineering.
They are produced from a polymer precursor through a series of spinning, stabilization, and carbonization processes. However, there is a major obstacle to producing high-quality carbon fibers. That is, when there exist ill-defined regions within the polymer matrixes, they result in disorder and defects within the produced carbon fibers.
As a solution to this problem, it was proposed that the introduction of carbon nanotubes (CNT) could enhance polymer orientation and crystallization. However, although the alignment geometry of the CNT-polymer interface apparently affects the quality of produced fibers, the atomistic understanding of the CNT-polymer interface has been so far lacking, hindering further developments.
To clarify the nature of CNT-polymer interactions, Professor Yong-Hoon Kim from the Graduate School of Energy, Environment, Water and Sustainability and his team employed a multiscale approach that combines first-principles density functional theory (DFT) calculations and force-fields molecular dynamics (MD) simulations and revealed the unique structural and electronic characteristics of polymer-CNT interfaces.
Here, they studied polyacrylonitrile (PAN)-CNT hybrid structures as a representative case of polymer-CNT composites. PAN is the most common polymer precursor, taking more than 90% of carbon fiber production.
Based on their DFT calculations, the team showed that the lying-down PAN configurations give a larger PAN-CNT binding energy than their standing-up counterparts. Moreover, maximizing the lying-down PAN configuration was shown to allow linear alignments of PANs on CNT, enabling the desirable ordered long-range PAN-PAN packing.
They also identified the CNT curvature as another significant factor, giving the largest PAN-CNT binding energy in the zero-curvature graphene limit. Conducting large-scale MD simulations, they then demonstrated that graphene nanoribbons are a promising carbon nano-reinforcement candidate by explicitly showing its strong propensity to induce linear alignments of PANs adsorbed on them.
Professor Kim said, “This research can be an exemplary case where the quantum mechanical simulations identify basic principles for developing advanced materials. Computer simulation studies will play an increasingly greater role thanks to the advances in the simulation theory and computer performance.”
This research, carried out by the PhD candidate Juho Lee, was published in the inside back cover of Advanced Functional Materials on April 11.
Figure 1. Inside back cover of Advanced Functional Materials
Figure 2. Research outline
2018.08.03 View 8375
Computer Simulation Identifies a Key Principle for Next-generation Carbon Fibers
(from left: Professor Yong-Hoon Kim and PhD candidate Juho Lee)
Performing state-of-the-art computer simulations, a KAIST research team identified an atomistic design principle to produce high-quality, next-generation carbon fibers.
Carbon fibers are light-weight yet excellent in mechanical strength and thermal resistance. Boasting these properties, they can be diversely applied in high-technology sectors, including automotive, aerospace, and nuclear engineering.
They are produced from a polymer precursor through a series of spinning, stabilization, and carbonization processes. However, there is a major obstacle to producing high-quality carbon fibers. That is, when there exist ill-defined regions within the polymer matrixes, they result in disorder and defects within the produced carbon fibers.
As a solution to this problem, it was proposed that the introduction of carbon nanotubes (CNT) could enhance polymer orientation and crystallization. However, although the alignment geometry of the CNT-polymer interface apparently affects the quality of produced fibers, the atomistic understanding of the CNT-polymer interface has been so far lacking, hindering further developments.
To clarify the nature of CNT-polymer interactions, Professor Yong-Hoon Kim from the Graduate School of Energy, Environment, Water and Sustainability and his team employed a multiscale approach that combines first-principles density functional theory (DFT) calculations and force-fields molecular dynamics (MD) simulations and revealed the unique structural and electronic characteristics of polymer-CNT interfaces.
Here, they studied polyacrylonitrile (PAN)-CNT hybrid structures as a representative case of polymer-CNT composites. PAN is the most common polymer precursor, taking more than 90% of carbon fiber production.
Based on their DFT calculations, the team showed that the lying-down PAN configurations give a larger PAN-CNT binding energy than their standing-up counterparts. Moreover, maximizing the lying-down PAN configuration was shown to allow linear alignments of PANs on CNT, enabling the desirable ordered long-range PAN-PAN packing.
They also identified the CNT curvature as another significant factor, giving the largest PAN-CNT binding energy in the zero-curvature graphene limit. Conducting large-scale MD simulations, they then demonstrated that graphene nanoribbons are a promising carbon nano-reinforcement candidate by explicitly showing its strong propensity to induce linear alignments of PANs adsorbed on them.
Professor Kim said, “This research can be an exemplary case where the quantum mechanical simulations identify basic principles for developing advanced materials. Computer simulation studies will play an increasingly greater role thanks to the advances in the simulation theory and computer performance.”
This research, carried out by the PhD candidate Juho Lee, was published in the inside back cover of Advanced Functional Materials on April 11.
Figure 1. Inside back cover of Advanced Functional Materials
Figure 2. Research outline
2018.08.03 View 8375 -
 Participation in the 2018 Bio-Digital City Workshop in Paris
(A student make a presentatiion during the Bio-Digital City Workshop in Paris last month.)
KAIST students explored ideas for developing future cities during the 2018 Bio-Digital City Workshop held in Paris last month.
This international workshop hosted by Cité des Sciences et de l'Industrie was held under the theme “Biomimicry, Digital City and Big Data.” During the workshop from July 10 to July 20, students teamed up with French counterparts to develop innovative urban design ideas. Cité des Sciences et de l'Industrie is the largest science museum in Europe and is operated by Universcience, a specialized institute of science and technology in France.
Professor Seongju Chang from the Department of Civil and Environmental Engineering and Professor Jihyun Lee of the Graduate School of Culture Technology Students led the students group.
Participants presented their ideas and findings on new urban solutions that combine biomimetic systems and digital technology. Each student group analyzed a special natural ecosystem such as sand dunes, jellyfish communities, or mangrove forests and conducted research to extract algorithms for constructing sustainable urban building complexes based on the results. The extracted algorithm was used to conceive a sustainable building complex forming a part of the urban environment by applying it to the actual Parisian city segment given as the virtual site for the workshop.
Students from diverse background in both countries participated in this convergence workshop. KAIST students included Ph.D. candidate Hyung Min Cho, undergraduates Min-Woo Jeong, Seung-Hwan Cha, and Sang-Jun Park from the Department of Civil and Environmental Engineering, undergraduate Kyeong-Keun Seo from the Department of Materials Science and Engineering, JiWhan Jeong (Master’s course) from the Department of Industrial and Systems Engineering, Ph.D. candidate Bo-Yoon Zang from the Graduate School of Culture Technology. They teamed up with French students from diverse backgrounds, including Design/Science, Visual Design, Geography, Computer Science and Humanities and Social Science.
This workshop will serve as another opportunity to expand academic and human exchange efforts in the domain of smart and sustainable cities with Europe in the future as the first international cooperation activity of KAIST and the Paris La Villette Science Museum.
Professor Seong-Ju Chang who led the research group said, "We will continue to establish a cooperative relationship between KAIST and the European scientific community. This workshop is a good opportunity to demonstrate the competence of KAIST students and their scientific and technological excellence on the international stage.”
2018.08.01 View 10680
Participation in the 2018 Bio-Digital City Workshop in Paris
(A student make a presentatiion during the Bio-Digital City Workshop in Paris last month.)
KAIST students explored ideas for developing future cities during the 2018 Bio-Digital City Workshop held in Paris last month.
This international workshop hosted by Cité des Sciences et de l'Industrie was held under the theme “Biomimicry, Digital City and Big Data.” During the workshop from July 10 to July 20, students teamed up with French counterparts to develop innovative urban design ideas. Cité des Sciences et de l'Industrie is the largest science museum in Europe and is operated by Universcience, a specialized institute of science and technology in France.
Professor Seongju Chang from the Department of Civil and Environmental Engineering and Professor Jihyun Lee of the Graduate School of Culture Technology Students led the students group.
Participants presented their ideas and findings on new urban solutions that combine biomimetic systems and digital technology. Each student group analyzed a special natural ecosystem such as sand dunes, jellyfish communities, or mangrove forests and conducted research to extract algorithms for constructing sustainable urban building complexes based on the results. The extracted algorithm was used to conceive a sustainable building complex forming a part of the urban environment by applying it to the actual Parisian city segment given as the virtual site for the workshop.
Students from diverse background in both countries participated in this convergence workshop. KAIST students included Ph.D. candidate Hyung Min Cho, undergraduates Min-Woo Jeong, Seung-Hwan Cha, and Sang-Jun Park from the Department of Civil and Environmental Engineering, undergraduate Kyeong-Keun Seo from the Department of Materials Science and Engineering, JiWhan Jeong (Master’s course) from the Department of Industrial and Systems Engineering, Ph.D. candidate Bo-Yoon Zang from the Graduate School of Culture Technology. They teamed up with French students from diverse backgrounds, including Design/Science, Visual Design, Geography, Computer Science and Humanities and Social Science.
This workshop will serve as another opportunity to expand academic and human exchange efforts in the domain of smart and sustainable cities with Europe in the future as the first international cooperation activity of KAIST and the Paris La Villette Science Museum.
Professor Seong-Ju Chang who led the research group said, "We will continue to establish a cooperative relationship between KAIST and the European scientific community. This workshop is a good opportunity to demonstrate the competence of KAIST students and their scientific and technological excellence on the international stage.”
2018.08.01 View 10680 -
 Formation of Burning Ice in Oceanic Clay Rich Sediment Disclosed
(from left: Professor Tae-Hyuk Kwon and PhD candidate Taehyung Park) A KAIST research team has identified the formation of natural gas hydrates, so-called flammable ice, formed in oceans.
Professor Tae-Hyuk Kwon from the Department of Civil & Environmental Engineering and his team found that clay minerals in oceanic clay-rich sedimentary deposits promote formation of gas hydrates and proposed the principle of gas hydrate formation in the clayey sedimentary layers.
Gas hydrates are ice-like crystalline structures composed of hydrogen-bonded water molecules encapsulating gas molecules. They are also known as burning ice. Their deposits are so huge that they gain attention for alternative energy.
Conventionally, it was believed that formation of gas hydrates is limited in clay sedimentary deposits; however, unexpected abundance of natural gas hydrates in oceanic clay-rich sedimentary deposits raised the issue of how they formed.
The surfaces of natural clay minerals are negatively charged and, thus, unavoidably generate physicochemical interactions between clay and water. Such clay-water interactions have a critical role in the occurrence of natural gas hydrates in clay-rich sedimentary formations.
However, there has been experimental difficulty in analyzing hydrate formation because of the cations contained in clay particles, which balance the clay surface charges. Therefore, clay particles inevitably release the cations when mixed with water, which complicates the interpretation of experimental results.
To overcome this limitation, the team polarized water molecules with an electric field and monitored the induction times of water molecules forming gas hydrates.
They found that the 10 kV/m of electric field promoted gas hydrate nucleation under certain conditions rather than slowing it down, due to the partial breakage of the hydrogen bonded water clusters and the lowered thermal energy of water molecules.
Professor Kwon said, “Through this research, we gained better insight into the origin of gas hydrates occurrence in clay-rich sedimentary deposits. In the near future, we will soon be able to commercially produce methane gas from natural gas hydrate deposits.”
This research, led by PhD candidate Taehyung Park, was published online in Environmental Science and Technology on February 3. (doi: 10.1021/acs.est.7b05477)
Figure 1. Formation of gas hydrates with water molecules
Figure 2. Enhancement and inhibition of gas hydrates
2018.04.09 View 6497
Formation of Burning Ice in Oceanic Clay Rich Sediment Disclosed
(from left: Professor Tae-Hyuk Kwon and PhD candidate Taehyung Park) A KAIST research team has identified the formation of natural gas hydrates, so-called flammable ice, formed in oceans.
Professor Tae-Hyuk Kwon from the Department of Civil & Environmental Engineering and his team found that clay minerals in oceanic clay-rich sedimentary deposits promote formation of gas hydrates and proposed the principle of gas hydrate formation in the clayey sedimentary layers.
Gas hydrates are ice-like crystalline structures composed of hydrogen-bonded water molecules encapsulating gas molecules. They are also known as burning ice. Their deposits are so huge that they gain attention for alternative energy.
Conventionally, it was believed that formation of gas hydrates is limited in clay sedimentary deposits; however, unexpected abundance of natural gas hydrates in oceanic clay-rich sedimentary deposits raised the issue of how they formed.
The surfaces of natural clay minerals are negatively charged and, thus, unavoidably generate physicochemical interactions between clay and water. Such clay-water interactions have a critical role in the occurrence of natural gas hydrates in clay-rich sedimentary formations.
However, there has been experimental difficulty in analyzing hydrate formation because of the cations contained in clay particles, which balance the clay surface charges. Therefore, clay particles inevitably release the cations when mixed with water, which complicates the interpretation of experimental results.
To overcome this limitation, the team polarized water molecules with an electric field and monitored the induction times of water molecules forming gas hydrates.
They found that the 10 kV/m of electric field promoted gas hydrate nucleation under certain conditions rather than slowing it down, due to the partial breakage of the hydrogen bonded water clusters and the lowered thermal energy of water molecules.
Professor Kwon said, “Through this research, we gained better insight into the origin of gas hydrates occurrence in clay-rich sedimentary deposits. In the near future, we will soon be able to commercially produce methane gas from natural gas hydrate deposits.”
This research, led by PhD candidate Taehyung Park, was published online in Environmental Science and Technology on February 3. (doi: 10.1021/acs.est.7b05477)
Figure 1. Formation of gas hydrates with water molecules
Figure 2. Enhancement and inhibition of gas hydrates
2018.04.09 View 6497 -
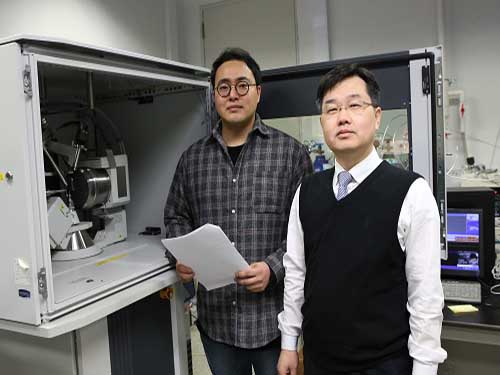 Aqueous Storage Device Needs Only 20 Seconds to Go
(from left: PhD candidate Il Woo Ock and Professor Jeung Ku Kang)
A KAIST research team developed a new hybrid energy storage device that can be charged in less than half a minute. It employs aqueous electrolytes instead of flammable organic solvents, so it is both environmentally friendly and safe. It also facilitates a boosting charge with high energy density, which makes it suitable for portable electronic devices.
Professor Jeung Ku Kang and his team from the Graduate School of Energy, Environment, Water, and Sustainability developed this hybrid energy storage with high energy and power densities along over a long cycle life by assembling fibre-like polymer chain anodes and sub-nanoscale metal oxide cathodes on graphene.
Conventional aqueous electrolyte-based energy storage devices have a limitation for boosting charges and high energy density due to low driving voltage and a shortage of anode materials.
Energy storage device capacity is determined by the two electrodes, and the balance between cathode and anode leads to high stability. In general, two electrodes show differences in electrical properties and differ in ion storage mechanism processes, resulting in poor storage and stability from the imbalance.
The research team came up with new structures and materials to facilitate rapid speed in energy exchange on the surfaces of the electrodes and minimize the energy loss between the two electrodes.
The team made anodes with graphene-based polymer chain materials. The web-like structure of graphene leads to a high surface area, thereby allowing higher capacitance.
For cathode materials, the team used metal oxide in sub-nanoscale structures to elevate atom-by-ion redox reactions. This method realized higher energy density and faster energy exchange while minimizing energy loss.
The developed device can be charged within 20 to 30 seconds using a low-power charging system, such as a USB switching charger or a flexible photovoltaic cell. The developed aqueous hybrid energy device shows more than 100-fold higher power density compared to conventional aqueous batteries and can be rapidly recharged. Further, the device showed high stability with its capacity maintained at 100% at a high charge/discharge current.
Professor Kang said, “This eco-friendly technology can be easily manufactured and is highly applicable. In particular, its high capacity and high stability, compared to existing technologies, could contribute to the commercialization of aqueous capacitors. The device can be rapidly charged using a low-power charging system, and thus can be applied to portable electronic device.”
This research, led by a PhD candidate Il Woo Ock, was published in Advanced Energy Materials on January 15.
Figure 1. Switching wearable LED kit with two AHCs in series charged by a flexible photovoltaic cell
Figure 2. Schematic diagram for aqueous hybrid capacitors
Figure 3. TEM images of anode and cathode
2018.02.28 View 12663
Aqueous Storage Device Needs Only 20 Seconds to Go
(from left: PhD candidate Il Woo Ock and Professor Jeung Ku Kang)
A KAIST research team developed a new hybrid energy storage device that can be charged in less than half a minute. It employs aqueous electrolytes instead of flammable organic solvents, so it is both environmentally friendly and safe. It also facilitates a boosting charge with high energy density, which makes it suitable for portable electronic devices.
Professor Jeung Ku Kang and his team from the Graduate School of Energy, Environment, Water, and Sustainability developed this hybrid energy storage with high energy and power densities along over a long cycle life by assembling fibre-like polymer chain anodes and sub-nanoscale metal oxide cathodes on graphene.
Conventional aqueous electrolyte-based energy storage devices have a limitation for boosting charges and high energy density due to low driving voltage and a shortage of anode materials.
Energy storage device capacity is determined by the two electrodes, and the balance between cathode and anode leads to high stability. In general, two electrodes show differences in electrical properties and differ in ion storage mechanism processes, resulting in poor storage and stability from the imbalance.
The research team came up with new structures and materials to facilitate rapid speed in energy exchange on the surfaces of the electrodes and minimize the energy loss between the two electrodes.
The team made anodes with graphene-based polymer chain materials. The web-like structure of graphene leads to a high surface area, thereby allowing higher capacitance.
For cathode materials, the team used metal oxide in sub-nanoscale structures to elevate atom-by-ion redox reactions. This method realized higher energy density and faster energy exchange while minimizing energy loss.
The developed device can be charged within 20 to 30 seconds using a low-power charging system, such as a USB switching charger or a flexible photovoltaic cell. The developed aqueous hybrid energy device shows more than 100-fold higher power density compared to conventional aqueous batteries and can be rapidly recharged. Further, the device showed high stability with its capacity maintained at 100% at a high charge/discharge current.
Professor Kang said, “This eco-friendly technology can be easily manufactured and is highly applicable. In particular, its high capacity and high stability, compared to existing technologies, could contribute to the commercialization of aqueous capacitors. The device can be rapidly charged using a low-power charging system, and thus can be applied to portable electronic device.”
This research, led by a PhD candidate Il Woo Ock, was published in Advanced Energy Materials on January 15.
Figure 1. Switching wearable LED kit with two AHCs in series charged by a flexible photovoltaic cell
Figure 2. Schematic diagram for aqueous hybrid capacitors
Figure 3. TEM images of anode and cathode
2018.02.28 View 12663 -
 Research Center for Smart Submerged Floating Tunnel Systems Opens
(Distinguished guests including President Shin (fourth from the right) and Director Lee (third from left) at the opening ceremony)
The Research Center for a Smart Submerged Floating Tunnel Systems was recently established at KAIST with the purpose of taking the lead in developing fundamental and applicable technology for submerged floating tunnels as well as fostering creative and talented people. Haeng-Ki Lee, a professor in the Department of Civil & Environmental Engineering at KAIST is heading the center.
KAIST held its opening ceremony on September 7, 2017 in the Applied Engineering Building located on the main campus.
Distinguished guests, including KAIST president Sung-Chul Shin, the President of the Korea Institute of Ocean Science and Technology Gi-Hoon Hong, the President of the Korean Society of Civil Engineering Young-Seok Park, and the Director in the Division of Engineering at the National Research Foundation of Korea Joong-Kon Park attended the ceremony.
The National Research Foundation of Korea provides Engineering Research Center (ERC) projects which find and foster groups with outstanding research performance in a field of engineering. The projects support these groups so that they can strengthen their global competitiveness while enhancing national competence in basic research.
The ‘Research Center for Smart Submerged Floating Tunnel Systems’ was selected as one of the ERC projects in 2017. For the next seven years, the research center will work to develop a submerged floating tunnel system resistant depths greater than 100 meters.
To achieve its goal, the center has defined crucial research topics including: i) a structural analysis program and integrated design technology specific for submerged floating tunnel systems, ii) high-durability marine construction materials and submerged construction integrated systems, and iii) safety and maintenance integrated technology for smart submerged floating tunnel systems.
The ‘Research Center for Smart Submerged Floating Tunnel Systems’ will devote itself to developing a variety of fundamental and applicable technology that will be leading global maritime construction. Moreover, it will concentrate on fostering professional research manpower in related areas.
The Director of the Center Lee said, “The center will cooperate with KAIST researchers who are experts in various fields, including structures, materials, construction, and maritime research. Based on this collaboration, the center will contribute to achieving autonomous technologies by developing fundamental and applicable technology related with submerged floating tunnel systems. It will also take the role of a leading global research hub in the field of submerged floating tunnels as well as construction technologies.”
2017.09.07 View 9322
Research Center for Smart Submerged Floating Tunnel Systems Opens
(Distinguished guests including President Shin (fourth from the right) and Director Lee (third from left) at the opening ceremony)
The Research Center for a Smart Submerged Floating Tunnel Systems was recently established at KAIST with the purpose of taking the lead in developing fundamental and applicable technology for submerged floating tunnels as well as fostering creative and talented people. Haeng-Ki Lee, a professor in the Department of Civil & Environmental Engineering at KAIST is heading the center.
KAIST held its opening ceremony on September 7, 2017 in the Applied Engineering Building located on the main campus.
Distinguished guests, including KAIST president Sung-Chul Shin, the President of the Korea Institute of Ocean Science and Technology Gi-Hoon Hong, the President of the Korean Society of Civil Engineering Young-Seok Park, and the Director in the Division of Engineering at the National Research Foundation of Korea Joong-Kon Park attended the ceremony.
The National Research Foundation of Korea provides Engineering Research Center (ERC) projects which find and foster groups with outstanding research performance in a field of engineering. The projects support these groups so that they can strengthen their global competitiveness while enhancing national competence in basic research.
The ‘Research Center for Smart Submerged Floating Tunnel Systems’ was selected as one of the ERC projects in 2017. For the next seven years, the research center will work to develop a submerged floating tunnel system resistant depths greater than 100 meters.
To achieve its goal, the center has defined crucial research topics including: i) a structural analysis program and integrated design technology specific for submerged floating tunnel systems, ii) high-durability marine construction materials and submerged construction integrated systems, and iii) safety and maintenance integrated technology for smart submerged floating tunnel systems.
The ‘Research Center for Smart Submerged Floating Tunnel Systems’ will devote itself to developing a variety of fundamental and applicable technology that will be leading global maritime construction. Moreover, it will concentrate on fostering professional research manpower in related areas.
The Director of the Center Lee said, “The center will cooperate with KAIST researchers who are experts in various fields, including structures, materials, construction, and maritime research. Based on this collaboration, the center will contribute to achieving autonomous technologies by developing fundamental and applicable technology related with submerged floating tunnel systems. It will also take the role of a leading global research hub in the field of submerged floating tunnels as well as construction technologies.”
2017.09.07 View 9322 -
 Highly-Efficient Photoelectrochemical CO2 Reduction
Direct CO2 conversion has continuously attracted a great deal of attention as a technology to produce fuels and chemical building blocks from renewable energy resources. Specifically, substances such as carbon feedstocks and fuels can be produced by utilizing sunlight, water, and CO2 as semiconductors and a water interface through photoelectrochemical CO2 reduction.
A KAIST research team demonstrated a novel photoelectrode structure for highly-selective and efficient photoelectrochemical CO2 reduction reactions. The research team led by Professor Jihun Oh of the Graduate School of EEWS (Energy, Environment, Water and Sustainability) presented a Si photoelectrode with a nanoporous Au thin film that is capable of reducing CO2 to CO with 90 percent selectivity in aqueous solution. The research team’s technology will provide a basic framework for designing the semiconductor photoelectrode structure necessary for photoelectrochemical conversion.
In order to achieve steady conversion of CO2, it is necessary to use a high-performance catalyst to lower overpotential. Among the metal catalysts, Au is known to be an electrocatalyst that converts CO2 to CO.
Conventionally, bare Au, as a catalyst, produces a lot of hydrogen gas due to its low CO selectivity. In addition, the high cost of Au remains a challenge in using the catalyst. Professor Oh’s research team addressed the issue by creating a nanoporous Au thin film formed by the electrochemical reduction of an anodized Au thin film.
As a result, the team could demonstrate an efficient, selective photoelectrochemical reduction reaction of CO2 to CO using electrochemically-treated Au thin films on a Si photoelectrode. The electrochemical reduction on anodized Au thin films forms a nanoporous thin layer exhibiting many grain boundaries of nanoparticles on the Au surface. This dramatically improves the selectivity of the reduction reaction with a maximum CO faradaic efficiency of over 90% at low overpotential and durability.
The research team also used an Au thin film of about 200 nanometers, 50,000 times thinner than previously reported nanostructured Au catalysts, resulting in a cost-effective catalyst. When depositing the catalyst on the semiconductor surface in the type of nanoparticles, the substrate of the thin film will be affected in the course of electrochemical reduction.
Thus, the research team designed a new Si photoelectrode with mesh-type co-catalysts that are independently wired at the front and back of the photoelectrode without influencing the photoelectrode, and made it possible for electrochemical reduction.
Due to the superior CO2 reduction reaction activity of the nanoporous Au mesh and high photovoltage from Si, the Si photoelectrode with the nanoporous Au thin film mesh shows conversion of CO2 to CO with 91% Faradaic efficiency at positive potential than CO equilibrium potential.
Professor Oh explained, “This technology will serve as a platform for diverse semiconductors and catalysts. Researchers can further improve the solar-to-CO2 conversion efficiency using this technology.
Dr. Jun Tae Song, the first author continued, “This new approach made it possible to develop a simple but very important type of electrode structure. It is the first time to achieve CO2 conversion at the potential lower than equilibrium potential. We believe that our research will contribute to efficient CO2 conversion.”
This research was published in the inside front cover of Advanced Energy Materials on February 8, 2017. The research was funded and supported by the Korea Carbon Capture & Sequestration R&D Center. Professor Sung-Yoon Chung of the EEWS also participated in this research.
(Figure: Schematic diagram of a Si photoelectrode that patterns with mesh-type nanoporous Au)
2017.03.08 View 9800
Highly-Efficient Photoelectrochemical CO2 Reduction
Direct CO2 conversion has continuously attracted a great deal of attention as a technology to produce fuels and chemical building blocks from renewable energy resources. Specifically, substances such as carbon feedstocks and fuels can be produced by utilizing sunlight, water, and CO2 as semiconductors and a water interface through photoelectrochemical CO2 reduction.
A KAIST research team demonstrated a novel photoelectrode structure for highly-selective and efficient photoelectrochemical CO2 reduction reactions. The research team led by Professor Jihun Oh of the Graduate School of EEWS (Energy, Environment, Water and Sustainability) presented a Si photoelectrode with a nanoporous Au thin film that is capable of reducing CO2 to CO with 90 percent selectivity in aqueous solution. The research team’s technology will provide a basic framework for designing the semiconductor photoelectrode structure necessary for photoelectrochemical conversion.
In order to achieve steady conversion of CO2, it is necessary to use a high-performance catalyst to lower overpotential. Among the metal catalysts, Au is known to be an electrocatalyst that converts CO2 to CO.
Conventionally, bare Au, as a catalyst, produces a lot of hydrogen gas due to its low CO selectivity. In addition, the high cost of Au remains a challenge in using the catalyst. Professor Oh’s research team addressed the issue by creating a nanoporous Au thin film formed by the electrochemical reduction of an anodized Au thin film.
As a result, the team could demonstrate an efficient, selective photoelectrochemical reduction reaction of CO2 to CO using electrochemically-treated Au thin films on a Si photoelectrode. The electrochemical reduction on anodized Au thin films forms a nanoporous thin layer exhibiting many grain boundaries of nanoparticles on the Au surface. This dramatically improves the selectivity of the reduction reaction with a maximum CO faradaic efficiency of over 90% at low overpotential and durability.
The research team also used an Au thin film of about 200 nanometers, 50,000 times thinner than previously reported nanostructured Au catalysts, resulting in a cost-effective catalyst. When depositing the catalyst on the semiconductor surface in the type of nanoparticles, the substrate of the thin film will be affected in the course of electrochemical reduction.
Thus, the research team designed a new Si photoelectrode with mesh-type co-catalysts that are independently wired at the front and back of the photoelectrode without influencing the photoelectrode, and made it possible for electrochemical reduction.
Due to the superior CO2 reduction reaction activity of the nanoporous Au mesh and high photovoltage from Si, the Si photoelectrode with the nanoporous Au thin film mesh shows conversion of CO2 to CO with 91% Faradaic efficiency at positive potential than CO equilibrium potential.
Professor Oh explained, “This technology will serve as a platform for diverse semiconductors and catalysts. Researchers can further improve the solar-to-CO2 conversion efficiency using this technology.
Dr. Jun Tae Song, the first author continued, “This new approach made it possible to develop a simple but very important type of electrode structure. It is the first time to achieve CO2 conversion at the potential lower than equilibrium potential. We believe that our research will contribute to efficient CO2 conversion.”
This research was published in the inside front cover of Advanced Energy Materials on February 8, 2017. The research was funded and supported by the Korea Carbon Capture & Sequestration R&D Center. Professor Sung-Yoon Chung of the EEWS also participated in this research.
(Figure: Schematic diagram of a Si photoelectrode that patterns with mesh-type nanoporous Au)
2017.03.08 View 9800 -
 A Firefighter Drone That Flies and Crawls Up Walls
KAIST researchers developed a wall-climbing scout drone to fight fires in high-rises, finding the source of the fires and locating people trapped inside.
The 1974 American disaster film Towering Inferno depicted well the earnest struggles of firefighters engaged in ending a fire at a 138-story skyscraper. To this day, fires at high-rise buildings are considered one of the most dangerous disasters.
Skyscraper fires are particularly difficult to contain because of their ability to spread rapidly in high-occupant density spaces and the challenge of fighting fires in the buildings’ complex vertical structure. Accessibility to skyscrapers at the time of the fire is limited, and it is hard to assess the initial situation.
A research team at KAIST led by Professor Hyun Myung of the Civil and Environmental Engineering Department developed an unmanned aerial vehicle, named the Fireproof Aerial RObot System (FAROS), which detects fires in skyscrapers, searches the inside of the building, and transfers data in real time from fire scenes to the ground station.
As an extended version of Climbing Aerial RObot System (CAROS) that was created in 2014 by the research team, the FAROS can also fly and climb walls.
The FAROS, whose movements rely on a quadrotor system, can freely change its flight mode into a spider’s crawling on walls, and vice versa, facilitating unimpeded navigation in the labyrinth of narrow spaces filled with debris and rubble inside the blazing building.
The drone “estimates” its pose by utilizing a 2-D laser scanner, an altimeter, and an Inertia Measurement Unit sensor to navigate autonomously. With the localization result and using a thermal-imaging camera to recognize objects or people inside a building, the FAROS can also detect and find the fire-ignition point by employing dedicated image-processing technology.
The FAROS is fireproof and flame-retardant. The drone’s body is covered with aramid fibers to protect its electric and mechanical components from the direct effects of the flame. The aramid fiber skin also has a buffer of air underneath it, and a thermoelectric cooling system based on the Peltier effect to help maintain the air layer within a specific temperature range.
The research team demonstrated the feasibility of the localization system and wall-climbing mechanism in a smoky indoor environment. The fireproof test showed that the drone could endure the heat of over 1,000° Celsius from butane gas and ethanol aerosol flames for over one minute.
Professor Myung said, “As cities become more crowded with skyscrapers and super structures, fire incidents in these high-rise buildings are life-threatening massive disasters. The FAROS can be aptly deployed to the disaster site at an early stage of such incidents to minimize the damage and maximize the safety and efficiency of rescue mission.”
The research team has recently started to enhance the performance of the fireproof design for the exteroceptive sensors including a 2-D laser scanner and a thermal-imaging camera because those sensors could be more exposed to fire than other inside sensors and electric components.
This research was funded by the KAIST Initiative for Disaster Studies and the KAIST Institute.
YouTube link: https://youtu.be/gPNRZi0EPQw
Picture 1: Demonstration of Wall-climbing
The Fireproof Aerial RObot System (FAROS) is a wall-climbing scout drone developed to conduct explorations into the site of skyscraper fires. It has an ability to climb walls in smoky, narrow spaces inside buildings.
Figure 2: An Ability to Withstand Fires
The FAROS can endure the heat of over 1,000° Celsius from butane gas and ethanol aerosol flames for over one minute.
2016.01.20 View 15452
A Firefighter Drone That Flies and Crawls Up Walls
KAIST researchers developed a wall-climbing scout drone to fight fires in high-rises, finding the source of the fires and locating people trapped inside.
The 1974 American disaster film Towering Inferno depicted well the earnest struggles of firefighters engaged in ending a fire at a 138-story skyscraper. To this day, fires at high-rise buildings are considered one of the most dangerous disasters.
Skyscraper fires are particularly difficult to contain because of their ability to spread rapidly in high-occupant density spaces and the challenge of fighting fires in the buildings’ complex vertical structure. Accessibility to skyscrapers at the time of the fire is limited, and it is hard to assess the initial situation.
A research team at KAIST led by Professor Hyun Myung of the Civil and Environmental Engineering Department developed an unmanned aerial vehicle, named the Fireproof Aerial RObot System (FAROS), which detects fires in skyscrapers, searches the inside of the building, and transfers data in real time from fire scenes to the ground station.
As an extended version of Climbing Aerial RObot System (CAROS) that was created in 2014 by the research team, the FAROS can also fly and climb walls.
The FAROS, whose movements rely on a quadrotor system, can freely change its flight mode into a spider’s crawling on walls, and vice versa, facilitating unimpeded navigation in the labyrinth of narrow spaces filled with debris and rubble inside the blazing building.
The drone “estimates” its pose by utilizing a 2-D laser scanner, an altimeter, and an Inertia Measurement Unit sensor to navigate autonomously. With the localization result and using a thermal-imaging camera to recognize objects or people inside a building, the FAROS can also detect and find the fire-ignition point by employing dedicated image-processing technology.
The FAROS is fireproof and flame-retardant. The drone’s body is covered with aramid fibers to protect its electric and mechanical components from the direct effects of the flame. The aramid fiber skin also has a buffer of air underneath it, and a thermoelectric cooling system based on the Peltier effect to help maintain the air layer within a specific temperature range.
The research team demonstrated the feasibility of the localization system and wall-climbing mechanism in a smoky indoor environment. The fireproof test showed that the drone could endure the heat of over 1,000° Celsius from butane gas and ethanol aerosol flames for over one minute.
Professor Myung said, “As cities become more crowded with skyscrapers and super structures, fire incidents in these high-rise buildings are life-threatening massive disasters. The FAROS can be aptly deployed to the disaster site at an early stage of such incidents to minimize the damage and maximize the safety and efficiency of rescue mission.”
The research team has recently started to enhance the performance of the fireproof design for the exteroceptive sensors including a 2-D laser scanner and a thermal-imaging camera because those sensors could be more exposed to fire than other inside sensors and electric components.
This research was funded by the KAIST Initiative for Disaster Studies and the KAIST Institute.
YouTube link: https://youtu.be/gPNRZi0EPQw
Picture 1: Demonstration of Wall-climbing
The Fireproof Aerial RObot System (FAROS) is a wall-climbing scout drone developed to conduct explorations into the site of skyscraper fires. It has an ability to climb walls in smoky, narrow spaces inside buildings.
Figure 2: An Ability to Withstand Fires
The FAROS can endure the heat of over 1,000° Celsius from butane gas and ethanol aerosol flames for over one minute.
2016.01.20 View 15452