Department+of+Bio
-
 KAIST Successfully Implements 3D Brain-Mimicking Platform with 6x Higher Precision
<(From left) Dr. Dongjo Yoon, Professor Je-Kyun Park from the Department of Bio and Brain Engineering, (upper right) Professor Yoonkey Nam, Dr. Soo Jee Kim>
Existing three-dimensional (3D) neuronal culture technology has limitations in brain research due to the difficulty of precisely replicating the brain's complex multilayered structure and the lack of a platform that can simultaneously analyze both structure and function. A KAIST research team has successfully developed an integrated platform that can implement brain-like layered neuronal structures using 3D printing technology and precisely measure neuronal activity within them.
KAIST (President Kwang Hyung Lee) announced on the 16th of July that a joint research team led by Professors Je-Kyun Park and Yoonkey Nam from the Department of Bio and Brain Engineering has developed an integrated platform capable of fabricating high-resolution 3D multilayer neuronal networks using low-viscosity natural hydrogels with mechanical properties similar to brain tissue, and simultaneously analyzing their structural and functional connectivity.
Conventional bioprinting technology uses high-viscosity bioinks for structural stability, but this limits neuronal proliferation and neurite growth. Conversely, neural cell-friendly low-viscosity hydrogels are difficult to precisely pattern, leading to a fundamental trade-off between structural stability and biological function. The research team completed a sophisticated and stable brain-mimicking platform by combining three key technologies that enable the precise creation of brain structure with dilute gels, accurate alignment between layers, and simultaneous observation of neuronal activity.
The three core technologies are: ▲ 'Capillary Pinning Effect' technology, which enables the dilute gel (hydrogel) to adhere firmly to a stainless steel mesh (micromesh) to prevent it from flowing, thereby reproducing brain structures with six times greater precision (resolution of 500 μm or less) than conventional methods; ▲ the '3D Printing Aligner,' a cylindrical design that ensures the printed layers are precisely stacked without misalignment, guaranteeing the accurate assembly of multilayer structures and stable integration with microelectrode chips; and ▲ 'Dual-mode Analysis System' technology, which simultaneously measures electrical signals from below and observes cell activity with light (calcium imaging) from above, allowing for the simultaneous verification of the functional operation of interlayer connections through multiple methods.
< Figure 1. Platform integrating brain-structure-mimicking neural network model construction and functional measurement technology>
The research team successfully implemented a three-layered mini-brain structure using 3D printing with a fibrin hydrogel, which has elastic properties similar to those of the brain, and experimentally verified the process of actual neural cells transmitting and receiving signals within it.
Cortical neurons were placed in the upper and lower layers, while the middle layer was left empty but designed to allow neurons to penetrate and connect through it. Electrical signals were measured from the lower layer using a microsensor (electrode chip), and cell activity was observed from the upper layer using light (calcium imaging). The results showed that when electrical stimulation was applied, neural cells in both upper and lower layers responded simultaneously. When a synapse-blocking agent (synaptic blocker) was introduced, the response decreased, proving that the neural cells were genuinely connected and transmitting signals.
Professor Je-Kyun Park of KAIST explained, "This research is a joint development achievement of an integrated platform that can simultaneously reproduce the complex multilayered structure and function of brain tissue. Compared to existing technologies where signal measurement was impossible for more than 14 days, this platform maintains a stable microelectrode chip interface for over 27 days, allowing the real-time analysis of structure-function relationships. It can be utilized in various brain research fields such as neurological disease modeling, brain function research, neurotoxicity assessment, and neuroprotective drug screening in the future."
<Figure 2. Integration process of stacked bioprinting technology and microelectrode chip>
The research, in which Dr. Soo Jee Kim and Dr. Dongjo Yoon from KAIST's Department of Bio and Brain Engineering participated as co-first authors, was published online in the international journal 'Biosensors and Bioelectronics' on June 11, 2025.
※Paper: Hybrid biofabrication of multilayered 3D neuronal networks with structural and functional interlayer connectivity
※DOI: https://doi.org/10.1016/j.bios.2025.117688
2025.07.16 View 76
KAIST Successfully Implements 3D Brain-Mimicking Platform with 6x Higher Precision
<(From left) Dr. Dongjo Yoon, Professor Je-Kyun Park from the Department of Bio and Brain Engineering, (upper right) Professor Yoonkey Nam, Dr. Soo Jee Kim>
Existing three-dimensional (3D) neuronal culture technology has limitations in brain research due to the difficulty of precisely replicating the brain's complex multilayered structure and the lack of a platform that can simultaneously analyze both structure and function. A KAIST research team has successfully developed an integrated platform that can implement brain-like layered neuronal structures using 3D printing technology and precisely measure neuronal activity within them.
KAIST (President Kwang Hyung Lee) announced on the 16th of July that a joint research team led by Professors Je-Kyun Park and Yoonkey Nam from the Department of Bio and Brain Engineering has developed an integrated platform capable of fabricating high-resolution 3D multilayer neuronal networks using low-viscosity natural hydrogels with mechanical properties similar to brain tissue, and simultaneously analyzing their structural and functional connectivity.
Conventional bioprinting technology uses high-viscosity bioinks for structural stability, but this limits neuronal proliferation and neurite growth. Conversely, neural cell-friendly low-viscosity hydrogels are difficult to precisely pattern, leading to a fundamental trade-off between structural stability and biological function. The research team completed a sophisticated and stable brain-mimicking platform by combining three key technologies that enable the precise creation of brain structure with dilute gels, accurate alignment between layers, and simultaneous observation of neuronal activity.
The three core technologies are: ▲ 'Capillary Pinning Effect' technology, which enables the dilute gel (hydrogel) to adhere firmly to a stainless steel mesh (micromesh) to prevent it from flowing, thereby reproducing brain structures with six times greater precision (resolution of 500 μm or less) than conventional methods; ▲ the '3D Printing Aligner,' a cylindrical design that ensures the printed layers are precisely stacked without misalignment, guaranteeing the accurate assembly of multilayer structures and stable integration with microelectrode chips; and ▲ 'Dual-mode Analysis System' technology, which simultaneously measures electrical signals from below and observes cell activity with light (calcium imaging) from above, allowing for the simultaneous verification of the functional operation of interlayer connections through multiple methods.
< Figure 1. Platform integrating brain-structure-mimicking neural network model construction and functional measurement technology>
The research team successfully implemented a three-layered mini-brain structure using 3D printing with a fibrin hydrogel, which has elastic properties similar to those of the brain, and experimentally verified the process of actual neural cells transmitting and receiving signals within it.
Cortical neurons were placed in the upper and lower layers, while the middle layer was left empty but designed to allow neurons to penetrate and connect through it. Electrical signals were measured from the lower layer using a microsensor (electrode chip), and cell activity was observed from the upper layer using light (calcium imaging). The results showed that when electrical stimulation was applied, neural cells in both upper and lower layers responded simultaneously. When a synapse-blocking agent (synaptic blocker) was introduced, the response decreased, proving that the neural cells were genuinely connected and transmitting signals.
Professor Je-Kyun Park of KAIST explained, "This research is a joint development achievement of an integrated platform that can simultaneously reproduce the complex multilayered structure and function of brain tissue. Compared to existing technologies where signal measurement was impossible for more than 14 days, this platform maintains a stable microelectrode chip interface for over 27 days, allowing the real-time analysis of structure-function relationships. It can be utilized in various brain research fields such as neurological disease modeling, brain function research, neurotoxicity assessment, and neuroprotective drug screening in the future."
<Figure 2. Integration process of stacked bioprinting technology and microelectrode chip>
The research, in which Dr. Soo Jee Kim and Dr. Dongjo Yoon from KAIST's Department of Bio and Brain Engineering participated as co-first authors, was published online in the international journal 'Biosensors and Bioelectronics' on June 11, 2025.
※Paper: Hybrid biofabrication of multilayered 3D neuronal networks with structural and functional interlayer connectivity
※DOI: https://doi.org/10.1016/j.bios.2025.117688
2025.07.16 View 76 -
 KAIST Shows That the Brain Can Distinguish Glucose: Clues to Treat Obesity and Diabetes
<(From left)Prof. Greg S.B Suh, Dr. Jieun Kim, Dr. Shinhye Kim, Researcher Wongyo Jeong)
“How does our brain distinguish glucose from the many nutrients absorbed in the gut?” Starting with this question, a KAIST research team has demonstrated that the brain can selectively recognize specific nutrients—particularly glucose—beyond simply detecting total calorie content. This study is expected to offer a new paradigm for appetite control and the treatment of metabolic diseases.
On the 9th, KAIST (President Kwang Hyung Lee) announced that Professor Greg S.B. Suh’s team in the Department of Biological Sciences, in collaboration with Professor Young-Gyun Park’s team (BarNeuro), Professor Seung-Hee Lee’s team (Department of Biological Sciences), and the Albert Einstein College of Medicine in New York, had identified the existence of a gut-brain circuit that allows animals in a hungry state to selectively detect and prefer glucose in the gut.
Organisms derive energy from various nutrients including sugars, proteins, and fats. Previous studies have shown that total caloric information in the gut suppresses hunger neurons in the hypothalamus to regulate appetite. However, the existence of a gut-brain circuit that specifically responds to glucose and corresponding brain cells had not been demonstrated until now.
In this study, the team successfully identified a “gut-brain circuit” that senses glucose—essential for brain function—and regulates food intake behavior for required nutrients.
They further proved, for the first time, that this circuit responds within seconds to not only hunger or external stimuli but also to specific caloric nutrients directly introduced into the small intestine, particularly D-glucose, through the activity of “CRF neurons*” in the brain’s hypothalamus.
*CRF neurons: These neurons secrete corticotropin-releasing factor (CRF) in the hypothalamus and are central to the hypothalamic-pituitary-adrenal (HPA) axis, the body’s core physiological system for responding to stress. CRF neurons are known to regulate neuroendocrine balance in response to stress stimuli.
Using optogenetics to precisely track neural activity in real time, the researchers injected various nutrients—D-glucose, L-glucose, amino acids, and fats—directly into the small intestines of mice and observed the results.
They discovered that among the CRF neurons located in the paraventricular nucleus (PVN)* of the hypothalamus, only those specific to D-glucose showed selective responses. These neurons did not respond—or showed inverse reactions—to other sugars or to proteins and fats. This is the first demonstration that single neurons in the brain can guide nutrient-specific responses depending on gut nutrient influx.
*PVN (Paraventricular Nucleus): A key nucleus within the hypothalamus responsible for maintaining bodily homeostasis.
The team also revealed that glucose-sensing signals in the small intestine are transmitted via the spinal cord to the dorsolateral parabrachial nucleus (PBNdl) of the brain, and from there to CRF neurons in the PVN. In contrast, signals for amino acids and fats are transmitted to the brain through the vagus nerve, a different pathway.
In optogenetic inhibition experiments, suppressing CRF neurons in fasting mice eliminated their preference for glucose, proving that this circuit is essential for glucose-specific nutrient preference.
This study was inspired by Professor Suh’s earlier research at NYU using fruit flies, where he identified “DH44 neurons” that selectively detect glucose and sugar in the gut. Based on the hypothesis that hypothalamic neurons in mammals would show similar functional responses to glucose, the current study was launched.
To test this hypothesis, Dr. Jineun Kim (KAIST Ph.D. graduate, now at Caltech) demonstrated during her doctoral research that hungry mice preferred glucose among various intragastrically infused nutrients and that CRF neurons exhibited rapid and specific responses.
Along with Wongyo Jung (KAIST B.S. graduate, now Ph.D. student at Caltech), they modeled and experimentally confirmed the critical role of CRF neurons. Dr. Shinhye Kim, through collaboration, revealed that specific spinal neurons play a key role in conveying intestinal nutrient information to the brain.
Dr. Jineun Kim and Dr. Shinhye Kim said, “This study started from a simple but fundamental question—‘How does the brain distinguish glucose from various nutrients absorbed in the gut?’ We have shown that spinal-based gut-brain circuits play a central role in energy metabolism and homeostasis by transmitting specific gut nutrient signals to the brain.”
Professor Suh added, “By identifying a gut-brain pathway specialized for glucose, this research offers a new therapeutic target for metabolic diseases such as obesity and diabetes. Our future research will explore similar circuits for sensing other essential nutrients like amino acids and fats and their interaction mechanisms.”
Ph.D. student Jineun Kim, Dr. Shinhye Kim, and student Wongyo Jung (co-first authors) contributed to this study, which was published online in the international journal Neuron on June 20, 2025.
※ Paper Title: Encoding the glucose identity by discrete hypothalamic neurons via the gut-brain axis ※ DOI: https://doi.org/10.1016/j.neuron.2025.05.024
This study was supported by the Samsung Science & Technology Foundation, the National Research Foundation of Korea (NRF) Leader Research Program, the POSCO Cheongam Science Fellowship, the Asan Foundation Biomedical Science Scholarship, the Institute for Basic Science (IBS), and the KAIST KAIX program.
2025.07.09 View 265
KAIST Shows That the Brain Can Distinguish Glucose: Clues to Treat Obesity and Diabetes
<(From left)Prof. Greg S.B Suh, Dr. Jieun Kim, Dr. Shinhye Kim, Researcher Wongyo Jeong)
“How does our brain distinguish glucose from the many nutrients absorbed in the gut?” Starting with this question, a KAIST research team has demonstrated that the brain can selectively recognize specific nutrients—particularly glucose—beyond simply detecting total calorie content. This study is expected to offer a new paradigm for appetite control and the treatment of metabolic diseases.
On the 9th, KAIST (President Kwang Hyung Lee) announced that Professor Greg S.B. Suh’s team in the Department of Biological Sciences, in collaboration with Professor Young-Gyun Park’s team (BarNeuro), Professor Seung-Hee Lee’s team (Department of Biological Sciences), and the Albert Einstein College of Medicine in New York, had identified the existence of a gut-brain circuit that allows animals in a hungry state to selectively detect and prefer glucose in the gut.
Organisms derive energy from various nutrients including sugars, proteins, and fats. Previous studies have shown that total caloric information in the gut suppresses hunger neurons in the hypothalamus to regulate appetite. However, the existence of a gut-brain circuit that specifically responds to glucose and corresponding brain cells had not been demonstrated until now.
In this study, the team successfully identified a “gut-brain circuit” that senses glucose—essential for brain function—and regulates food intake behavior for required nutrients.
They further proved, for the first time, that this circuit responds within seconds to not only hunger or external stimuli but also to specific caloric nutrients directly introduced into the small intestine, particularly D-glucose, through the activity of “CRF neurons*” in the brain’s hypothalamus.
*CRF neurons: These neurons secrete corticotropin-releasing factor (CRF) in the hypothalamus and are central to the hypothalamic-pituitary-adrenal (HPA) axis, the body’s core physiological system for responding to stress. CRF neurons are known to regulate neuroendocrine balance in response to stress stimuli.
Using optogenetics to precisely track neural activity in real time, the researchers injected various nutrients—D-glucose, L-glucose, amino acids, and fats—directly into the small intestines of mice and observed the results.
They discovered that among the CRF neurons located in the paraventricular nucleus (PVN)* of the hypothalamus, only those specific to D-glucose showed selective responses. These neurons did not respond—or showed inverse reactions—to other sugars or to proteins and fats. This is the first demonstration that single neurons in the brain can guide nutrient-specific responses depending on gut nutrient influx.
*PVN (Paraventricular Nucleus): A key nucleus within the hypothalamus responsible for maintaining bodily homeostasis.
The team also revealed that glucose-sensing signals in the small intestine are transmitted via the spinal cord to the dorsolateral parabrachial nucleus (PBNdl) of the brain, and from there to CRF neurons in the PVN. In contrast, signals for amino acids and fats are transmitted to the brain through the vagus nerve, a different pathway.
In optogenetic inhibition experiments, suppressing CRF neurons in fasting mice eliminated their preference for glucose, proving that this circuit is essential for glucose-specific nutrient preference.
This study was inspired by Professor Suh’s earlier research at NYU using fruit flies, where he identified “DH44 neurons” that selectively detect glucose and sugar in the gut. Based on the hypothesis that hypothalamic neurons in mammals would show similar functional responses to glucose, the current study was launched.
To test this hypothesis, Dr. Jineun Kim (KAIST Ph.D. graduate, now at Caltech) demonstrated during her doctoral research that hungry mice preferred glucose among various intragastrically infused nutrients and that CRF neurons exhibited rapid and specific responses.
Along with Wongyo Jung (KAIST B.S. graduate, now Ph.D. student at Caltech), they modeled and experimentally confirmed the critical role of CRF neurons. Dr. Shinhye Kim, through collaboration, revealed that specific spinal neurons play a key role in conveying intestinal nutrient information to the brain.
Dr. Jineun Kim and Dr. Shinhye Kim said, “This study started from a simple but fundamental question—‘How does the brain distinguish glucose from various nutrients absorbed in the gut?’ We have shown that spinal-based gut-brain circuits play a central role in energy metabolism and homeostasis by transmitting specific gut nutrient signals to the brain.”
Professor Suh added, “By identifying a gut-brain pathway specialized for glucose, this research offers a new therapeutic target for metabolic diseases such as obesity and diabetes. Our future research will explore similar circuits for sensing other essential nutrients like amino acids and fats and their interaction mechanisms.”
Ph.D. student Jineun Kim, Dr. Shinhye Kim, and student Wongyo Jung (co-first authors) contributed to this study, which was published online in the international journal Neuron on June 20, 2025.
※ Paper Title: Encoding the glucose identity by discrete hypothalamic neurons via the gut-brain axis ※ DOI: https://doi.org/10.1016/j.neuron.2025.05.024
This study was supported by the Samsung Science & Technology Foundation, the National Research Foundation of Korea (NRF) Leader Research Program, the POSCO Cheongam Science Fellowship, the Asan Foundation Biomedical Science Scholarship, the Institute for Basic Science (IBS), and the KAIST KAIX program.
2025.07.09 View 265 -
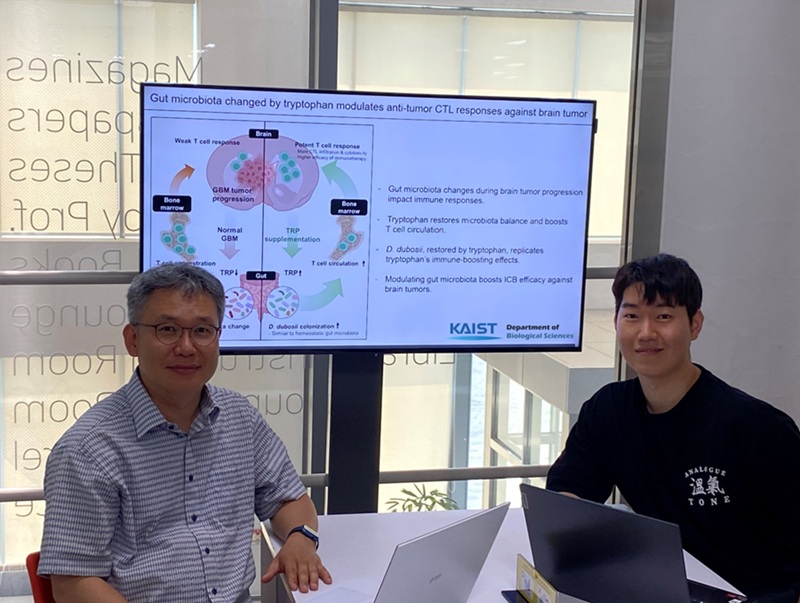 KAIST Enhances Immunotherapy for Difficult-to-Treat Brain Tumors with Gut Microbiota
< Photo 1.(From left) Prof. Heung Kyu Lee, Department of Biological Sciences,
and Dr. Hyeon Cheol Kim>
Advanced treatments, known as immunotherapies that activate T cells—our body's immune cells—to eliminate cancer cells, have shown limited efficacy as standalone therapies for glioblastoma, the most lethal form of brain tumor. This is due to their minimal response to glioblastoma and high resistance to treatment.
Now, a KAIST research team has now demonstrated a new therapeutic strategy that can enhance the efficacy of immunotherapy for brain tumors by utilizing gut microbes and their metabolites. This also opens up possibilities for developing microbiome-based immunotherapy supplements in the future.
KAIST (President Kwang Hyung Lee) announced on July 1 that a research team led by Professor Heung Kyu Lee of the Department of Biological Sciences discovered and demonstrated a method to significantly improve the efficiency of glioblastoma immunotherapy by focusing on changes in the gut microbial ecosystem.
The research team noted that as glioblastoma progresses, the concentration of ‘tryptophan’, an important amino acid in the gut, sharply decreases, leading to changes in the gut microbial ecosystem. They discovered that by supplementing tryptophan to restore microbial diversity, specific beneficial strains activate CD8 T cells (a type of immune cell) and induce their infiltration into tumor tissues. Through a mouse model of glioblastoma, the research team confirmed that tryptophan supplementation enhanced the response of cancer-attacking T cells (especially CD8 T cells), leading to their increased migration to tumor sites such as lymph nodes and the brain.
In this process, they also revealed that ‘Duncaniella dubosii’, a beneficial commensal bacterium present in the gut, plays a crucial role. This bacterium helped T cells effectively redistribute within the body, and survival rates significantly improved when used in combination with immunotherapy (anti-PD-1).
Furthermore, it was demonstrated that even when this commensal bacterium was administered alone to germ-free mice (mice without any commensal microbes), the survival rate for glioblastoma increased. This is because the bacterium utilizes tryptophan to regulate the gut environment, and the metabolites produced in this process strengthen the ability of CD8 T cells to attack cancer cells.
Professor Heung Kyu Lee explained, "This research is a meaningful achievement, showing that even in intractable brain tumors where immune checkpoint inhibitors had no effect, a combined strategy utilizing gut microbes can significantly enhance treatment response."
Dr. Hyeon Cheol Kim of KAIST (currently a postdoctoral researcher at the Institute for Biological Sciences) participated as the first author. The research findings were published online in Cell Reports, an international journal in the life sciences, on June 26.
This research was conducted as part of the Basic Research Program and Bio & Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
※Paper Title: Gut microbiota dysbiosis induced by brain tumor modulates the efficacy of immunotherapy
※DOI: https://doi.org/10.1016/j.celrep.2025.115825
2025.07.02 View 840
KAIST Enhances Immunotherapy for Difficult-to-Treat Brain Tumors with Gut Microbiota
< Photo 1.(From left) Prof. Heung Kyu Lee, Department of Biological Sciences,
and Dr. Hyeon Cheol Kim>
Advanced treatments, known as immunotherapies that activate T cells—our body's immune cells—to eliminate cancer cells, have shown limited efficacy as standalone therapies for glioblastoma, the most lethal form of brain tumor. This is due to their minimal response to glioblastoma and high resistance to treatment.
Now, a KAIST research team has now demonstrated a new therapeutic strategy that can enhance the efficacy of immunotherapy for brain tumors by utilizing gut microbes and their metabolites. This also opens up possibilities for developing microbiome-based immunotherapy supplements in the future.
KAIST (President Kwang Hyung Lee) announced on July 1 that a research team led by Professor Heung Kyu Lee of the Department of Biological Sciences discovered and demonstrated a method to significantly improve the efficiency of glioblastoma immunotherapy by focusing on changes in the gut microbial ecosystem.
The research team noted that as glioblastoma progresses, the concentration of ‘tryptophan’, an important amino acid in the gut, sharply decreases, leading to changes in the gut microbial ecosystem. They discovered that by supplementing tryptophan to restore microbial diversity, specific beneficial strains activate CD8 T cells (a type of immune cell) and induce their infiltration into tumor tissues. Through a mouse model of glioblastoma, the research team confirmed that tryptophan supplementation enhanced the response of cancer-attacking T cells (especially CD8 T cells), leading to their increased migration to tumor sites such as lymph nodes and the brain.
In this process, they also revealed that ‘Duncaniella dubosii’, a beneficial commensal bacterium present in the gut, plays a crucial role. This bacterium helped T cells effectively redistribute within the body, and survival rates significantly improved when used in combination with immunotherapy (anti-PD-1).
Furthermore, it was demonstrated that even when this commensal bacterium was administered alone to germ-free mice (mice without any commensal microbes), the survival rate for glioblastoma increased. This is because the bacterium utilizes tryptophan to regulate the gut environment, and the metabolites produced in this process strengthen the ability of CD8 T cells to attack cancer cells.
Professor Heung Kyu Lee explained, "This research is a meaningful achievement, showing that even in intractable brain tumors where immune checkpoint inhibitors had no effect, a combined strategy utilizing gut microbes can significantly enhance treatment response."
Dr. Hyeon Cheol Kim of KAIST (currently a postdoctoral researcher at the Institute for Biological Sciences) participated as the first author. The research findings were published online in Cell Reports, an international journal in the life sciences, on June 26.
This research was conducted as part of the Basic Research Program and Bio & Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
※Paper Title: Gut microbiota dysbiosis induced by brain tumor modulates the efficacy of immunotherapy
※DOI: https://doi.org/10.1016/j.celrep.2025.115825
2025.07.02 View 840 -
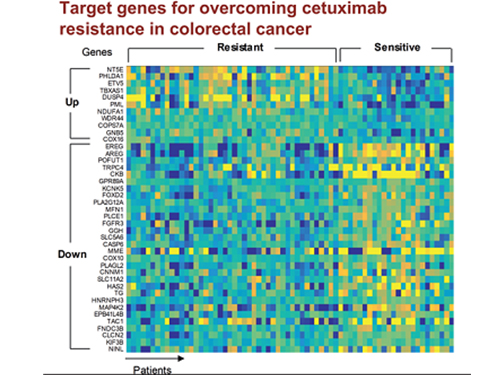 5 Biomarkers for Overcoming Colorectal Cancer Drug Resistance Identified
< Professor Kwang-Hyun Cho's Team >
KAIST researchers have identified five biomarkers that will help them address resistance to cancer-targeting therapeutics. This new treatment strategy will bring us one step closer to precision medicine for patients who showed resistance.
Colorectal cancer is one of the most common types of cancer worldwide. The number of patients has surpassed 1 million, and its five-year survival rate significantly drops to about 20 percent when metastasized. In Korea, the surge of colorectal cancer has been the highest in the last 10 years due to increasing Westernized dietary patterns and obesity. It is expected that the number and mortality rates of colorectal cancer patients will increase sharply as the nation is rapidly facing an increase in its aging population.
Recently, anticancer agents targeting only specific molecules of colon cancer cells have been developed. Unlike conventional anticancer medications, these selectively treat only specific target factors, so they can significantly reduce some of the side-effects of anticancer therapy while enhancing drug efficacy.
Cetuximab is the most well-known FDA approved anticancer medication. It is a biomarker that predicts drug reactivity and utilizes the presence of the ‘KRAS’ gene mutation. Cetuximab is prescribed to patients who don’t carry the KRAS gene mutation.
However, even in patients without the KRAS gene mutation, the response rate of Cetuximab is only about fifty percent, and there is also resistance to drugs after targeted chemotherapy. Compared with conventional chemotherapy alone, the life expectancy only lasts five months on average.
In research featured in the FEBS Journal as the cover paper for the April 7 edition, the KAIST research team led by Professor Kwang-Hyun Cho at the Department of Bio and Brain Engineering presented five additional biomarkers that could increase Cetuximab responsiveness using systems biology approach that combines genomic data analysis, mathematical modeling, and cell experiments. The experimental inhibition of newly discovered biomarkers DUSP4, ETV5, GNB5, NT5E, and PHLDA1 in colorectal cancer cells has been shown to overcome Cetuximab resistance in KRAS-normal genes. The research team confirmed that when suppressing GNB5, one of the new biomarkers, it was shown to overcome resistance to Cetuximab regardless of having a mutation in the KRAS gene.
Professor Cho said, “There has not been an example of colorectal cancer treatment involving regulation of the GNB5 gene.” He continued, “Identifying the principle of drug resistance in cancer cells through systems biology and discovering new biomarkers that could be a new molecular target to overcome drug resistance suggest real potential to actualize precision medicine.”
This study was supported by the National Research Foundation of Korea (NRF) and funded by the Ministry of Science and ICT (2017R1A2A1A17069642 and 2015M3A9A7067220).
Image 1. The cover of FEBS Journal for April 2019
2019.05.27 View 61612
5 Biomarkers for Overcoming Colorectal Cancer Drug Resistance Identified
< Professor Kwang-Hyun Cho's Team >
KAIST researchers have identified five biomarkers that will help them address resistance to cancer-targeting therapeutics. This new treatment strategy will bring us one step closer to precision medicine for patients who showed resistance.
Colorectal cancer is one of the most common types of cancer worldwide. The number of patients has surpassed 1 million, and its five-year survival rate significantly drops to about 20 percent when metastasized. In Korea, the surge of colorectal cancer has been the highest in the last 10 years due to increasing Westernized dietary patterns and obesity. It is expected that the number and mortality rates of colorectal cancer patients will increase sharply as the nation is rapidly facing an increase in its aging population.
Recently, anticancer agents targeting only specific molecules of colon cancer cells have been developed. Unlike conventional anticancer medications, these selectively treat only specific target factors, so they can significantly reduce some of the side-effects of anticancer therapy while enhancing drug efficacy.
Cetuximab is the most well-known FDA approved anticancer medication. It is a biomarker that predicts drug reactivity and utilizes the presence of the ‘KRAS’ gene mutation. Cetuximab is prescribed to patients who don’t carry the KRAS gene mutation.
However, even in patients without the KRAS gene mutation, the response rate of Cetuximab is only about fifty percent, and there is also resistance to drugs after targeted chemotherapy. Compared with conventional chemotherapy alone, the life expectancy only lasts five months on average.
In research featured in the FEBS Journal as the cover paper for the April 7 edition, the KAIST research team led by Professor Kwang-Hyun Cho at the Department of Bio and Brain Engineering presented five additional biomarkers that could increase Cetuximab responsiveness using systems biology approach that combines genomic data analysis, mathematical modeling, and cell experiments. The experimental inhibition of newly discovered biomarkers DUSP4, ETV5, GNB5, NT5E, and PHLDA1 in colorectal cancer cells has been shown to overcome Cetuximab resistance in KRAS-normal genes. The research team confirmed that when suppressing GNB5, one of the new biomarkers, it was shown to overcome resistance to Cetuximab regardless of having a mutation in the KRAS gene.
Professor Cho said, “There has not been an example of colorectal cancer treatment involving regulation of the GNB5 gene.” He continued, “Identifying the principle of drug resistance in cancer cells through systems biology and discovering new biomarkers that could be a new molecular target to overcome drug resistance suggest real potential to actualize precision medicine.”
This study was supported by the National Research Foundation of Korea (NRF) and funded by the Ministry of Science and ICT (2017R1A2A1A17069642 and 2015M3A9A7067220).
Image 1. The cover of FEBS Journal for April 2019
2019.05.27 View 61612 -
 KAIST Unveils the Hidden Control Architecture of Brain Networks
(Professor Kwang-Hyun Cho and his team)
A KAIST research team identified the intrinsic control architecture of brain networks. The control properties will contribute to providing a fundamental basis for the exogenous control of brain networks and, therefore, has broad implications in cognitive and clinical neuroscience.
Although efficiency and robustness are often regarded as having a trade-off relationship, the human brain usually exhibits both attributes when it performs complex cognitive functions. Such optimality must be rooted in a specific coordinated control of interconnected brain regions, but the understanding of the intrinsic control architecture of brain networks is lacking.
Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering and his team investigated the intrinsic control architecture of brain networks. They employed an interdisciplinary approach that spans connectomics, neuroscience, control engineering, network science, and systems biology to examine the structural brain networks of various species and compared them with the control architecture of other biological networks, as well as man-made ones, such as social, infrastructural and technological networks.
In particular, the team reconstructed the structural brain networks of 100 healthy human adults by performing brain parcellation and tractography with structural and diffusion imaging data obtained from the Human Connectome Project database of the US National Institutes of Health.
The team developed a framework for analyzing the control architecture of brain networks based on the minimum dominating set (MDSet), which refers to a minimal subset of nodes (MD-nodes) that control the remaining nodes with a one-step direct interaction. MD-nodes play a crucial role in various complex networks including biomolecular networks, but they have not been investigated in brain networks.
By exploring and comparing the structural principles underlying the composition of MDSets of various complex networks, the team delineated their distinct control architectures. Interestingly, the team found that the proportion of MDSets in brain networks is remarkably small compared to those of other complex networks. This finding implies that brain networks may have been optimized for minimizing the cost required for controlling networks. Furthermore, the team found that the MDSets of brain networks are not solely determined by the degree of nodes, but rather strategically placed to form a particular control architecture.
Consequently, the team revealed the hidden control architecture of brain networks, namely, the distributed and overlapping control architecture that is distinct from other complex networks. The team found that such a particular control architecture brings about robustness against targeted attacks (i.e., preferential attacks on high-degree nodes) which might be a fundamental basis of robust brain functions against preferential damage of high-degree nodes (i.e., brain regions).
Moreover, the team found that the particular control architecture of brain networks also enables high efficiency in switching from one network state, defined by a set of node activities, to another – a capability that is crucial for traversing diverse cognitive states.
Professor Cho said, “This study is the first attempt to make a quantitative comparison between brain networks and other real-world complex networks. Understanding of intrinsic control architecture underlying brain networks may enable the development of optimal interventions for therapeutic purposes or cognitive enhancement.”
This research, led by Byeongwook Lee, Uiryong Kang and Hongjun Chang, was published in iScience (10.1016/j.isci.2019.02.017) on March 29, 2019.
Figure 1. Schematic of identification of control architecture of brain networks.
Figure 2. Identified control architectures of brain networks and other real-world complex networks.
2019.04.23 View 39380
KAIST Unveils the Hidden Control Architecture of Brain Networks
(Professor Kwang-Hyun Cho and his team)
A KAIST research team identified the intrinsic control architecture of brain networks. The control properties will contribute to providing a fundamental basis for the exogenous control of brain networks and, therefore, has broad implications in cognitive and clinical neuroscience.
Although efficiency and robustness are often regarded as having a trade-off relationship, the human brain usually exhibits both attributes when it performs complex cognitive functions. Such optimality must be rooted in a specific coordinated control of interconnected brain regions, but the understanding of the intrinsic control architecture of brain networks is lacking.
Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering and his team investigated the intrinsic control architecture of brain networks. They employed an interdisciplinary approach that spans connectomics, neuroscience, control engineering, network science, and systems biology to examine the structural brain networks of various species and compared them with the control architecture of other biological networks, as well as man-made ones, such as social, infrastructural and technological networks.
In particular, the team reconstructed the structural brain networks of 100 healthy human adults by performing brain parcellation and tractography with structural and diffusion imaging data obtained from the Human Connectome Project database of the US National Institutes of Health.
The team developed a framework for analyzing the control architecture of brain networks based on the minimum dominating set (MDSet), which refers to a minimal subset of nodes (MD-nodes) that control the remaining nodes with a one-step direct interaction. MD-nodes play a crucial role in various complex networks including biomolecular networks, but they have not been investigated in brain networks.
By exploring and comparing the structural principles underlying the composition of MDSets of various complex networks, the team delineated their distinct control architectures. Interestingly, the team found that the proportion of MDSets in brain networks is remarkably small compared to those of other complex networks. This finding implies that brain networks may have been optimized for minimizing the cost required for controlling networks. Furthermore, the team found that the MDSets of brain networks are not solely determined by the degree of nodes, but rather strategically placed to form a particular control architecture.
Consequently, the team revealed the hidden control architecture of brain networks, namely, the distributed and overlapping control architecture that is distinct from other complex networks. The team found that such a particular control architecture brings about robustness against targeted attacks (i.e., preferential attacks on high-degree nodes) which might be a fundamental basis of robust brain functions against preferential damage of high-degree nodes (i.e., brain regions).
Moreover, the team found that the particular control architecture of brain networks also enables high efficiency in switching from one network state, defined by a set of node activities, to another – a capability that is crucial for traversing diverse cognitive states.
Professor Cho said, “This study is the first attempt to make a quantitative comparison between brain networks and other real-world complex networks. Understanding of intrinsic control architecture underlying brain networks may enable the development of optimal interventions for therapeutic purposes or cognitive enhancement.”
This research, led by Byeongwook Lee, Uiryong Kang and Hongjun Chang, was published in iScience (10.1016/j.isci.2019.02.017) on March 29, 2019.
Figure 1. Schematic of identification of control architecture of brain networks.
Figure 2. Identified control architectures of brain networks and other real-world complex networks.
2019.04.23 View 39380 -
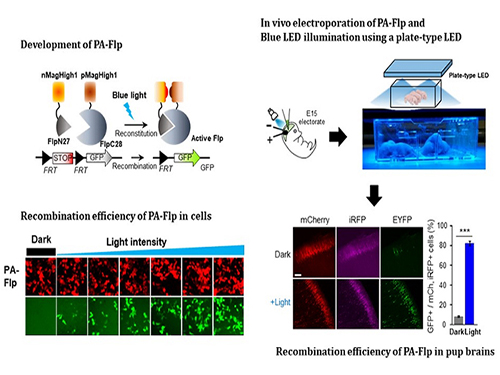 Noninvasive Light-Sensitive Recombinase for Deep Brain Genetic Manipulation
A KAIST team presented a noninvasive light-sensitive photoactivatable recombinase suitable for genetic manipulation in vivo. The highly light-sensitive property of photoactivatable Flp recombinase will be ideal for controlling genetic manipulation in deep mouse brain regions by illumination with a noninvasive light-emitting diode. This easy-to-use optogenetic module made by Professor Won Do Heo and his team will provide a side-effect free and expandable genetic manipulation tool for neuroscience research.
Spatiotemporal control of gene expression has been acclaimed as a valuable strategy for identifying functions of genes with complex neural circuits. Studies of complex brain functions require highly sophisticated and robust technologies that enable specific labeling and rapid genetic modification in live animals. A number of approaches for controlling the activity of proteins or expression of genes in a spatiotemporal manner using light, small molecules, hormones, and peptides have been developed for manipulating intact circuits or functions.
Among them, recombination-employing, chemically inducible systems are the most commonly used in vivo gene-modification systems. Other approaches include selective or conditional Cre-activation systems within subsets of green fluorescent protein-expressing cells or dual-promoter-driven intersectional populations of cells.
However, these methods are limited by the considerable time and effort required to establish knock-in mouse lines and by constraints on spatiotemporal control, which relies on a limited set of available genetic promoters and transgenic mouse resources.
Beyond these constraints, optogenetic approaches allow the activity of genetically defined neurons in the mouse brain to be controlled with high spatiotemporal resolution. However, an optogenetic module for gene-manipulation capable of revealing the spatiotemporal functions of specific target genes in the mouse brain has remained a challenge.
In the study published at Nature Communication on Jan. 18, the team featured photoactivatable Flp recombinase by searching out split sites of Flp recombinase that were not previously identified, being capable of reconstitution to be active. The team validated the highly light-sensitive, efficient performance of photoactivatable Flp recombinase through precise light targeting by showing transgene expression within anatomically confined mouse brain regions.
The concept of local genetic labeling presented here suggests a new approach for genetically identifying subpopulations of cells defined by the spatial and temporal characteristics of light delivery. To date, an optogenetic module for gene-manipulation capable of revealing spatiotemporal functions of specific target genes in the mouse brain has remained out of reach and no such light-inducible Flp system has been developed. Accordingly, the team sought to develop a photoactivatable Flp recombinase that takes full advantage of the high spatiotemporal control offered by light stimulation.
This activation through noninvasive light illumination deep inside the brain is advantageous in that it avoids chemical or optic fiber implantation-mediated side effects, such as off-target cytotoxicity or physical lesions that might influence animal physiology or behaviors. The technique provides expandable utilities for transgene expression systems upon Flp recombinase activity in vivo, by designing a viral vector for minimal leaky expression influenced by viral nascent promoters.
The team demonstrated the utility of PA-Flp as a noninvasive in vivo optogenetic manipulation tool for use in the mouse brain, even applicable for deep brain structures as it can reach the hippocampus or medial septum using external LED light illumination.
The study is the result of five years of research by Professor Heo, who has led the bio-imaging and optogenetics fields by developing his own bio-imaging and optogenetics technologies. “It will be a great advantage to control specific gene expression desired by LEDs with little physical and chemical stimulation that can affect the physiological phenomenon in living animals,” he explained.
2019.01.22 View 8294
Noninvasive Light-Sensitive Recombinase for Deep Brain Genetic Manipulation
A KAIST team presented a noninvasive light-sensitive photoactivatable recombinase suitable for genetic manipulation in vivo. The highly light-sensitive property of photoactivatable Flp recombinase will be ideal for controlling genetic manipulation in deep mouse brain regions by illumination with a noninvasive light-emitting diode. This easy-to-use optogenetic module made by Professor Won Do Heo and his team will provide a side-effect free and expandable genetic manipulation tool for neuroscience research.
Spatiotemporal control of gene expression has been acclaimed as a valuable strategy for identifying functions of genes with complex neural circuits. Studies of complex brain functions require highly sophisticated and robust technologies that enable specific labeling and rapid genetic modification in live animals. A number of approaches for controlling the activity of proteins or expression of genes in a spatiotemporal manner using light, small molecules, hormones, and peptides have been developed for manipulating intact circuits or functions.
Among them, recombination-employing, chemically inducible systems are the most commonly used in vivo gene-modification systems. Other approaches include selective or conditional Cre-activation systems within subsets of green fluorescent protein-expressing cells or dual-promoter-driven intersectional populations of cells.
However, these methods are limited by the considerable time and effort required to establish knock-in mouse lines and by constraints on spatiotemporal control, which relies on a limited set of available genetic promoters and transgenic mouse resources.
Beyond these constraints, optogenetic approaches allow the activity of genetically defined neurons in the mouse brain to be controlled with high spatiotemporal resolution. However, an optogenetic module for gene-manipulation capable of revealing the spatiotemporal functions of specific target genes in the mouse brain has remained a challenge.
In the study published at Nature Communication on Jan. 18, the team featured photoactivatable Flp recombinase by searching out split sites of Flp recombinase that were not previously identified, being capable of reconstitution to be active. The team validated the highly light-sensitive, efficient performance of photoactivatable Flp recombinase through precise light targeting by showing transgene expression within anatomically confined mouse brain regions.
The concept of local genetic labeling presented here suggests a new approach for genetically identifying subpopulations of cells defined by the spatial and temporal characteristics of light delivery. To date, an optogenetic module for gene-manipulation capable of revealing spatiotemporal functions of specific target genes in the mouse brain has remained out of reach and no such light-inducible Flp system has been developed. Accordingly, the team sought to develop a photoactivatable Flp recombinase that takes full advantage of the high spatiotemporal control offered by light stimulation.
This activation through noninvasive light illumination deep inside the brain is advantageous in that it avoids chemical or optic fiber implantation-mediated side effects, such as off-target cytotoxicity or physical lesions that might influence animal physiology or behaviors. The technique provides expandable utilities for transgene expression systems upon Flp recombinase activity in vivo, by designing a viral vector for minimal leaky expression influenced by viral nascent promoters.
The team demonstrated the utility of PA-Flp as a noninvasive in vivo optogenetic manipulation tool for use in the mouse brain, even applicable for deep brain structures as it can reach the hippocampus or medial septum using external LED light illumination.
The study is the result of five years of research by Professor Heo, who has led the bio-imaging and optogenetics fields by developing his own bio-imaging and optogenetics technologies. “It will be a great advantage to control specific gene expression desired by LEDs with little physical and chemical stimulation that can affect the physiological phenomenon in living animals,” he explained.
2019.01.22 View 8294 -
 Ultrathin Digital Camera Inspired by Xenos Peckii Eyes
(Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering)
The visual system of Xenos peckii, an endoparasite of paper wasps, demonstrates distinct benefits for high sensitivity and high resolution, differing from the compound eyes of most insects. Taking their unique features, a KAIST team developed an ultrathin digital camera that emulates the unique eyes of Xenos peckii.
The ultrathin digital camera offers a wide field of view and high resolution in a slimmer body compared to existing imaging systems. It is expected to support various applications, such as monitoring equipment, medical imaging devices, and mobile imaging systems.
Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering and his team are known for mimicking biological visual organs. The team’s past research includes an LED lens based on the abdominal segments of fireflies and biologically inspired anti-reflective structures.
Recently, the demand for ultrathin digital cameras has increased, due to the miniaturization of electronic and optical devices. However, most camera modules use multiple lenses along the optical axis to compensate for optical aberrations, resulting in a larger volume as well as a thicker total track length of digital cameras. Resolution and sensitivity would be compromised if these modules were to be simply reduced in size and thickness.
To address this issue, the team have developed micro-optical components, inspired from the visual system of Xenos peckii, and combined them with a CMOS (complementary metal oxide semiconductor) image sensor to achieve an ultrathin digital camera.
This new camera, measuring less than 2mm in thickness, emulates the eyes of Xenos peckii by using dozens of microprism arrays and microlens arrays. A microprism and microlens pair form a channel and the light-absorbing medium between the channels reduces optical crosstalk. Each channel captures the partial image at slightly different orientation, and the retrieved partial images are combined into a single image, thereby ensuring a wide field of view and high resolution.
Professor Jeong said, “We have proposed a novel method of fabricating an ultrathin camera. As the first insect-inspired, ultrathin camera that integrates a microcamera on a conventional CMOS image sensor array, our study will have a significant impact in optics and related fields.”
This research, led by PhD candidates Dongmin Keum and Kyung-Won Jang, was published in Light: Science & Applications on October 24, 2018.
Figure 1. Natural Xenos peckii eye and the biological inspiration for the ultrathin digital camera (Light: Science & Applications 2018)
Figure 2. Optical images captured by the bioinspired ultrathin digital camera (Light: Science & Applications 2018)
2018.12.31 View 9795
Ultrathin Digital Camera Inspired by Xenos Peckii Eyes
(Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering)
The visual system of Xenos peckii, an endoparasite of paper wasps, demonstrates distinct benefits for high sensitivity and high resolution, differing from the compound eyes of most insects. Taking their unique features, a KAIST team developed an ultrathin digital camera that emulates the unique eyes of Xenos peckii.
The ultrathin digital camera offers a wide field of view and high resolution in a slimmer body compared to existing imaging systems. It is expected to support various applications, such as monitoring equipment, medical imaging devices, and mobile imaging systems.
Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering and his team are known for mimicking biological visual organs. The team’s past research includes an LED lens based on the abdominal segments of fireflies and biologically inspired anti-reflective structures.
Recently, the demand for ultrathin digital cameras has increased, due to the miniaturization of electronic and optical devices. However, most camera modules use multiple lenses along the optical axis to compensate for optical aberrations, resulting in a larger volume as well as a thicker total track length of digital cameras. Resolution and sensitivity would be compromised if these modules were to be simply reduced in size and thickness.
To address this issue, the team have developed micro-optical components, inspired from the visual system of Xenos peckii, and combined them with a CMOS (complementary metal oxide semiconductor) image sensor to achieve an ultrathin digital camera.
This new camera, measuring less than 2mm in thickness, emulates the eyes of Xenos peckii by using dozens of microprism arrays and microlens arrays. A microprism and microlens pair form a channel and the light-absorbing medium between the channels reduces optical crosstalk. Each channel captures the partial image at slightly different orientation, and the retrieved partial images are combined into a single image, thereby ensuring a wide field of view and high resolution.
Professor Jeong said, “We have proposed a novel method of fabricating an ultrathin camera. As the first insect-inspired, ultrathin camera that integrates a microcamera on a conventional CMOS image sensor array, our study will have a significant impact in optics and related fields.”
This research, led by PhD candidates Dongmin Keum and Kyung-Won Jang, was published in Light: Science & Applications on October 24, 2018.
Figure 1. Natural Xenos peckii eye and the biological inspiration for the ultrathin digital camera (Light: Science & Applications 2018)
Figure 2. Optical images captured by the bioinspired ultrathin digital camera (Light: Science & Applications 2018)
2018.12.31 View 9795 -
 Skin Hardness to Estimate Better Human Thermal Status
(Professor Young-Ho Cho and Researcher Sunghyun Yoon)
Under the same temperature and humidity, human thermal status may vary due to individual body constitution and climatic environment. A KAIST research team previously developed a wearable sweat rate sensor for human thermal comfort monitoring. Furthering the development, this time they proposed skin hardness as an additional, independent physiological sign to estimate human thermal status more accurately. This novel approach can be applied to developing systems incorporating human-machine interaction, which requires accurate information about human thermal status.
Professor Young-Ho Cho and his team from the Department of Bio and Brain Engineering had previously studied skin temperature and sweat rate to determine human thermal comfort, and developed a watch-type sweat rate sensor that accurately and steadily detects thermal comfort last February (title: Wearable Sweat Rate Sensors for Human Thermal Comfort Monitoring ).
However, skin temperature and sweat rate are still not enough to estimate exact human thermal comfort. Hence, an additional indicator is required for enhancing the accuracy and reliability of the estimation and the team selected skin hardness. When people feel hot or cold, arrector pili muscles connected to hair follicles contract and expand, and skin hardness comes from this contraction and relaxation of the muscles. Based on the phenomenon of changing skin hardness, the team proposed skin hardness as a new indicator for measuring human thermal sensation.
With this new estimation model using three physiological signs for estimating human thermal status, the team conducted human experiments and verified that skin hardness is effective and independent from the two conventional physiological signs. Adding skin hardness to the conventional model can reduce errors by 23.5%, which makes its estimation more reliable.
The team will develop a sensor that detects skin hardness and applies it to cognitive air-conditioning and heating systems that better interact with humans than existing systems.
Professor Cho said, “Introducing this new indicator, skin hardness, elevates the reliability of measuring human thermal comfort regardless of individual body constitution and climatic environment. Based on this method, we can develop a personalized air conditioning and heating system that will allow affective interaction between humans and machines by sharing both physical and mental health conditions and emotions.”
This research, led by researchers Sunghyun Yoon and Jai Kyoung Sim, was published in Scientific Reports, Vol.8, Article No.12027 on August 13, 2018. (pp.1-6)
Figure 1. Measuring human thermal status through skin hardness
Figure 2. The instrument used for measuring human thermal status through skin hardness
2018.10.17 View 7374
Skin Hardness to Estimate Better Human Thermal Status
(Professor Young-Ho Cho and Researcher Sunghyun Yoon)
Under the same temperature and humidity, human thermal status may vary due to individual body constitution and climatic environment. A KAIST research team previously developed a wearable sweat rate sensor for human thermal comfort monitoring. Furthering the development, this time they proposed skin hardness as an additional, independent physiological sign to estimate human thermal status more accurately. This novel approach can be applied to developing systems incorporating human-machine interaction, which requires accurate information about human thermal status.
Professor Young-Ho Cho and his team from the Department of Bio and Brain Engineering had previously studied skin temperature and sweat rate to determine human thermal comfort, and developed a watch-type sweat rate sensor that accurately and steadily detects thermal comfort last February (title: Wearable Sweat Rate Sensors for Human Thermal Comfort Monitoring ).
However, skin temperature and sweat rate are still not enough to estimate exact human thermal comfort. Hence, an additional indicator is required for enhancing the accuracy and reliability of the estimation and the team selected skin hardness. When people feel hot or cold, arrector pili muscles connected to hair follicles contract and expand, and skin hardness comes from this contraction and relaxation of the muscles. Based on the phenomenon of changing skin hardness, the team proposed skin hardness as a new indicator for measuring human thermal sensation.
With this new estimation model using three physiological signs for estimating human thermal status, the team conducted human experiments and verified that skin hardness is effective and independent from the two conventional physiological signs. Adding skin hardness to the conventional model can reduce errors by 23.5%, which makes its estimation more reliable.
The team will develop a sensor that detects skin hardness and applies it to cognitive air-conditioning and heating systems that better interact with humans than existing systems.
Professor Cho said, “Introducing this new indicator, skin hardness, elevates the reliability of measuring human thermal comfort regardless of individual body constitution and climatic environment. Based on this method, we can develop a personalized air conditioning and heating system that will allow affective interaction between humans and machines by sharing both physical and mental health conditions and emotions.”
This research, led by researchers Sunghyun Yoon and Jai Kyoung Sim, was published in Scientific Reports, Vol.8, Article No.12027 on August 13, 2018. (pp.1-6)
Figure 1. Measuring human thermal status through skin hardness
Figure 2. The instrument used for measuring human thermal status through skin hardness
2018.10.17 View 7374 -
 Trigger of the Hyperactivation of Fibrosis Identified
(Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering)
Scientists have been investigating the negative effects that the hyperactivation of fibrosis has on fibrotic diseases and cancer. A KAIST research team unveiled a positive feedback loop that bi-stably activates fibroblasts in collaboration with Samsung Medical Center. This finding will contribute to developing therapeutic targets for both fibrosis and cancer.
Human fibroblasts are dormant in normal tissue, but show radical activation during wound healing. However, the principle that induces their explosive activation has not yet been identified.
Here, Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering, in collaboration with Professor Seok-Hyung Kim from Samsung Medical Center, discovered the principle of a circuit that continuously activates fibroblasts.
They constructed a positive feedback loops (PFLs) where Twist1, Prrx1, and Tenascin-C (TNC) molecules consecutively activate fibroblasts. They confirmed that these are the main inducers of fibroblast activation by conducting various experiments, including molecular biological tests, mathematical modeling, animal testing, and computer simulations to conclude that they are the main inducers of fibroblast activation.
According to their research, Twist 1 is a key regulator of cancer-associated fibroblasts, which directly upregulates Prrx1 and then triggers TNC, which also increases Twist1 expression. This circuit consequently forms a Twist-Prrx1-TNC positive feedback loop.
Activated fibroblasts need to be deactivated after wounds are healed. However, if the PFLs continue, the fibroblasts become the major cause of worsening fibrotic diseases and cancers.
Therefore, the team expects that Twist1-Prrx1-TNC positive PFLs will be applied for novel and effective therapeutic targeting of fibrotic diseases and cancers.
This research was published in Nature Communications on August 1, 2018.
Figure 1. Twist1 increases tenascin-c expression in cancer-associated fibroblasts. Twist1 is a potent but indirect inducer of tenascin-c (TNC), which is essential for maintaining Twist1 expression in cancer-associated fibroblasts (CAFs).
Figure 2. Summary of the study. The Twist1-Prrx1-TNC positive feedback regulation provides clues for understanding the activation of fibroblasts during wound healing under normal conditions, as well as abnormally activated fibroblasts in pathological conditions such as cancerous and fibrotic diseases. Under normal conditions, the PFL acts as a reversible bistable switch by which the activation of fibroblasts is “ON" above a sufficient level of stimulation and “OFF" for the withdrawal of the stimulus. However, this switch can be permanently turned on under pathologic conditions by continued activation of the PFL, resulting in sustained proliferation of fibroblasts.
2018.10.11 View 7156
Trigger of the Hyperactivation of Fibrosis Identified
(Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering)
Scientists have been investigating the negative effects that the hyperactivation of fibrosis has on fibrotic diseases and cancer. A KAIST research team unveiled a positive feedback loop that bi-stably activates fibroblasts in collaboration with Samsung Medical Center. This finding will contribute to developing therapeutic targets for both fibrosis and cancer.
Human fibroblasts are dormant in normal tissue, but show radical activation during wound healing. However, the principle that induces their explosive activation has not yet been identified.
Here, Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering, in collaboration with Professor Seok-Hyung Kim from Samsung Medical Center, discovered the principle of a circuit that continuously activates fibroblasts.
They constructed a positive feedback loops (PFLs) where Twist1, Prrx1, and Tenascin-C (TNC) molecules consecutively activate fibroblasts. They confirmed that these are the main inducers of fibroblast activation by conducting various experiments, including molecular biological tests, mathematical modeling, animal testing, and computer simulations to conclude that they are the main inducers of fibroblast activation.
According to their research, Twist 1 is a key regulator of cancer-associated fibroblasts, which directly upregulates Prrx1 and then triggers TNC, which also increases Twist1 expression. This circuit consequently forms a Twist-Prrx1-TNC positive feedback loop.
Activated fibroblasts need to be deactivated after wounds are healed. However, if the PFLs continue, the fibroblasts become the major cause of worsening fibrotic diseases and cancers.
Therefore, the team expects that Twist1-Prrx1-TNC positive PFLs will be applied for novel and effective therapeutic targeting of fibrotic diseases and cancers.
This research was published in Nature Communications on August 1, 2018.
Figure 1. Twist1 increases tenascin-c expression in cancer-associated fibroblasts. Twist1 is a potent but indirect inducer of tenascin-c (TNC), which is essential for maintaining Twist1 expression in cancer-associated fibroblasts (CAFs).
Figure 2. Summary of the study. The Twist1-Prrx1-TNC positive feedback regulation provides clues for understanding the activation of fibroblasts during wound healing under normal conditions, as well as abnormally activated fibroblasts in pathological conditions such as cancerous and fibrotic diseases. Under normal conditions, the PFL acts as a reversible bistable switch by which the activation of fibroblasts is “ON" above a sufficient level of stimulation and “OFF" for the withdrawal of the stimulus. However, this switch can be permanently turned on under pathologic conditions by continued activation of the PFL, resulting in sustained proliferation of fibroblasts.
2018.10.11 View 7156 -
 Mathematical Principle behind AI's 'Black Box'
(from left: Professor Jong Chul Ye, PhD candidates Yoseob Han and Eunju Cha)
A KAIST research team identified the geometrical structure of artificial intelligence (AI) and discovered the mathematical principles of highly performing artificial neural networks, which can be applicable in fields such as medical imaging.
Deep neural networks are an exemplary method of implementing deep learning, which is at the core of the AI technology, and have shown explosive growth in recent years. This technique has been used in various fields, such as image and speech recognition as well as image processing.
Despite its excellent performance and usefulness, the exact working principles of deep neural networks has not been well understood, and they often suffer from unexpected results or errors. Hence, there is an increasing social and technical demand for interpretable deep neural network models.
To address these issues, Professor Jong Chul Ye from the Department of Bio & Brain Engineering and his team attempted to find the geometric structure in a higher dimensional space where the structure of the deep neural network can be easily understood. They proposed a general deep learning framework, called deep convolutional framelets, to understand the mathematical principle of a deep neural network in terms of the mathematical tools in Harmonic analysis.
As a result, it was found that deep neural networks’ structure appears during the process of decomposition of high dimensionally lifted signal via Hankel matrix, which is a high-dimensional structure formerly studied intensively in the field of signal processing.
In the process of decomposing the lifted signal, two bases categorized as local and non-local basis emerge. The researchers found that non-local and local basis functions play a role in pooling and filtering operation in convolutional neural network, respectively.
Previously, when implementing AI, deep neural networks were usually constructed through empirical trial and errors. The significance of the research lies in the fact that it provides a mathematical understanding on the neural network structure in high dimensional space, which guides users to design an optimized neural network.
They demonstrated improved performance of the deep convolutional framelets’ neural networks in the applications of image denoising, image pixel in painting, and medical image restoration.
Professor Ye said, “Unlike conventional neural networks designed through trial-and-error, our theory shows that neural network structure can be optimized to each desired application and are easily predictable in their effects by exploiting the high dimensional geometry. This technology can be applied to a variety of fields requiring interpretation of the architecture, such as medical imaging.”
This research, led by PhD candidates Yoseob Han and Eunju Cha, was published in the April 26th issue of the SIAM Journal on Imaging Sciences.
Figure 1. The design of deep neural network using mathematical principles
Figure 2. The results of image noise cancelling
Figure 3. The artificial neural network restoration results in the case where 80% of the pixels are lost
2018.09.12 View 6979
Mathematical Principle behind AI's 'Black Box'
(from left: Professor Jong Chul Ye, PhD candidates Yoseob Han and Eunju Cha)
A KAIST research team identified the geometrical structure of artificial intelligence (AI) and discovered the mathematical principles of highly performing artificial neural networks, which can be applicable in fields such as medical imaging.
Deep neural networks are an exemplary method of implementing deep learning, which is at the core of the AI technology, and have shown explosive growth in recent years. This technique has been used in various fields, such as image and speech recognition as well as image processing.
Despite its excellent performance and usefulness, the exact working principles of deep neural networks has not been well understood, and they often suffer from unexpected results or errors. Hence, there is an increasing social and technical demand for interpretable deep neural network models.
To address these issues, Professor Jong Chul Ye from the Department of Bio & Brain Engineering and his team attempted to find the geometric structure in a higher dimensional space where the structure of the deep neural network can be easily understood. They proposed a general deep learning framework, called deep convolutional framelets, to understand the mathematical principle of a deep neural network in terms of the mathematical tools in Harmonic analysis.
As a result, it was found that deep neural networks’ structure appears during the process of decomposition of high dimensionally lifted signal via Hankel matrix, which is a high-dimensional structure formerly studied intensively in the field of signal processing.
In the process of decomposing the lifted signal, two bases categorized as local and non-local basis emerge. The researchers found that non-local and local basis functions play a role in pooling and filtering operation in convolutional neural network, respectively.
Previously, when implementing AI, deep neural networks were usually constructed through empirical trial and errors. The significance of the research lies in the fact that it provides a mathematical understanding on the neural network structure in high dimensional space, which guides users to design an optimized neural network.
They demonstrated improved performance of the deep convolutional framelets’ neural networks in the applications of image denoising, image pixel in painting, and medical image restoration.
Professor Ye said, “Unlike conventional neural networks designed through trial-and-error, our theory shows that neural network structure can be optimized to each desired application and are easily predictable in their effects by exploiting the high dimensional geometry. This technology can be applied to a variety of fields requiring interpretation of the architecture, such as medical imaging.”
This research, led by PhD candidates Yoseob Han and Eunju Cha, was published in the April 26th issue of the SIAM Journal on Imaging Sciences.
Figure 1. The design of deep neural network using mathematical principles
Figure 2. The results of image noise cancelling
Figure 3. The artificial neural network restoration results in the case where 80% of the pixels are lost
2018.09.12 View 6979 -
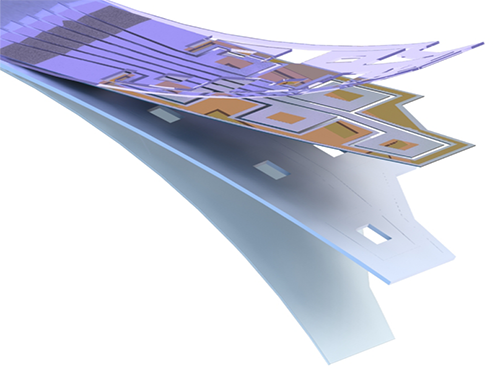 Flexible Drug Delivery Microdevice to Advance Precision Medicine
(Schematic view of flexible microdevice: The flexible drug delivery device for controlled release fabricated via inorganic laser lift off.)
A KAIST research team has developed a flexible drug delivery device with controlled release for personalized medicine, blazing the path toward theragnosis.
Theragnosis, an emerging medical technology, is gaining attention as key factor to advance precision medicine for its featuring simultaneous diagnosis and therapeutics. Theragnosis devices including smart contact lenses and microneedle patches integrate physiological data sensors and drug delivery devices. The controlled drug delivery boasts fewer side-effects, uniform therapeutic results, and minimal dosages compared to oral ingestion. Recently, some research groups conducted in-human applications of controlled-release bulky microchips for osteoporosis treatment. However they failed to demonstrate successful human-friendly flexible drug delivery systems for controlled release.
For this microdevice, the team under Professor Daesoo Kim from the Department of Biological Science and Professor Keon Jae Lee from the Department of Materials Science and Engineering, fabricated a device on a rigid substrate and transferred a 50 µm-thick active drug delivery layer to the flexible substrate via inorganic laser lift off. The fabricated device shows mechanical flexibility while maintaining the capability of precise administration of exact dosages at desired times. The core technology is to produce a freestanding gold capping layer directly on top of the microreservoir with the drugs inside, which had been regarded as impossible in conventional microfabrication.
The developed flexible drug delivery system can be applied to smart contact lenses or the brain disease treatments by implanting them into cramped and corrugated organs. In addition, when powered wirelessly, it will represent a novel platform for personalized medicine. The team already proved through animal experimentation that treatment for brain epilepsy made progress by releasing anti-epileptic medication through the device.
Professor Lee believes the flexible microdevice will further expand the applications of smart contact lenses, therapeutic treatments for brain disease, and subcutaneous implantations for daily healthcare system. This study “Flexible Wireless Powered Drug Delivery System for Targeted Administration on Cerebral Cortex” was described in the June online issue of Nano Energy.
(Photo: The flexible drug delivery device for contolled relase attached on a glass rod.)
2018.08.13 View 10133
Flexible Drug Delivery Microdevice to Advance Precision Medicine
(Schematic view of flexible microdevice: The flexible drug delivery device for controlled release fabricated via inorganic laser lift off.)
A KAIST research team has developed a flexible drug delivery device with controlled release for personalized medicine, blazing the path toward theragnosis.
Theragnosis, an emerging medical technology, is gaining attention as key factor to advance precision medicine for its featuring simultaneous diagnosis and therapeutics. Theragnosis devices including smart contact lenses and microneedle patches integrate physiological data sensors and drug delivery devices. The controlled drug delivery boasts fewer side-effects, uniform therapeutic results, and minimal dosages compared to oral ingestion. Recently, some research groups conducted in-human applications of controlled-release bulky microchips for osteoporosis treatment. However they failed to demonstrate successful human-friendly flexible drug delivery systems for controlled release.
For this microdevice, the team under Professor Daesoo Kim from the Department of Biological Science and Professor Keon Jae Lee from the Department of Materials Science and Engineering, fabricated a device on a rigid substrate and transferred a 50 µm-thick active drug delivery layer to the flexible substrate via inorganic laser lift off. The fabricated device shows mechanical flexibility while maintaining the capability of precise administration of exact dosages at desired times. The core technology is to produce a freestanding gold capping layer directly on top of the microreservoir with the drugs inside, which had been regarded as impossible in conventional microfabrication.
The developed flexible drug delivery system can be applied to smart contact lenses or the brain disease treatments by implanting them into cramped and corrugated organs. In addition, when powered wirelessly, it will represent a novel platform for personalized medicine. The team already proved through animal experimentation that treatment for brain epilepsy made progress by releasing anti-epileptic medication through the device.
Professor Lee believes the flexible microdevice will further expand the applications of smart contact lenses, therapeutic treatments for brain disease, and subcutaneous implantations for daily healthcare system. This study “Flexible Wireless Powered Drug Delivery System for Targeted Administration on Cerebral Cortex” was described in the June online issue of Nano Energy.
(Photo: The flexible drug delivery device for contolled relase attached on a glass rod.)
2018.08.13 View 10133 -
 How to Trigger Innate Fear Response?
(Figure:This illustration describes how ACC-BLA circuit controls innate freezing response depending on its activity level.)
When animals encounter danger, they usually respond to the situation in one of two ways: to freeze or to flee. How do they make this quick decision in a life or death moment?
According to KAIST neuroscientists, there are two types of fear: learned versus innate. The latter is known to be induced without any prior experience and is thus naturally encoded in the brain. A research team under Professor Jin-Hee Han in the Department of Biological Sciences identified the brain circuit responsible for regulating the innate fear response.
The study, which appeared in the July 24 issue of Nature Communications represents a significant step toward understanding how the neural circuits in the prefrontal cortex create behavioral responses to external threats. This also represents a new paradigm in therapeutic development for fear-related mental disorders.
Responses of freezing or fleeing when facing external threats reflect behavioral and physiological changes in an instinctive move to adapt to the new environment for survival. These responses are controlled by the emotional circuit systems of the brain and the malfunction of this circuit leads to fear-related disorders.
The anterior cingulate cortex (ACC) is a sub-region within the prefrontal cortex, comprising a part of the brain circuitry that regulates behavioral and physiological fear responses. This area is capable of high-order processing of the perceived sensory information and conveys ‘top-down’ information toward the amygdala and brainstem areas, known as the response outlet.
Many studies have already demonstrated that the brain regions in the prefrontal cortex regulate the response against learned threats. However, it has been unknown how innate responses against fear are encoded in the neural circuits in the prefrontal cortex.
Dr. Jinho Jhang, the lead author of the study explains how the team achieved their key idea. “Many overseas studies have already proved that the prefrontal cortex circuit works to regulate the fear response. However, researchers have paid little attention to the innate response against predators. Professor Han suggested we do research on the instinctive fear response instead of the learned response. We particularly focused on the anterior cingulate region, which has been connected with memory, pain, and sympathy, but not the fear response itself. Since we turned in this new direction, we have accumulated some significant data,” said Dr. Jhang.
For this study, Professor Han’s team investigated how mice react when exposed to the olfactory stimuli of predators. Based on the results of optogenetic manipulation, neural circuit tracing, and ex vivo slice electrophysiology experiments, the team demonstrated that the anterior cingulate cortex and its projection input to the basolateral amygdala play a role in the inhibitory regulation of innate fear responses to predators’ odors in mice.
Professor Han believes these results will extend the understanding of how instinctive fear responses can be encoded in our brain circuits. “Our findings will help to develop therapeutic treatments for mental disorders aroused from fear such as panic disorders and post-traumatic stress disorder,” said Professor Han.
2018.08.08 View 8529
How to Trigger Innate Fear Response?
(Figure:This illustration describes how ACC-BLA circuit controls innate freezing response depending on its activity level.)
When animals encounter danger, they usually respond to the situation in one of two ways: to freeze or to flee. How do they make this quick decision in a life or death moment?
According to KAIST neuroscientists, there are two types of fear: learned versus innate. The latter is known to be induced without any prior experience and is thus naturally encoded in the brain. A research team under Professor Jin-Hee Han in the Department of Biological Sciences identified the brain circuit responsible for regulating the innate fear response.
The study, which appeared in the July 24 issue of Nature Communications represents a significant step toward understanding how the neural circuits in the prefrontal cortex create behavioral responses to external threats. This also represents a new paradigm in therapeutic development for fear-related mental disorders.
Responses of freezing or fleeing when facing external threats reflect behavioral and physiological changes in an instinctive move to adapt to the new environment for survival. These responses are controlled by the emotional circuit systems of the brain and the malfunction of this circuit leads to fear-related disorders.
The anterior cingulate cortex (ACC) is a sub-region within the prefrontal cortex, comprising a part of the brain circuitry that regulates behavioral and physiological fear responses. This area is capable of high-order processing of the perceived sensory information and conveys ‘top-down’ information toward the amygdala and brainstem areas, known as the response outlet.
Many studies have already demonstrated that the brain regions in the prefrontal cortex regulate the response against learned threats. However, it has been unknown how innate responses against fear are encoded in the neural circuits in the prefrontal cortex.
Dr. Jinho Jhang, the lead author of the study explains how the team achieved their key idea. “Many overseas studies have already proved that the prefrontal cortex circuit works to regulate the fear response. However, researchers have paid little attention to the innate response against predators. Professor Han suggested we do research on the instinctive fear response instead of the learned response. We particularly focused on the anterior cingulate region, which has been connected with memory, pain, and sympathy, but not the fear response itself. Since we turned in this new direction, we have accumulated some significant data,” said Dr. Jhang.
For this study, Professor Han’s team investigated how mice react when exposed to the olfactory stimuli of predators. Based on the results of optogenetic manipulation, neural circuit tracing, and ex vivo slice electrophysiology experiments, the team demonstrated that the anterior cingulate cortex and its projection input to the basolateral amygdala play a role in the inhibitory regulation of innate fear responses to predators’ odors in mice.
Professor Han believes these results will extend the understanding of how instinctive fear responses can be encoded in our brain circuits. “Our findings will help to develop therapeutic treatments for mental disorders aroused from fear such as panic disorders and post-traumatic stress disorder,” said Professor Han.
2018.08.08 View 8529