Nano+Letter
-
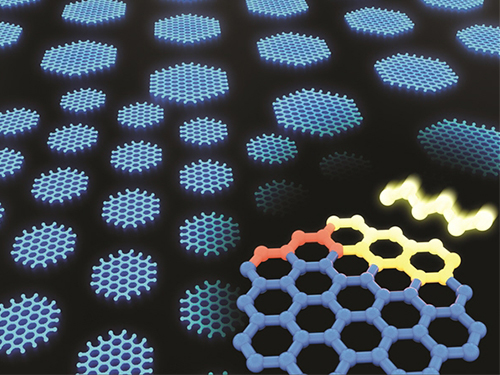 Synthesizing Single-Crystalline Hexagonal Graphene Quantum Dots
(Figure: Uniformly ordered single-crystalline graphene quantum dots of various sizes synthesized through solution chemistry.)
A KAIST team has designed a novel strategy for synthesizing single-crystalline graphene quantum dots, which emit stable blue light. The research team confirmed that a display made of their synthesized graphene quantum dots successfully emitted blue light with stable electric pressure, reportedly resolving the long-standing challenges of blue light emission in manufactured displays. The study, led by Professor O Ok Park in the Department of Chemical and Biological Engineering, was featured online in Nano Letters on July 5.
Graphene has gained increased attention as a next-generation material for its heat and electrical conductivity as well as its transparency. However, single and multi-layered graphene have characteristics of a conductor so that it is difficult to apply into semiconductor. Only when downsized to the nanoscale, semiconductor’s distinct feature of bandgap will be exhibited to emit the light in the graphene. This illuminating featuring of dot is referred to as a graphene quantum dot.
Conventionally, single-crystalline graphene has been fabricated by chemical vapor deposition (CVD) on copper or nickel thin films, or by peeling graphite physically and chemically. However, graphene made via chemical vapor deposition is mainly used for large-surface transparent electrodes. Meanwhile, graphene made by chemical and physical peeling carries uneven size defects.
The research team explained that their graphene quantum dots exhibited a very stable single-phase reaction when they mixed amine and acetic acid with an aqueous solution of glucose. Then, they synthesized single-crystalline graphene quantum dots from the self-assembly of the reaction intermediate. In the course of fabrication, the team developed a new separation method at a low-temperature precipitation, which led to successfully creating a homogeneous nucleation of graphene quantum dots via a single-phase reaction.
Professor Park and his colleagues have developed solution phase synthesis technology that allows for the creation of the desired crystal size for single nanocrystals down to 100 nano meters. It is reportedly the first synthesis of the homogeneous nucleation of graphene through a single-phase reaction.
Professor Park said, "This solution method will significantly contribute to the grafting of graphene in various fields. The application of this new graphene will expand the scope of its applications such as for flexible displays and varistors.”
This research was a joint project with a team from Korea University under Professor Sang Hyuk Im from the Department of Chemical and Biological Engineering, and was supported by the National Research Foundation of Korea, the Nano-Material Technology Development Program from the Electronics and Telecommunications Research Institute (ETRI), KAIST EEWS, and the BK21+ project from the Korean government.
2019.08.02 View 35542
Synthesizing Single-Crystalline Hexagonal Graphene Quantum Dots
(Figure: Uniformly ordered single-crystalline graphene quantum dots of various sizes synthesized through solution chemistry.)
A KAIST team has designed a novel strategy for synthesizing single-crystalline graphene quantum dots, which emit stable blue light. The research team confirmed that a display made of their synthesized graphene quantum dots successfully emitted blue light with stable electric pressure, reportedly resolving the long-standing challenges of blue light emission in manufactured displays. The study, led by Professor O Ok Park in the Department of Chemical and Biological Engineering, was featured online in Nano Letters on July 5.
Graphene has gained increased attention as a next-generation material for its heat and electrical conductivity as well as its transparency. However, single and multi-layered graphene have characteristics of a conductor so that it is difficult to apply into semiconductor. Only when downsized to the nanoscale, semiconductor’s distinct feature of bandgap will be exhibited to emit the light in the graphene. This illuminating featuring of dot is referred to as a graphene quantum dot.
Conventionally, single-crystalline graphene has been fabricated by chemical vapor deposition (CVD) on copper or nickel thin films, or by peeling graphite physically and chemically. However, graphene made via chemical vapor deposition is mainly used for large-surface transparent electrodes. Meanwhile, graphene made by chemical and physical peeling carries uneven size defects.
The research team explained that their graphene quantum dots exhibited a very stable single-phase reaction when they mixed amine and acetic acid with an aqueous solution of glucose. Then, they synthesized single-crystalline graphene quantum dots from the self-assembly of the reaction intermediate. In the course of fabrication, the team developed a new separation method at a low-temperature precipitation, which led to successfully creating a homogeneous nucleation of graphene quantum dots via a single-phase reaction.
Professor Park and his colleagues have developed solution phase synthesis technology that allows for the creation of the desired crystal size for single nanocrystals down to 100 nano meters. It is reportedly the first synthesis of the homogeneous nucleation of graphene through a single-phase reaction.
Professor Park said, "This solution method will significantly contribute to the grafting of graphene in various fields. The application of this new graphene will expand the scope of its applications such as for flexible displays and varistors.”
This research was a joint project with a team from Korea University under Professor Sang Hyuk Im from the Department of Chemical and Biological Engineering, and was supported by the National Research Foundation of Korea, the Nano-Material Technology Development Program from the Electronics and Telecommunications Research Institute (ETRI), KAIST EEWS, and the BK21+ project from the Korean government.
2019.08.02 View 35542 -
 KAIST's Doctoral Student Receives a Hoffman Scholarship Award
Hyo-Sun Lee, a doctoral student at the Graduate School of EEWS (Environment, Energy, Water and Sustainability), KAIST, is a recipient of the 2016 Dorothy M. and Earl S. Hoffman Scholarships presented by the American Vacuum Society (AVS). The award ceremony took place during the Society’s 63rd International Symposium and Exhibition on November 6-11, 2016 in Nashville, Tennessee.
Lee is the first Korean and foreign student to receive this scholarship.
The Hoffman Scholarships were established in 2002 to recognize and encourage excellence in graduate studies in the sciences and technologies of interest to AVS. The scholarships are funded by a bequest from Dorothy M. Hoffman, who was a pioneering member of the Society of Women Engineers and served as the president of AVS in 1974.
Lee received the scholarship for her research that detects hot electrons from chemical reactions on catalytic surface using nanodevices. Nano Letters, an academic journal published by the American Chemical Society, described her work in its February 2016 issue as a technology that allows quantitative analysis of hot electrons by employing a new nanodevice and therefore helps researchers understand better the mechanism of chemical reactions on nanocatalytic surface. She also published her work to detect the flow of hot electrons that occur on metal nanocatalytic surface during hydrogen oxidation reactions in Angewandte Chemie.
Lee said, “I am pleased to receive this honor from such a world-renowned academic society. Certainly, this will be a great support for my future study and research.”
Founded in 1953, AVS is an interdisciplinary, professional society composed of approximately 4,500 members worldwide. It supports networking among academic, industrial, government, and consulting professionals involved in a range of established and emerging science and technology areas such as chemistry, physics, engineering, business, and technology development.
2016.11.17 View 11260
KAIST's Doctoral Student Receives a Hoffman Scholarship Award
Hyo-Sun Lee, a doctoral student at the Graduate School of EEWS (Environment, Energy, Water and Sustainability), KAIST, is a recipient of the 2016 Dorothy M. and Earl S. Hoffman Scholarships presented by the American Vacuum Society (AVS). The award ceremony took place during the Society’s 63rd International Symposium and Exhibition on November 6-11, 2016 in Nashville, Tennessee.
Lee is the first Korean and foreign student to receive this scholarship.
The Hoffman Scholarships were established in 2002 to recognize and encourage excellence in graduate studies in the sciences and technologies of interest to AVS. The scholarships are funded by a bequest from Dorothy M. Hoffman, who was a pioneering member of the Society of Women Engineers and served as the president of AVS in 1974.
Lee received the scholarship for her research that detects hot electrons from chemical reactions on catalytic surface using nanodevices. Nano Letters, an academic journal published by the American Chemical Society, described her work in its February 2016 issue as a technology that allows quantitative analysis of hot electrons by employing a new nanodevice and therefore helps researchers understand better the mechanism of chemical reactions on nanocatalytic surface. She also published her work to detect the flow of hot electrons that occur on metal nanocatalytic surface during hydrogen oxidation reactions in Angewandte Chemie.
Lee said, “I am pleased to receive this honor from such a world-renowned academic society. Certainly, this will be a great support for my future study and research.”
Founded in 1953, AVS is an interdisciplinary, professional society composed of approximately 4,500 members worldwide. It supports networking among academic, industrial, government, and consulting professionals involved in a range of established and emerging science and technology areas such as chemistry, physics, engineering, business, and technology development.
2016.11.17 View 11260 -
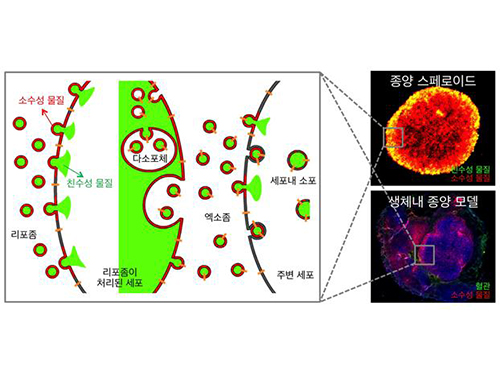 Anti-Cancer Therapy Delivering Drug to an Entire Tumor Developed
KAIST’s Department of Bio and Brain Engineering Professor Ji-Ho Park and his team successfully developed a new highly efficacious anti-cancer nanotechnology by delivering anti-cancer drugs uniformly to an entire tumor. Their research results were published in Nano Letters online on March 31, 2015.
To treat inoperable tumors, anti-cancer medicine is commonly used. However, efficient drug delivery to tumor cells is often difficult, treating an entire tumor with drugs even more so.
Using the existing drug delivery systems, including nanotechnology, a drug can be delivered only to tumor cells near blood vessels, leaving cells at the heart of a tumor intact. Since most drugs are injected into the bloodstream, tumor recurrence post medication is frequent.
Therefore, the team used liposomes that can fuse to the cell membrane and enter the cell. Once inside liposomes the drug can travel into the bloodstream, enter tumor cells near blood vessels, where they are loaded to exosomes, which are naturally occurring nanoparticles in the body. Since exosomes can travel between cells, the drug can be delivered efficiently into inner cells of the tumor.
Exosomes, which are secreted by cells that exist in the tumor microenvironment, is known to have an important role in tumor progression and metastasis since they transfer biological materials between cells. The research team started the investigation recognizing the possibility of delivering the anti-cancer drug to the entire tumor using exosomes.
The team injected the light-sensitive anti-cancer drug using their new delivery technique into experimental mice. The researchers applied light to the tumor site to activate the anti-cancer treatment and analyzed a tissue sample. They observed the effects of the anti-cancer drug in the entire tumor tissue.
The team’s results establish a ground-breaking foothold in drug delivery technology development that can be tailored to specific diseases by understanding its microenvironment. The work paves the way to more effective drug delivery systems for many chronic diseases, including cancer tumors that were difficult to treat due to the inability to penetrate deep into the tissue.
The team is currently conducting experiments with other anti-cancer drugs, which are being developed by pharmaceutical companies, using their tumor-penetrating drug delivery nanotechnology, to identify its effects on malignant tumors.
Professor Park said, “This research is the first to apply biological nanoparticles, exosomes that are continuously secreted and can transfer materials to neighboring cells, to deliver drugs directly to the heart of tumor.”
Picture: Incorporation of hydrophilic and hydrophobic compounds into membrane vesicles by engineering the parental cells via synthetic liposomes.
2015.04.07 View 13000
Anti-Cancer Therapy Delivering Drug to an Entire Tumor Developed
KAIST’s Department of Bio and Brain Engineering Professor Ji-Ho Park and his team successfully developed a new highly efficacious anti-cancer nanotechnology by delivering anti-cancer drugs uniformly to an entire tumor. Their research results were published in Nano Letters online on March 31, 2015.
To treat inoperable tumors, anti-cancer medicine is commonly used. However, efficient drug delivery to tumor cells is often difficult, treating an entire tumor with drugs even more so.
Using the existing drug delivery systems, including nanotechnology, a drug can be delivered only to tumor cells near blood vessels, leaving cells at the heart of a tumor intact. Since most drugs are injected into the bloodstream, tumor recurrence post medication is frequent.
Therefore, the team used liposomes that can fuse to the cell membrane and enter the cell. Once inside liposomes the drug can travel into the bloodstream, enter tumor cells near blood vessels, where they are loaded to exosomes, which are naturally occurring nanoparticles in the body. Since exosomes can travel between cells, the drug can be delivered efficiently into inner cells of the tumor.
Exosomes, which are secreted by cells that exist in the tumor microenvironment, is known to have an important role in tumor progression and metastasis since they transfer biological materials between cells. The research team started the investigation recognizing the possibility of delivering the anti-cancer drug to the entire tumor using exosomes.
The team injected the light-sensitive anti-cancer drug using their new delivery technique into experimental mice. The researchers applied light to the tumor site to activate the anti-cancer treatment and analyzed a tissue sample. They observed the effects of the anti-cancer drug in the entire tumor tissue.
The team’s results establish a ground-breaking foothold in drug delivery technology development that can be tailored to specific diseases by understanding its microenvironment. The work paves the way to more effective drug delivery systems for many chronic diseases, including cancer tumors that were difficult to treat due to the inability to penetrate deep into the tissue.
The team is currently conducting experiments with other anti-cancer drugs, which are being developed by pharmaceutical companies, using their tumor-penetrating drug delivery nanotechnology, to identify its effects on malignant tumors.
Professor Park said, “This research is the first to apply biological nanoparticles, exosomes that are continuously secreted and can transfer materials to neighboring cells, to deliver drugs directly to the heart of tumor.”
Picture: Incorporation of hydrophilic and hydrophobic compounds into membrane vesicles by engineering the parental cells via synthetic liposomes.
2015.04.07 View 13000 -
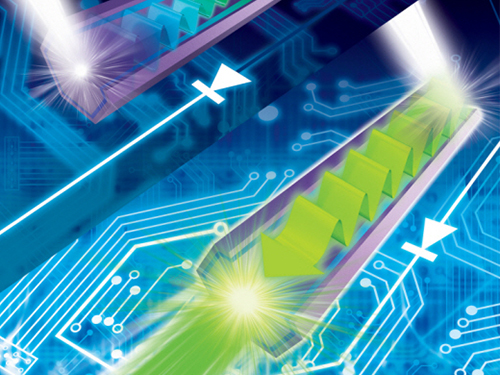 Development of a Photonic Diode with Light Speed, Single-Direction Transfer
A photonic diode using a nitride semiconductor rod can increase the possibility of developing all-optical integrated circuits, an alternative to conventional integrated circuits.
Professor Yong-Hoon Cho's research team from the Department of Physics, KAIST, developed a photonic diode which can selectively transfer light in one way, using semiconductor rods.
The photonic diode has a diameter of hundreds of nanometers (nm) and a length of few micrometers. This size enables its use in large-scale integration (LSI). The diode’s less sensitivity towards polarized light angle makes it more useful.
In an integrated circuit, a diode controls the flow of electrons. If this diode controls light rather than electrons, data can be transferred at high speed, and its loss is minimized to a greater extent. Since these implementations conserve more energy, this is a very promising future technology.
However, conventional electronic diodes, made up of asymmetric meta-materials or photonic crystalline structures, are large, which makes them difficult to be used in LSI. These diodes could only be implemented under limited conditions due to its sensitivity towards polarized light angle.
The research team used nitride semiconductor rods to develop a highly efficient photonic diode with distinct light intensities from opposite ends.
The semiconductor rod yields different amount of energy horizontally. According to the research team, this is because the width of the quantum well and its indium quantity is continuously controlled.
Professor Cho said, "A large energy difference in a horizontal direction causes asymmetrical light propagation, enabling it to be operated as a photonic diode." He added that “If light, instead of electrons, were adopted in integrated circuits, the transfer speed would be expected as great as that of light.”
The research findings were published in the September 10th issue of Nano Letters as the cover paper.
Under the guidance of Professor Cho, two Ph.D. candidates, Suk-Min Ko and Su-Hyun Gong, conducted this research. This research project was sponsored by the National Research Foundation of Korea and KAIST’s EEWS (energy, environment, water, and sustainability) Research Center.
Figure Description: Computer simulated image of photonic diode made of semiconductor rod implemented in an all-optical integrated circuit
2014.09.23 View 12727
Development of a Photonic Diode with Light Speed, Single-Direction Transfer
A photonic diode using a nitride semiconductor rod can increase the possibility of developing all-optical integrated circuits, an alternative to conventional integrated circuits.
Professor Yong-Hoon Cho's research team from the Department of Physics, KAIST, developed a photonic diode which can selectively transfer light in one way, using semiconductor rods.
The photonic diode has a diameter of hundreds of nanometers (nm) and a length of few micrometers. This size enables its use in large-scale integration (LSI). The diode’s less sensitivity towards polarized light angle makes it more useful.
In an integrated circuit, a diode controls the flow of electrons. If this diode controls light rather than electrons, data can be transferred at high speed, and its loss is minimized to a greater extent. Since these implementations conserve more energy, this is a very promising future technology.
However, conventional electronic diodes, made up of asymmetric meta-materials or photonic crystalline structures, are large, which makes them difficult to be used in LSI. These diodes could only be implemented under limited conditions due to its sensitivity towards polarized light angle.
The research team used nitride semiconductor rods to develop a highly efficient photonic diode with distinct light intensities from opposite ends.
The semiconductor rod yields different amount of energy horizontally. According to the research team, this is because the width of the quantum well and its indium quantity is continuously controlled.
Professor Cho said, "A large energy difference in a horizontal direction causes asymmetrical light propagation, enabling it to be operated as a photonic diode." He added that “If light, instead of electrons, were adopted in integrated circuits, the transfer speed would be expected as great as that of light.”
The research findings were published in the September 10th issue of Nano Letters as the cover paper.
Under the guidance of Professor Cho, two Ph.D. candidates, Suk-Min Ko and Su-Hyun Gong, conducted this research. This research project was sponsored by the National Research Foundation of Korea and KAIST’s EEWS (energy, environment, water, and sustainability) Research Center.
Figure Description: Computer simulated image of photonic diode made of semiconductor rod implemented in an all-optical integrated circuit
2014.09.23 View 12727 -
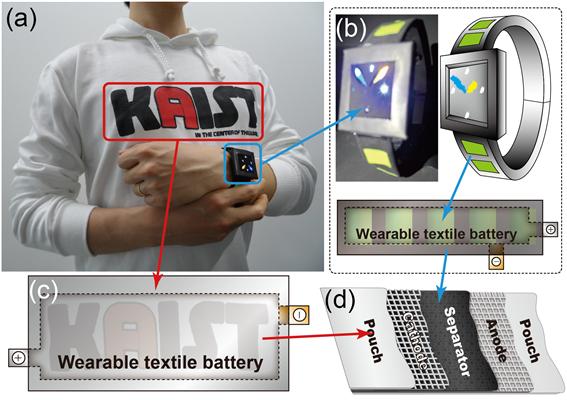 Technology Developed for Flexible, Foldable & Rechargeable Battery
Flexible, Foldable & Rechargeable Battery
The research group of professors Jang-Wook Choi & Jung-Yong Lee from the Graduate School of EEWS and Taek-Soo Kim from the Department of Mechanical Engineering at KAIST has developed technology for flexible and foldable batteries which are rechargeable using solar energy. The research result was published in the online issue of Nano Letters on November 5.
Trial versions of flexible and wearable electronics are being developed and introduced in the market such as Galaxy Gear, Apple’s i-Watch, and Google Glass. Research is being conducted to make the batteries softer and more wearable and to compete in the fast-growing market for flexible electronics.
This new technology is expected to be applied to the development of wearable computers as well as winter outdoor clothing since it is flexible and light. The research group expects that the new technology can be applied to current battery production lines without additional investment.
Professor Choi said, “It can be used as a core-source technology in the rechargeable battery industry in the future. Various wearable mobile electronic products can be developed through cooperation and collaboration within the industry.”
2013.11.21 View 13338
Technology Developed for Flexible, Foldable & Rechargeable Battery
Flexible, Foldable & Rechargeable Battery
The research group of professors Jang-Wook Choi & Jung-Yong Lee from the Graduate School of EEWS and Taek-Soo Kim from the Department of Mechanical Engineering at KAIST has developed technology for flexible and foldable batteries which are rechargeable using solar energy. The research result was published in the online issue of Nano Letters on November 5.
Trial versions of flexible and wearable electronics are being developed and introduced in the market such as Galaxy Gear, Apple’s i-Watch, and Google Glass. Research is being conducted to make the batteries softer and more wearable and to compete in the fast-growing market for flexible electronics.
This new technology is expected to be applied to the development of wearable computers as well as winter outdoor clothing since it is flexible and light. The research group expects that the new technology can be applied to current battery production lines without additional investment.
Professor Choi said, “It can be used as a core-source technology in the rechargeable battery industry in the future. Various wearable mobile electronic products can be developed through cooperation and collaboration within the industry.”
2013.11.21 View 13338 -
 Nanowire Made of Diverse Materials May Become Marketable
- Technology to commercialize nanowire developed after 2 years of industrial-academic joint research -
- 2 million strands of 50nm-width, 20 cm-length nanowire mass producible in 2 hours –
A South Korean joint industrial-academic research team has developed the technology to put forward the commercialization of nanowire that is only a few nanometers wide. It is expected to be applied in various fields such as semiconductors, high performance sensors, and biodevices.
In cooperation with LG Innotek and the National Nanofab center, Professor Jun-Bo Yoon, from KAIST Department of Electrical Engineering, developed the technology to mass produce nanowire at any length with various materials. The research results are published on the online edition of Nano Letters on July 30th.
Nanowire has a long linear structure with its width at 100 nanometers at maximum. It is a multifunctional material that has yet undiscovered thermal, electric, and mechanical properties. Nanowire is highly acclaimed as a cutting-edge material with unique nano-level properties that can be applied in semiconductors, energy, biodevices, and optic devices.
Previously, nanowires had an extremely low synthesis rate that required three or four days to grow few millimeters. It was therefore difficult to produce the desired products using nanowires. Moreover, nanowires needed to be evenly arranged for practical application, but the traditional technology required complex post-treatment, not to mention the arrangement was not immaculate.
The research team applied semiconductor process instead of chemical synthesis to resolve these issues. The team first formed a pattern greater that of the target frequency by using a photo-engraving process on a silicon wafer board whose diameter was 20 centimeters, then repeatedly reduced the frequency to produce 100 nm ultrafine linear grid pattern. Based on this pattern, the research team applied the sputtering process to mass-produce nanowires in perfect shapes of 50 nm width and 20 cm maximum length.
The new technology requires neither a lengthy synthesis process nor post-cleaning to attain a perfectly aligned state. Thus, academic and industrial circles consider the technology has high possibilities for commercialization.
“The significance is in resolving the issues in traditional technology, such as low productivity, long manufacturing time, restrictions in material synthesis, and nanowire alignment,” commented Professor Yoon on this research. “Nanowires have not been widely applied in the industry, but this technology will bring forward the commercialization of high performance semiconductors, optic devices, and biodevices that make use of nanowires.”
2013.10.18 View 10515
Nanowire Made of Diverse Materials May Become Marketable
- Technology to commercialize nanowire developed after 2 years of industrial-academic joint research -
- 2 million strands of 50nm-width, 20 cm-length nanowire mass producible in 2 hours –
A South Korean joint industrial-academic research team has developed the technology to put forward the commercialization of nanowire that is only a few nanometers wide. It is expected to be applied in various fields such as semiconductors, high performance sensors, and biodevices.
In cooperation with LG Innotek and the National Nanofab center, Professor Jun-Bo Yoon, from KAIST Department of Electrical Engineering, developed the technology to mass produce nanowire at any length with various materials. The research results are published on the online edition of Nano Letters on July 30th.
Nanowire has a long linear structure with its width at 100 nanometers at maximum. It is a multifunctional material that has yet undiscovered thermal, electric, and mechanical properties. Nanowire is highly acclaimed as a cutting-edge material with unique nano-level properties that can be applied in semiconductors, energy, biodevices, and optic devices.
Previously, nanowires had an extremely low synthesis rate that required three or four days to grow few millimeters. It was therefore difficult to produce the desired products using nanowires. Moreover, nanowires needed to be evenly arranged for practical application, but the traditional technology required complex post-treatment, not to mention the arrangement was not immaculate.
The research team applied semiconductor process instead of chemical synthesis to resolve these issues. The team first formed a pattern greater that of the target frequency by using a photo-engraving process on a silicon wafer board whose diameter was 20 centimeters, then repeatedly reduced the frequency to produce 100 nm ultrafine linear grid pattern. Based on this pattern, the research team applied the sputtering process to mass-produce nanowires in perfect shapes of 50 nm width and 20 cm maximum length.
The new technology requires neither a lengthy synthesis process nor post-cleaning to attain a perfectly aligned state. Thus, academic and industrial circles consider the technology has high possibilities for commercialization.
“The significance is in resolving the issues in traditional technology, such as low productivity, long manufacturing time, restrictions in material synthesis, and nanowire alignment,” commented Professor Yoon on this research. “Nanowires have not been widely applied in the industry, but this technology will bring forward the commercialization of high performance semiconductors, optic devices, and biodevices that make use of nanowires.”
2013.10.18 View 10515 -
 Prof. Jang-Uk Choi develops Strong, Long-lasting Lithium-ion Battery
Lithium-ion secondary battery with high power, as well asmuch longer life span, has been developed using nanotechnology. Professor Jang-Uk Choi and his colleagues at KAIST University EEWS graduate school has succeeded in developing a new lithium-ion secondary battery that has more than five times the output and three times the life span of the conventional batteries.
The industry expects the new battery to significantly improve the acceleration performance and solve the drawbacks of slow electric cars, which occurred due to failure of battery performance to keep up with the output of the motors during acceleration.
It is also expected that the new battery could be utilized in various fields that require high power batteries such as Smart Grid, which is the next generation intelligent electrical grid, as well as electric tools and many others.
Currently, the most widely used commercial lithium ion batteries’ lithium-cobalt-based cathode material has the disadvantage of expensive cost, high toxicity, short life expectancy and long-charge/discharge time. Also, it has been difficult to apply in electric cars that require a large current density and are vulnerable to heat generated during charging/discharging.
On the other hand, Professor Choi and his colleagues’ lithium-manganese based cathode material is gaining popularity for having the advantages such as abundant raw materials, cheap prices, eco-friendliness and especially excellent high-temperature stability and high output, which are suitable for use as electrode material in electric cars.
The pure lithium manganese based cathode material has a critical drawback of a very short life expectancy, only lasting about average of 1-2 years, which is due to the elution when the melted manganese flows out into the electrolyte. There have been various studies to solve this problem; however, the unique crystal structure of the material remained as a challenge for many scientists.
Professor Choi’s team analyzed the structure of the crystal at the time shortly before manganese oxides were formed, while controlling the reaction temperature at the step of synthesizing nanomaterial. It has been found that, at 220℃, there are simultaneously existing two crystal faces, one that inhibits the dissolution of manganese ions and the other that enables lithium ions to move smoothly.
Each of these crystal faces improves both the life span and output, increasing the output more than five times and life expectancy over three times. In addition, the existing high temperature life span, that was known to be especially vulnerable, has improved ten-fold.
“By controlling the crystal face of lithium manganese anode material, which has previously existed in the battery as chunks of about 10 micro-meter particles, both output and life span has significantly improved,” said Professor Choi, “Domestic and international patent application for the regarding technology has been finished and we have plans to work with companies in the future for commercialization within 2-3 years.”
Professor Yi Cui of Stanford University, the world’s leading scholar on the secondary battery, has evaluated that “This research exemplifies how nanotechnology can innovatively develop the field of secondary battery.”
Meanwhile, the research led by Professor Jang-Uk Choi and participated by researcher Ju-Seong Kim has been published on the online edition (dated Nov 27th) of Nanoletters, the world’s leading authority on Nanoscience.
2012.12.21 View 10672
Prof. Jang-Uk Choi develops Strong, Long-lasting Lithium-ion Battery
Lithium-ion secondary battery with high power, as well asmuch longer life span, has been developed using nanotechnology. Professor Jang-Uk Choi and his colleagues at KAIST University EEWS graduate school has succeeded in developing a new lithium-ion secondary battery that has more than five times the output and three times the life span of the conventional batteries.
The industry expects the new battery to significantly improve the acceleration performance and solve the drawbacks of slow electric cars, which occurred due to failure of battery performance to keep up with the output of the motors during acceleration.
It is also expected that the new battery could be utilized in various fields that require high power batteries such as Smart Grid, which is the next generation intelligent electrical grid, as well as electric tools and many others.
Currently, the most widely used commercial lithium ion batteries’ lithium-cobalt-based cathode material has the disadvantage of expensive cost, high toxicity, short life expectancy and long-charge/discharge time. Also, it has been difficult to apply in electric cars that require a large current density and are vulnerable to heat generated during charging/discharging.
On the other hand, Professor Choi and his colleagues’ lithium-manganese based cathode material is gaining popularity for having the advantages such as abundant raw materials, cheap prices, eco-friendliness and especially excellent high-temperature stability and high output, which are suitable for use as electrode material in electric cars.
The pure lithium manganese based cathode material has a critical drawback of a very short life expectancy, only lasting about average of 1-2 years, which is due to the elution when the melted manganese flows out into the electrolyte. There have been various studies to solve this problem; however, the unique crystal structure of the material remained as a challenge for many scientists.
Professor Choi’s team analyzed the structure of the crystal at the time shortly before manganese oxides were formed, while controlling the reaction temperature at the step of synthesizing nanomaterial. It has been found that, at 220℃, there are simultaneously existing two crystal faces, one that inhibits the dissolution of manganese ions and the other that enables lithium ions to move smoothly.
Each of these crystal faces improves both the life span and output, increasing the output more than five times and life expectancy over three times. In addition, the existing high temperature life span, that was known to be especially vulnerable, has improved ten-fold.
“By controlling the crystal face of lithium manganese anode material, which has previously existed in the battery as chunks of about 10 micro-meter particles, both output and life span has significantly improved,” said Professor Choi, “Domestic and international patent application for the regarding technology has been finished and we have plans to work with companies in the future for commercialization within 2-3 years.”
Professor Yi Cui of Stanford University, the world’s leading scholar on the secondary battery, has evaluated that “This research exemplifies how nanotechnology can innovatively develop the field of secondary battery.”
Meanwhile, the research led by Professor Jang-Uk Choi and participated by researcher Ju-Seong Kim has been published on the online edition (dated Nov 27th) of Nanoletters, the world’s leading authority on Nanoscience.
2012.12.21 View 10672 -
 Principle behind increasing the catalytic property of nanocatalysts proven
The technology that allows full control of the catalytic property of nanocatalysts using oxide formation on nanocatalysts has been developed by KAIST researchers. The breakthrough opens up the possibility of the development of a new kind of catalysts that maximizes catalytic property and minimizes waste.
*nanocatalyst is a material that catalyzes gas reactions on its surface. It is composed of a high surface area oxide scaffold with nano-sized metal particles dispersed.
The team was led by Professor Park Jeong Young of the KAIST EEWS Graduate School and consists of Kamran Qadir Ph.D. candidate (1st Author), Professor Joo Sang Hoon of UNIST, Professor Moon Bong Jin of Hanyang University, and Professor Gabor Somorajai of UC Berkeley. Support for the research was provided from Ministry of Education Science and Technology, National Research Foundation, and Ministry of Knowledge Economy. The results were published as the online edition of Nano Letters: “Intrinsic Relation between Catalytic Activity of CO Oxidation on Ru Nanoparticles and Ru Oxides Uncovered with Ambient Pressure XPS”.
Catalysts are included in above 80% of all the products used in everyday life and are therefore included in most aspects of our lives.
The focus on nanocatalysts is based on finding solutions to increasing the efficiency for application to energy production and for solving environmental issues.
Most nanocatalysts are composed of nanoparticles and oxides where the nanoparticles increase the surface area of the catalyst to increase its activity.
The efficiency of a nanocatalyst is affected by the surface oxide of the nanoparticles. However the proving of this assumption remained difficult to do as it required in-situ measurement of the oxide state of the nanoparticles in the specific environment. Thus far, the experiments were conducted in a vacuum and therefore did not reflect the actual behavior in real life. The recently developed X-ray Photoelectron Spectroscopy allows for measurement of the oxidization state at standard atmospheric pressure.
Professor Park’s research team successfully measured the oxidization state of the nanoparticle using the atmospheric pressure X-ray Photoelectron Spectroscopy in the specified environment.
They confirmed the effect the oxidization state on the catalytic effect of the nanoparticles and additionally found that a thin layer of oxide can increase the catalytic effect and the effectiveness of the nanoparticle can controlled by the oxidation state.
2012.11.29 View 9752
Principle behind increasing the catalytic property of nanocatalysts proven
The technology that allows full control of the catalytic property of nanocatalysts using oxide formation on nanocatalysts has been developed by KAIST researchers. The breakthrough opens up the possibility of the development of a new kind of catalysts that maximizes catalytic property and minimizes waste.
*nanocatalyst is a material that catalyzes gas reactions on its surface. It is composed of a high surface area oxide scaffold with nano-sized metal particles dispersed.
The team was led by Professor Park Jeong Young of the KAIST EEWS Graduate School and consists of Kamran Qadir Ph.D. candidate (1st Author), Professor Joo Sang Hoon of UNIST, Professor Moon Bong Jin of Hanyang University, and Professor Gabor Somorajai of UC Berkeley. Support for the research was provided from Ministry of Education Science and Technology, National Research Foundation, and Ministry of Knowledge Economy. The results were published as the online edition of Nano Letters: “Intrinsic Relation between Catalytic Activity of CO Oxidation on Ru Nanoparticles and Ru Oxides Uncovered with Ambient Pressure XPS”.
Catalysts are included in above 80% of all the products used in everyday life and are therefore included in most aspects of our lives.
The focus on nanocatalysts is based on finding solutions to increasing the efficiency for application to energy production and for solving environmental issues.
Most nanocatalysts are composed of nanoparticles and oxides where the nanoparticles increase the surface area of the catalyst to increase its activity.
The efficiency of a nanocatalyst is affected by the surface oxide of the nanoparticles. However the proving of this assumption remained difficult to do as it required in-situ measurement of the oxide state of the nanoparticles in the specific environment. Thus far, the experiments were conducted in a vacuum and therefore did not reflect the actual behavior in real life. The recently developed X-ray Photoelectron Spectroscopy allows for measurement of the oxidization state at standard atmospheric pressure.
Professor Park’s research team successfully measured the oxidization state of the nanoparticle using the atmospheric pressure X-ray Photoelectron Spectroscopy in the specified environment.
They confirmed the effect the oxidization state on the catalytic effect of the nanoparticles and additionally found that a thin layer of oxide can increase the catalytic effect and the effectiveness of the nanoparticle can controlled by the oxidation state.
2012.11.29 View 9752 -
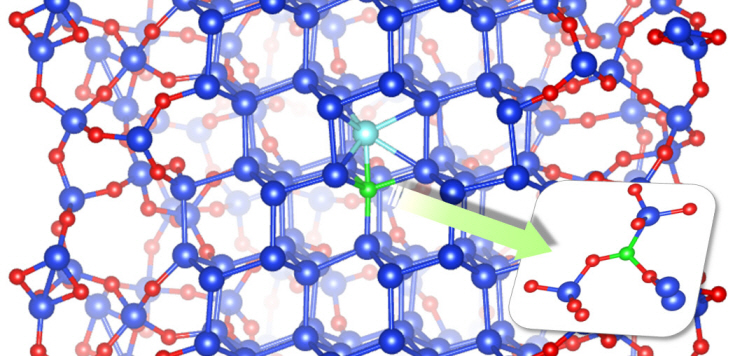 Dopant properties of silicon nanowires investigated
Professor Chang Kee Joo
Professor Kee Joo Chang’s research team from the Department of Physics at KAIST has successfully unearthed the properties of boron and phosphorous dopants in silicon nanowires, a material expected to be used in next generation semiconductors. The research team was the first in the world to investigate the movement of boron and phosphorous (impurities or ‘dopants’ added for electrical flow) in oxidized silicon nanowires and study the mechanism behind its deactivation.
It is nearly impossible to develop a silicon based semiconductor thinner than 10nm, even using the most advanced modern technology. However, the thickness of silicon nanowires are within the nano level and hence, allows a higher degree of integration in semiconductors.
For silicon nanowires to carry electricity, small amounts of boron and phosphorous need to be added (‘doping’ process). Compared to silicon, nanowires are harder to create due to the difficulties in the doping process as well as the control of electrical conduction properties.
Professor Chang’s research team improved upon the existing simple model by applying revolutionary quantum simulation theory to create a realistic core-shell atomic model. This research successfully investigated the cause of the escape of boron dopants from the silicon core during oxidation. It was also found that although phosphorous dopants do not escape as oxides, they form electrically deactivated pairs which decreases the efficiency. These phenomena were attributed to the film shape of the nano-wires, which increases the relative surface area compared to a same volume of silicon.
The research results were published in the online September edition of the world renowned Nano Letters.
Figure: The longitudinal section diagram of the Silicon/oxide core-shell model
2012.11.28 View 9148
Dopant properties of silicon nanowires investigated
Professor Chang Kee Joo
Professor Kee Joo Chang’s research team from the Department of Physics at KAIST has successfully unearthed the properties of boron and phosphorous dopants in silicon nanowires, a material expected to be used in next generation semiconductors. The research team was the first in the world to investigate the movement of boron and phosphorous (impurities or ‘dopants’ added for electrical flow) in oxidized silicon nanowires and study the mechanism behind its deactivation.
It is nearly impossible to develop a silicon based semiconductor thinner than 10nm, even using the most advanced modern technology. However, the thickness of silicon nanowires are within the nano level and hence, allows a higher degree of integration in semiconductors.
For silicon nanowires to carry electricity, small amounts of boron and phosphorous need to be added (‘doping’ process). Compared to silicon, nanowires are harder to create due to the difficulties in the doping process as well as the control of electrical conduction properties.
Professor Chang’s research team improved upon the existing simple model by applying revolutionary quantum simulation theory to create a realistic core-shell atomic model. This research successfully investigated the cause of the escape of boron dopants from the silicon core during oxidation. It was also found that although phosphorous dopants do not escape as oxides, they form electrically deactivated pairs which decreases the efficiency. These phenomena were attributed to the film shape of the nano-wires, which increases the relative surface area compared to a same volume of silicon.
The research results were published in the online September edition of the world renowned Nano Letters.
Figure: The longitudinal section diagram of the Silicon/oxide core-shell model
2012.11.28 View 9148 -
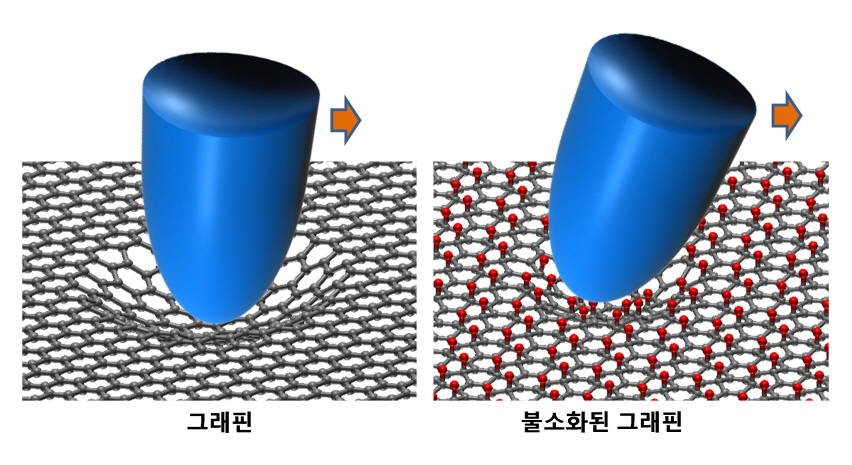 KAIST researchers verify and control the mechanical properties of graphene
KAIST researchers have successfully verified and controlled the mechanical properties of graphene, a next-generation material. Professor Park Jung Yong from the EEWS Graduate School and Professor Kim Yong Hyun from the Graduate School of Nanoscience and Technology have succeeded in fluorinating a single atomic-layered graphene sample and controlling its frictional and adhesive properties. This is the first time the frictional properties of graphene have been examined at the atomic level, and the technology is expected to be applied to nano-sized robots and microscopic joints.
Graphene is often dubbed “the dream material” because of its ability to conduct high amounts of electricity even when bent, making it the next-generation substitute for silicon semiconductors, paving the way for flexible display and wearable computer technologies. Graphene also has high potential applications in mechanical engineering because of its great material strength, but its mechanical properties remained elusive until now.
Professor Park’s research team successfully produced individual graphene samples with fluorine-deficiency at the atomic level by placing the samples in Fluoro-xenon (XeF2) gas and applying heat. The surface of the graphene was scanned using a micro probe and a high vacuum atomic microscope to measure its dynamic properties.
The research team found that the fluorinated graphene sample had 6 times more friction and 0.7 times more adhesiveness than the original graphene. Electrical measurements confirmed the fluorination process, and the analysis of the findings helped setup the theory of frictional changes in graphene.
Professor Park stated that “graphene can be used for the lubrication of joints in nano-sized devices” and that this research has numerous applications such as the coating of graphene-based microdynamic devices.
This research was published in the online June edition of Nano Letters and was supported by the Ministry of Science, Technology, and Education and the National Research Foundation as part of the World Class University (WCU) program.
2012.07.24 View 18234
KAIST researchers verify and control the mechanical properties of graphene
KAIST researchers have successfully verified and controlled the mechanical properties of graphene, a next-generation material. Professor Park Jung Yong from the EEWS Graduate School and Professor Kim Yong Hyun from the Graduate School of Nanoscience and Technology have succeeded in fluorinating a single atomic-layered graphene sample and controlling its frictional and adhesive properties. This is the first time the frictional properties of graphene have been examined at the atomic level, and the technology is expected to be applied to nano-sized robots and microscopic joints.
Graphene is often dubbed “the dream material” because of its ability to conduct high amounts of electricity even when bent, making it the next-generation substitute for silicon semiconductors, paving the way for flexible display and wearable computer technologies. Graphene also has high potential applications in mechanical engineering because of its great material strength, but its mechanical properties remained elusive until now.
Professor Park’s research team successfully produced individual graphene samples with fluorine-deficiency at the atomic level by placing the samples in Fluoro-xenon (XeF2) gas and applying heat. The surface of the graphene was scanned using a micro probe and a high vacuum atomic microscope to measure its dynamic properties.
The research team found that the fluorinated graphene sample had 6 times more friction and 0.7 times more adhesiveness than the original graphene. Electrical measurements confirmed the fluorination process, and the analysis of the findings helped setup the theory of frictional changes in graphene.
Professor Park stated that “graphene can be used for the lubrication of joints in nano-sized devices” and that this research has numerous applications such as the coating of graphene-based microdynamic devices.
This research was published in the online June edition of Nano Letters and was supported by the Ministry of Science, Technology, and Education and the National Research Foundation as part of the World Class University (WCU) program.
2012.07.24 View 18234 -
 Inexpensive Separation Method of Graphene Developed
The problem with commercializing graphene that is synthesized onto metals over a wide area is that it can not be separated from the metal. However, a groundbreaking separation technology which is both cheap and environment friendly has been developed.
Prof. Taek soo Kim and Prof. Byung Jin Cho"s research teams have conducted this research under the support of the Global Frontier program and Researcher Support Program initiated by The Ministry of Education and Science and Korea Research Foundation. The research results have been posted on the online news flash of Nano Letters on februrary 29th. (Thesis title: Direct Measurement of Adhesion Energy of Monolayer Graphene As-Grown on Copper and Its Application to Renewable Transfer Process)
The research has generated exact results on the interfacial adhesive energy of graphene and its surface material for the first time. Through this, the catalyst metal are no longer to be used just once, but will be used for an infinite number of times, thereby being ecofriendly and efficient.
Wide area graphine synthesized onto the catalyst meatal are used in various ways such as for display and for solar cells. There has been much research going on in this field. However, in order to use this wide area graphene, the graphene must be removed from the catalyst metal without damage.
Until now, the metal had been melted away through the use of chemical substances in order to separate the graphene. However, this method has been very problematic. The metal can not be reused, the costs are very high, much harmful wastes were created in the process of melting the metals, and the process was very complicated.
The research teams of Professors Taek Su Kim and Byung Jin Cho measured the interfacial adhesive energy of the synthesized graphene and learned that it could be easily removed.
Also, the mechanically removed graphene was successfully used in creating molecular electronic devices directly. This has thus innovatively shortened the graphene manufacturing process. Also, it has been confirmed that the metalic board can be reused multiple times after the graphene is removed. A new, ecofriendly and cost friendly method of graphene manufacturing has been paved.
Through this discovery, it is expected that graphene will become easier to manufacture and that the period til the commercialization date of graphene will therefore be greatly reduced
Prof. Cho stated " This reserach has much academical meaning significance in that it has successfully defined the surfacial adhesive energy between the graphene and its catalyst material and it should receive much attention in that it solved the largest technical problem involved in the production of graphene.
2012.04.04 View 16264
Inexpensive Separation Method of Graphene Developed
The problem with commercializing graphene that is synthesized onto metals over a wide area is that it can not be separated from the metal. However, a groundbreaking separation technology which is both cheap and environment friendly has been developed.
Prof. Taek soo Kim and Prof. Byung Jin Cho"s research teams have conducted this research under the support of the Global Frontier program and Researcher Support Program initiated by The Ministry of Education and Science and Korea Research Foundation. The research results have been posted on the online news flash of Nano Letters on februrary 29th. (Thesis title: Direct Measurement of Adhesion Energy of Monolayer Graphene As-Grown on Copper and Its Application to Renewable Transfer Process)
The research has generated exact results on the interfacial adhesive energy of graphene and its surface material for the first time. Through this, the catalyst metal are no longer to be used just once, but will be used for an infinite number of times, thereby being ecofriendly and efficient.
Wide area graphine synthesized onto the catalyst meatal are used in various ways such as for display and for solar cells. There has been much research going on in this field. However, in order to use this wide area graphene, the graphene must be removed from the catalyst metal without damage.
Until now, the metal had been melted away through the use of chemical substances in order to separate the graphene. However, this method has been very problematic. The metal can not be reused, the costs are very high, much harmful wastes were created in the process of melting the metals, and the process was very complicated.
The research teams of Professors Taek Su Kim and Byung Jin Cho measured the interfacial adhesive energy of the synthesized graphene and learned that it could be easily removed.
Also, the mechanically removed graphene was successfully used in creating molecular electronic devices directly. This has thus innovatively shortened the graphene manufacturing process. Also, it has been confirmed that the metalic board can be reused multiple times after the graphene is removed. A new, ecofriendly and cost friendly method of graphene manufacturing has been paved.
Through this discovery, it is expected that graphene will become easier to manufacture and that the period til the commercialization date of graphene will therefore be greatly reduced
Prof. Cho stated " This reserach has much academical meaning significance in that it has successfully defined the surfacial adhesive energy between the graphene and its catalyst material and it should receive much attention in that it solved the largest technical problem involved in the production of graphene.
2012.04.04 View 16264 -
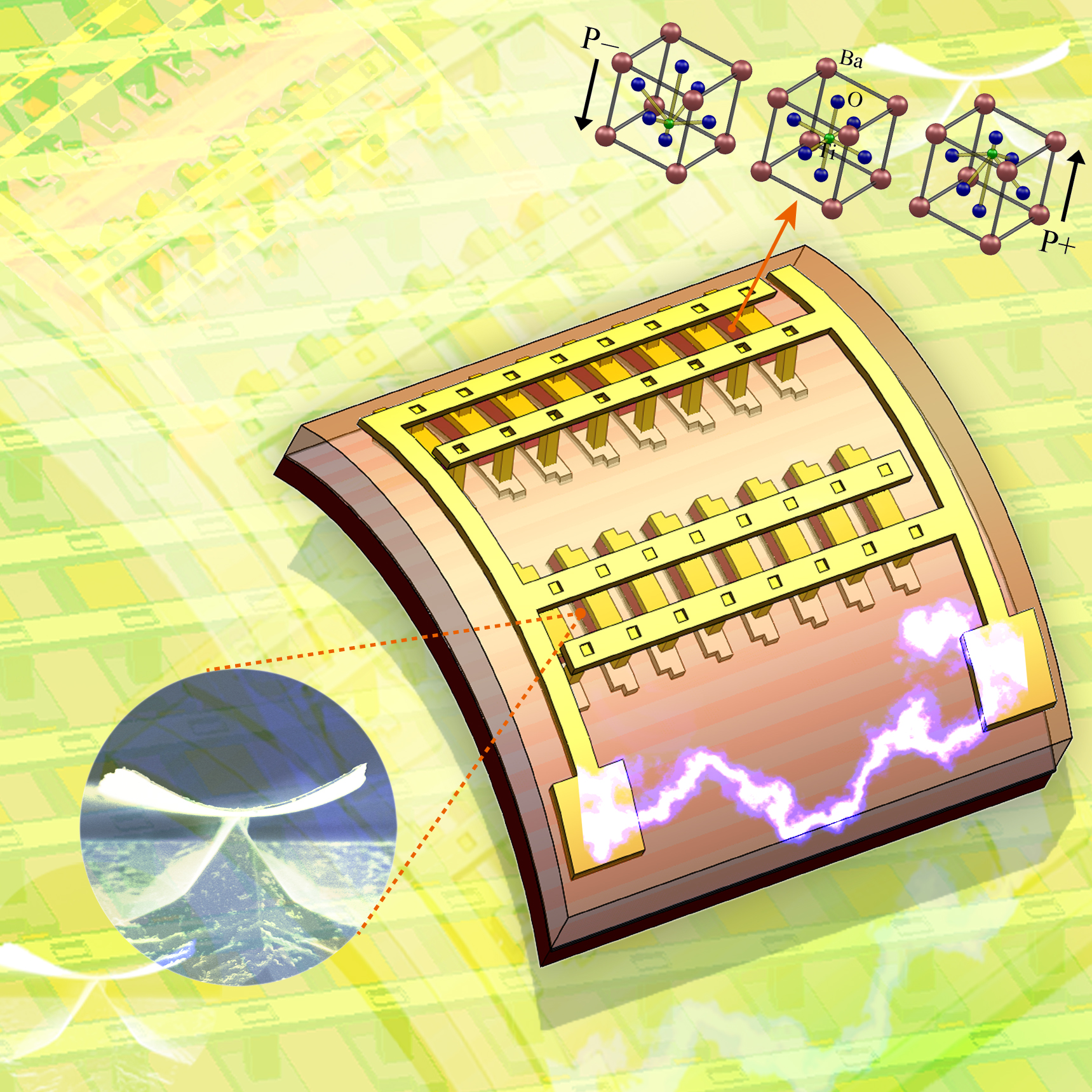 The KAIST & GIT team developed a power generation technology using bendable thin film nano-materials.
Figure description: Flexible thin film nanomaterials produce electricity.
Can a heart implanted micro robot operate permanently?
Can cell phones and tiny robots implanted in the heart operate permanently without having their batteries charged?
It might sound like science fiction, but these things seem to be possible in the near future. The team of Prof. Keon Jae Lee (KAIST, Dept. of Materials Science and Engineering) and Prof. Zhong Lin Wang (Georgia Institute of Technology, Dept. of Materials Science and Engineering) has developed new forms of highly efficient, flexible nanogenerator technology using the freely bendable piezoelectric ceramic thin film nano-materials that can convert tiny movements of the human body (such as heart beats and blood flow) into electrical energy.
The piezoelectric effect refers to voltage generation when pressure or bending strength is applied to piezoelectric materials. The ceramics, containing a perovskite structure, have a high piezoelectric efficiency. Until now, it has been very difficult to use these ceramic materials to fabricate flexible electronic systems due to their brittle property.
The research team, however, has succeeded in developing a bio-eco-friendly ceramic thin film nanogenerator that is freely bendable without breakdown.
Nanogenerator technology, a power generating system without wires or batteries, combines nanotechnology with piezoelectrics that can be used not only in personal mobile electronics but also in bio-implantable sensors or as an energy source for micro robots. Energy sources in nature (wind, vibration, and sound) and biomechanical forces produced by the human body (heart beats, blood flow, and muscle contraction/relaxation) can infinitely produce nonpolluting energy. (Nanogenerator produces electricity by external forces: http://www.youtube.com/watch?v=tvj0SsBqpBw)
Prof. Keon Jae Lee (KAIST) was involved in the first co-invention of “High Performance Flexible Single Crystal Electronics” during his PhD course at the University of Illinois at Urbana-Champaign. This nanogenerator technology, based on the previous invention, utilized the similar protocol of transferring ceramic thin film nano-materials on flexible substrates and produced voltage generation between electrodes.
Prof. Zhong Lin Wang (Georgia Tech, inventor of the nanogenerator) said, “This technology can be used to turn on an LED by slightly modifying circuits and operate touchable flexible displays. In addition, thin film nano-materials (‘barium titanate’) of this research have the property of both high efficiency and lead-free bio compatibility, which can be used in future medical applications.” This result is published in November online issue of ‘Nano Letters’ ACS journal.
<Video>
Youtube link: http://www.youtube.com/watch?v=tvj0SsBqpBw
Thin Film Nanogenerator produces electricity by external forces.
2010.11.23 View 17403
The KAIST & GIT team developed a power generation technology using bendable thin film nano-materials.
Figure description: Flexible thin film nanomaterials produce electricity.
Can a heart implanted micro robot operate permanently?
Can cell phones and tiny robots implanted in the heart operate permanently without having their batteries charged?
It might sound like science fiction, but these things seem to be possible in the near future. The team of Prof. Keon Jae Lee (KAIST, Dept. of Materials Science and Engineering) and Prof. Zhong Lin Wang (Georgia Institute of Technology, Dept. of Materials Science and Engineering) has developed new forms of highly efficient, flexible nanogenerator technology using the freely bendable piezoelectric ceramic thin film nano-materials that can convert tiny movements of the human body (such as heart beats and blood flow) into electrical energy.
The piezoelectric effect refers to voltage generation when pressure or bending strength is applied to piezoelectric materials. The ceramics, containing a perovskite structure, have a high piezoelectric efficiency. Until now, it has been very difficult to use these ceramic materials to fabricate flexible electronic systems due to their brittle property.
The research team, however, has succeeded in developing a bio-eco-friendly ceramic thin film nanogenerator that is freely bendable without breakdown.
Nanogenerator technology, a power generating system without wires or batteries, combines nanotechnology with piezoelectrics that can be used not only in personal mobile electronics but also in bio-implantable sensors or as an energy source for micro robots. Energy sources in nature (wind, vibration, and sound) and biomechanical forces produced by the human body (heart beats, blood flow, and muscle contraction/relaxation) can infinitely produce nonpolluting energy. (Nanogenerator produces electricity by external forces: http://www.youtube.com/watch?v=tvj0SsBqpBw)
Prof. Keon Jae Lee (KAIST) was involved in the first co-invention of “High Performance Flexible Single Crystal Electronics” during his PhD course at the University of Illinois at Urbana-Champaign. This nanogenerator technology, based on the previous invention, utilized the similar protocol of transferring ceramic thin film nano-materials on flexible substrates and produced voltage generation between electrodes.
Prof. Zhong Lin Wang (Georgia Tech, inventor of the nanogenerator) said, “This technology can be used to turn on an LED by slightly modifying circuits and operate touchable flexible displays. In addition, thin film nano-materials (‘barium titanate’) of this research have the property of both high efficiency and lead-free bio compatibility, which can be used in future medical applications.” This result is published in November online issue of ‘Nano Letters’ ACS journal.
<Video>
Youtube link: http://www.youtube.com/watch?v=tvj0SsBqpBw
Thin Film Nanogenerator produces electricity by external forces.
2010.11.23 View 17403