CTA
-
 KAIST Wins CES 2025 Innovation Award, Showcasing Innovative Technologies
KAIST will showcase innovative technologies at the world’s largest technology fair, the Consumer Electronics Show (CES 2025). In addition, KAIST startups VIRNECT Inc., Standard Energy Inc., A2US Inc., and Panmnesia, Inc. won the 2025 CES Innovation Awards.
< Image 1. 3D-Graphical Profile of CES 2025 KAIST Exhibition Booth >
KAIST (President Kwang-Hyung Lee) announced on the 31st that it will operate a 140㎡ standalone booth at CES Eureka Park, which will be held in Las Vegas, USA from January 7th to 10th next year, to showcase KAIST's innovative technologies to global companies and investors.
KAIST startups VIRNECT, Standard Energy, A2US, and Panmnesia, Inc. won the 2025 CES Innovation Awards. ▴VIRNECT won the Innovation Award in the ‘Industrial Equipment and Machinery’ category for ‘VisionX’, an AI-based smart glass for industrial sites; ▴Standard Energy Co., Ltd. won the Innovation Award in the ‘Smart City’ category for developing the world’s first vanadium-ion battery; ▴A2US won the Innovation Award in the ‘Environment & Energy’ category for its portable air purifier that eliminates bacteria, odors, and fine dust in the air with just water droplets; ▴Panmnesia, Inc. won the Innovation Award in the ‘Computer Peripherals and Accessories’ category for its ‘CXL-based GPU Memory Expansion Kit’ that can drastically reduce the cost of building AI infrastructure.
< Image 2. (From left on the top row) VIRNECT, Standard Energy, (From left on the bottom row) A2US, Panmnesia, Inc. >
This exhibition will feature 15 startups that are standing out in cutting-edge technologies such as artificial intelligence (AI), robotics, mobility, and sustainability. In particular, AI-based deep tech startups in various industries such as logistics, architecture, and medicine will take up half of the total, showcasing the companies’ innovative AI technologies.
Polyphenol Factory Co.,Ltd introduces ‘Grabity’, a hair loss shampoo launched domestically, which applies the patented ingredient ‘LiftMax 308™’ that forms an instantaneous protective layer on the hair during the shampooing process. A real-time demonstration will be held at this exhibition hall so that visitors can experience the effects of the ingredient directly, and plans to enter the global market starting with the launch on Amazon in the US in January 2025.
VIRNECT will present ‘VisionX’, a prototype that won the Innovation Award this time. The product provides a chatbot AI through an AI voice interface, and has a function that allows users to check the status of the equipment in real time through conversations with the AI and receive troubleshooting guidance through voice conversations, so users can experience it directly at the KAIST Hall.
‘Standard Energy’ plans to exhibit ‘Energy Tile’, an indoor ESS that utilizes the world’s first vanadium ion battery (hereinafter referred to as VIB). VIB is absolutely safe from fire and has high installation flexibility, so it can be applied to smart cities and AI data centers.
‘A2US’ is the only company in the world that has hydroxyl radical water production technology, and won the Innovation Award for its first product, an air purifier. In the future, it is expected to be widely commercialized in air and water purification, smart farms, food tech, and semiconductor cleaning using safe and environmentally friendly hydroxyl radical water.
Panmnesia, Inc. won the CES Innovation Award for its GPU memory expansion solution equipped with its CXL 3.1 IP. By connecting a memory expansion device using Panmnesia’s CXL IP, the GPU’s memory capacity can be expanded to the terabyte level. Following the Innovation Award for ‘CXL-equipped AI Accelerator’ at CES 2024 last year, it is the only company to have won the Innovation Award for its AI-oriented CXL solution for two consecutive years.
In addition, technologies from a total of 15 companies will be introduced, including ▴Omelet ▴NEXTWAVE ▴Planby Technologies ▴Cosmo Bee ▴ImpactAI ▴Roen Surgical ▴DIDEN Roboticss ▴Autopedia ▴OAQ ▴HydroXpand ▴BOOKEND ▴Sterri.
On the central stage of the KAIST Hall, KAIST students selected as CES Student Supporters will conduct interviews with participating companies and promote the companies' innovative technologies and solutions. On the 8th, from 5 PM to 7 PM, a KAIST NIGHT event will be held where pre-invited investors and participating companies can network.
Keon Jae Lee, the head of the Institute of Technology Value Creation, said, “Through CES 2025, we will showcase innovative technologies and solutions from startups based on KAIST’s deep science and deep tech, and lead commercialization in cutting-edge technology fields such as AI, robotics, mobility, and environment/energy. KAIST plans to further promote technology commercialization by supporting the growth and marketing of innovative startups through the Institute of Technology Value Creation and by strengthening global networks and expanding cooperation opportunities.”
2024.12.31 View 2810
KAIST Wins CES 2025 Innovation Award, Showcasing Innovative Technologies
KAIST will showcase innovative technologies at the world’s largest technology fair, the Consumer Electronics Show (CES 2025). In addition, KAIST startups VIRNECT Inc., Standard Energy Inc., A2US Inc., and Panmnesia, Inc. won the 2025 CES Innovation Awards.
< Image 1. 3D-Graphical Profile of CES 2025 KAIST Exhibition Booth >
KAIST (President Kwang-Hyung Lee) announced on the 31st that it will operate a 140㎡ standalone booth at CES Eureka Park, which will be held in Las Vegas, USA from January 7th to 10th next year, to showcase KAIST's innovative technologies to global companies and investors.
KAIST startups VIRNECT, Standard Energy, A2US, and Panmnesia, Inc. won the 2025 CES Innovation Awards. ▴VIRNECT won the Innovation Award in the ‘Industrial Equipment and Machinery’ category for ‘VisionX’, an AI-based smart glass for industrial sites; ▴Standard Energy Co., Ltd. won the Innovation Award in the ‘Smart City’ category for developing the world’s first vanadium-ion battery; ▴A2US won the Innovation Award in the ‘Environment & Energy’ category for its portable air purifier that eliminates bacteria, odors, and fine dust in the air with just water droplets; ▴Panmnesia, Inc. won the Innovation Award in the ‘Computer Peripherals and Accessories’ category for its ‘CXL-based GPU Memory Expansion Kit’ that can drastically reduce the cost of building AI infrastructure.
< Image 2. (From left on the top row) VIRNECT, Standard Energy, (From left on the bottom row) A2US, Panmnesia, Inc. >
This exhibition will feature 15 startups that are standing out in cutting-edge technologies such as artificial intelligence (AI), robotics, mobility, and sustainability. In particular, AI-based deep tech startups in various industries such as logistics, architecture, and medicine will take up half of the total, showcasing the companies’ innovative AI technologies.
Polyphenol Factory Co.,Ltd introduces ‘Grabity’, a hair loss shampoo launched domestically, which applies the patented ingredient ‘LiftMax 308™’ that forms an instantaneous protective layer on the hair during the shampooing process. A real-time demonstration will be held at this exhibition hall so that visitors can experience the effects of the ingredient directly, and plans to enter the global market starting with the launch on Amazon in the US in January 2025.
VIRNECT will present ‘VisionX’, a prototype that won the Innovation Award this time. The product provides a chatbot AI through an AI voice interface, and has a function that allows users to check the status of the equipment in real time through conversations with the AI and receive troubleshooting guidance through voice conversations, so users can experience it directly at the KAIST Hall.
‘Standard Energy’ plans to exhibit ‘Energy Tile’, an indoor ESS that utilizes the world’s first vanadium ion battery (hereinafter referred to as VIB). VIB is absolutely safe from fire and has high installation flexibility, so it can be applied to smart cities and AI data centers.
‘A2US’ is the only company in the world that has hydroxyl radical water production technology, and won the Innovation Award for its first product, an air purifier. In the future, it is expected to be widely commercialized in air and water purification, smart farms, food tech, and semiconductor cleaning using safe and environmentally friendly hydroxyl radical water.
Panmnesia, Inc. won the CES Innovation Award for its GPU memory expansion solution equipped with its CXL 3.1 IP. By connecting a memory expansion device using Panmnesia’s CXL IP, the GPU’s memory capacity can be expanded to the terabyte level. Following the Innovation Award for ‘CXL-equipped AI Accelerator’ at CES 2024 last year, it is the only company to have won the Innovation Award for its AI-oriented CXL solution for two consecutive years.
In addition, technologies from a total of 15 companies will be introduced, including ▴Omelet ▴NEXTWAVE ▴Planby Technologies ▴Cosmo Bee ▴ImpactAI ▴Roen Surgical ▴DIDEN Roboticss ▴Autopedia ▴OAQ ▴HydroXpand ▴BOOKEND ▴Sterri.
On the central stage of the KAIST Hall, KAIST students selected as CES Student Supporters will conduct interviews with participating companies and promote the companies' innovative technologies and solutions. On the 8th, from 5 PM to 7 PM, a KAIST NIGHT event will be held where pre-invited investors and participating companies can network.
Keon Jae Lee, the head of the Institute of Technology Value Creation, said, “Through CES 2025, we will showcase innovative technologies and solutions from startups based on KAIST’s deep science and deep tech, and lead commercialization in cutting-edge technology fields such as AI, robotics, mobility, and environment/energy. KAIST plans to further promote technology commercialization by supporting the growth and marketing of innovative startups through the Institute of Technology Value Creation and by strengthening global networks and expanding cooperation opportunities.”
2024.12.31 View 2810 -
 A KAIST Research Team Produces Eco-Friendly Nylon with Engineered Bacterium
With worsening climate change and environmental issues, in recent years, there has been increased interest in the eco-friendly production of polymers like nylon.
On August 10, Dr. Taehee Han from a KAIST research team led by Distinguished Professor Sang Yup Lee in the Department of Chemical and Biomolecular Engineering revealed the successful development of a microbial strain that produces valerolactam, a monomer of nylon-5.
Valerolactam is an important monomer that constitutes nylon-5 and nylon-6,5. Nylon is the oldest synthetic polymer, and nylon-5 is one of its derivatives composed of monomers with five carbons, while nylon-5,6 is composed of two types of monomers with either five or six carbons. They not only have excellent processability, but are also light and tough, which allows them to be applied in a wide range of industrial sectors including clothing, badminton rackets, fishing nets, tents, and gear parts. Monomers are materials that can be built into polymers, and synthetic processes are what connects them into a polymer.
The chemical production of valerolactam, however, is based on petrochemistry, where extreme reaction conditions are required and toxic waste is produced. To solve these problems, efforts are being made to develop environmentally friendly and highly efficient microbial cell factories for lactam production. Systems metabolic engineering, a key strategy for effective microbial strain development, is a research field pioneered by Professor Sang Yup Lee.
Professor Lee’s team used metabolic engineering, a technique for manipulating microbial metabolic pathways, to construct a synthetic metabolic pathway for valerolactam production in Corynebacteriam glutamicum, a bacterium commonly used for amino acid production. With this, they successfully developed a microbial strain that utilizes biomass-derived glucose as a carbon source to produce high-value valerolactam.
In 2017, the team suggested a novel method that metabolically manipulates Escherichia coli to produce valerolactam. However, there were several limitations at the time including low producibility and the generation of harmful byproducts.
< Figure 1. Schematic graphical representation of the development of microorganisms that produce valerolactam, a nylon-5 monomer >
In this research, the team improved valerolactam producibility and incorporated an additional systems metabolic strategy to the developed microbial strain while eliminating the harmful byproducts. By removing the gene involved in the production of the main byproduct and through gene screening, the team successfully converted 5-aminovaleric acid, a byproduct and a precursor, into valerolactam.
Furthermore, by employing a strategy where the 5-aminovaleric acid-converting gene is inserted multiple times into the genome, the team strengthened the metabolic flux for valerolactam production. As a result, they reached a world-record concentration of 76.1 g/L, which is 6.17 times greater than what was previously reported.
This study was published in Metabolic Engineering on July 12, under the title, “Metabolic engineering of Corynebacterium glutamicum for the high-level production of valerolactam, a nylon-5 monomer”.
Dr. Taehee Han, the first author of the paper, said, “The significance of this research lies in our development of an environmentally friendly technology that efficiently produces monomer lactam for nylon production using microorganisms.” She added, “Through this technology, we will be able to take a step forward in replacing the petrochemical industry with a microorganism-based biopolymer industry.”
This work was supported by the “Development of Next-Generation Biofinery Platform Technologies for Leading Bio-based Chemicals Industry Project” funded by the Korean Ministry of Science and ICT.
2023.08.24 View 5107
A KAIST Research Team Produces Eco-Friendly Nylon with Engineered Bacterium
With worsening climate change and environmental issues, in recent years, there has been increased interest in the eco-friendly production of polymers like nylon.
On August 10, Dr. Taehee Han from a KAIST research team led by Distinguished Professor Sang Yup Lee in the Department of Chemical and Biomolecular Engineering revealed the successful development of a microbial strain that produces valerolactam, a monomer of nylon-5.
Valerolactam is an important monomer that constitutes nylon-5 and nylon-6,5. Nylon is the oldest synthetic polymer, and nylon-5 is one of its derivatives composed of monomers with five carbons, while nylon-5,6 is composed of two types of monomers with either five or six carbons. They not only have excellent processability, but are also light and tough, which allows them to be applied in a wide range of industrial sectors including clothing, badminton rackets, fishing nets, tents, and gear parts. Monomers are materials that can be built into polymers, and synthetic processes are what connects them into a polymer.
The chemical production of valerolactam, however, is based on petrochemistry, where extreme reaction conditions are required and toxic waste is produced. To solve these problems, efforts are being made to develop environmentally friendly and highly efficient microbial cell factories for lactam production. Systems metabolic engineering, a key strategy for effective microbial strain development, is a research field pioneered by Professor Sang Yup Lee.
Professor Lee’s team used metabolic engineering, a technique for manipulating microbial metabolic pathways, to construct a synthetic metabolic pathway for valerolactam production in Corynebacteriam glutamicum, a bacterium commonly used for amino acid production. With this, they successfully developed a microbial strain that utilizes biomass-derived glucose as a carbon source to produce high-value valerolactam.
In 2017, the team suggested a novel method that metabolically manipulates Escherichia coli to produce valerolactam. However, there were several limitations at the time including low producibility and the generation of harmful byproducts.
< Figure 1. Schematic graphical representation of the development of microorganisms that produce valerolactam, a nylon-5 monomer >
In this research, the team improved valerolactam producibility and incorporated an additional systems metabolic strategy to the developed microbial strain while eliminating the harmful byproducts. By removing the gene involved in the production of the main byproduct and through gene screening, the team successfully converted 5-aminovaleric acid, a byproduct and a precursor, into valerolactam.
Furthermore, by employing a strategy where the 5-aminovaleric acid-converting gene is inserted multiple times into the genome, the team strengthened the metabolic flux for valerolactam production. As a result, they reached a world-record concentration of 76.1 g/L, which is 6.17 times greater than what was previously reported.
This study was published in Metabolic Engineering on July 12, under the title, “Metabolic engineering of Corynebacterium glutamicum for the high-level production of valerolactam, a nylon-5 monomer”.
Dr. Taehee Han, the first author of the paper, said, “The significance of this research lies in our development of an environmentally friendly technology that efficiently produces monomer lactam for nylon production using microorganisms.” She added, “Through this technology, we will be able to take a step forward in replacing the petrochemical industry with a microorganism-based biopolymer industry.”
This work was supported by the “Development of Next-Generation Biofinery Platform Technologies for Leading Bio-based Chemicals Industry Project” funded by the Korean Ministry of Science and ICT.
2023.08.24 View 5107 -
 Breastfeeding Helps Prevent Mothers from Developing Diabetes after Childbirth
A team of South Korean researchers found that lactation can lower the incidence and reduce the risk of maternal postpartum diabetes. The researchers identified that lactation increases the mass and function of pancreatic beta cells through serotonin production. The team suggested that sustained improvements in pancreatic beta cells, which can last for years even after the cessation of lactation, improve mothers’ metabolic health in addition to providing health benefits for infants.
Pregnancy imposes a substantial metabolic burden on women through weight gain and increased insulin resistance. Various other factors, including a history of gestational diabetes, maternal age, and obesity, further affect women’s risk of progressing to diabetes after delivery, and the risk of postpartum diabetes increases more in women who have had gestational diabetes and/or repeated deliveries.
Diabetes-related complications include damage to blood vessels, which can lead to cardiovascular and cerebrovascular diseases such as heart attack and stroke, and problems with the nerves, eyes, kidneys, and many more. Since diabetes can pose a serious threat to mothers’ metabolic health, the management of maternal metabolic risk factors is important, especially in the peripartum period. Previous epidemiological studies have reported that lactation reduces the risk of postpartum diabetes, but the mechanisms underlying this benefit have remained elusive.
The study, published in Science Translational Medicine on April 29, explains the biology underpinning this observation on the beneficial effects of lactation. Professor Hail Kim from the Graduate School of Medical Science and Engineering at KAIST led and jointly conducted the study in conjunction with researchers from the Seoul National University Bundang Hospital (SNUBH) and Chungnam National University (CNU) in Korea, and the University of California, San Francisco (UCSF) in the US.
In their study, the team observed that the milk-secreting hormone ‘prolactin’ in lactating mothers not only promotes milk production, but also plays a major role in stimulating insulin-secreting pancreatic beta cells that regulate blood glucose in the body.
The researchers also found that ‘serotonin’, known as a chemical that contributes to wellbeing and happiness, is produced in pancreatic beta cells during lactation. Serotonin in pancreatic beta cells act as an antioxidant and reduce oxidative stress, making mothers’ beta cells healthier. Serotonin also induces the proliferation of beta cells, thereby increasing the beta cell mass and helping maintain proper glucose levels.
The research team conducted follow-up examinations on a total of 174 postpartum women, 85 lactated and 99 non-lactated, at two months postpartum and annually thereafter for at least three years. The results demonstrated that mothers who had undergone lactation improved pancreatic beta cell mass and function, and showed improved glucose homeostasis with approximately 20mg/dL lower glucose levels, thereby reducing the risk of postpartum diabetes in women. Surprisingly, this beneficial effect was maintained after the cessation of lactation, for more than three years after delivery.
Professor Kim said, “We are happy to prove that lactation benefits female metabolic health by improving beta cell mass and function as well as glycemic control.”
“Our future studies on the modulation of the molecular serotonergic pathway in accordance with the management of maternal metabolic risk factors may lead to new therapeutics to help prevent mothers from developing metabolic disorders,” he added.
This work was supported by grants from the National Research Foundation (NRF) and the National Research Council of Science and Technology (NST) of Korea, the National Institutes of Health (NIH), the Larry L. Hillblom Foundation, and the Health Fellowship Foundation.
Image credit: Professor Hail Kim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Moon, J. H et al. (2020) ‘Lactation improves pancreatic β cell mass and function through serotonin production.’ Science Translational Medicine, 12, eaay0455. Available online at https://doi.org/10.1126/scitranslmed.aay0455
Profile: Hail Kim, MD, PhD
hailkim@kaist.edu
Associate Professor
Graduate School of Medical Science and Engineering (GSMSE)
Korea Advanced Institute of Science and Technology (KAIST)
Profile: Hak Chul Jang, MD, PhD
janghak@snu.ac.kr
Professor
Division of Endocrinology and Metabolism
Seoul National University Bundang Hospital (SNUBH)
President
Korean Diabetes Association
Profile: Joon Ho Moon, MD, PhD
moonjoonho@gmail.com
Clinical Fellow
Division of Endocrinology and Metabolism
SNUBH
Profile: Hyeongseok Kim, MD, PhD
hskim85kor@gmail.com
Assistant Professor
Chungnam National University (CNU)
Profile: Professor Michael S. German, MD
Michael.German@ucsf.edu
Professor
Diabetes Center
University of California, San Francisco (UCSF)
(END)
2020.04.29 View 18635
Breastfeeding Helps Prevent Mothers from Developing Diabetes after Childbirth
A team of South Korean researchers found that lactation can lower the incidence and reduce the risk of maternal postpartum diabetes. The researchers identified that lactation increases the mass and function of pancreatic beta cells through serotonin production. The team suggested that sustained improvements in pancreatic beta cells, which can last for years even after the cessation of lactation, improve mothers’ metabolic health in addition to providing health benefits for infants.
Pregnancy imposes a substantial metabolic burden on women through weight gain and increased insulin resistance. Various other factors, including a history of gestational diabetes, maternal age, and obesity, further affect women’s risk of progressing to diabetes after delivery, and the risk of postpartum diabetes increases more in women who have had gestational diabetes and/or repeated deliveries.
Diabetes-related complications include damage to blood vessels, which can lead to cardiovascular and cerebrovascular diseases such as heart attack and stroke, and problems with the nerves, eyes, kidneys, and many more. Since diabetes can pose a serious threat to mothers’ metabolic health, the management of maternal metabolic risk factors is important, especially in the peripartum period. Previous epidemiological studies have reported that lactation reduces the risk of postpartum diabetes, but the mechanisms underlying this benefit have remained elusive.
The study, published in Science Translational Medicine on April 29, explains the biology underpinning this observation on the beneficial effects of lactation. Professor Hail Kim from the Graduate School of Medical Science and Engineering at KAIST led and jointly conducted the study in conjunction with researchers from the Seoul National University Bundang Hospital (SNUBH) and Chungnam National University (CNU) in Korea, and the University of California, San Francisco (UCSF) in the US.
In their study, the team observed that the milk-secreting hormone ‘prolactin’ in lactating mothers not only promotes milk production, but also plays a major role in stimulating insulin-secreting pancreatic beta cells that regulate blood glucose in the body.
The researchers also found that ‘serotonin’, known as a chemical that contributes to wellbeing and happiness, is produced in pancreatic beta cells during lactation. Serotonin in pancreatic beta cells act as an antioxidant and reduce oxidative stress, making mothers’ beta cells healthier. Serotonin also induces the proliferation of beta cells, thereby increasing the beta cell mass and helping maintain proper glucose levels.
The research team conducted follow-up examinations on a total of 174 postpartum women, 85 lactated and 99 non-lactated, at two months postpartum and annually thereafter for at least three years. The results demonstrated that mothers who had undergone lactation improved pancreatic beta cell mass and function, and showed improved glucose homeostasis with approximately 20mg/dL lower glucose levels, thereby reducing the risk of postpartum diabetes in women. Surprisingly, this beneficial effect was maintained after the cessation of lactation, for more than three years after delivery.
Professor Kim said, “We are happy to prove that lactation benefits female metabolic health by improving beta cell mass and function as well as glycemic control.”
“Our future studies on the modulation of the molecular serotonergic pathway in accordance with the management of maternal metabolic risk factors may lead to new therapeutics to help prevent mothers from developing metabolic disorders,” he added.
This work was supported by grants from the National Research Foundation (NRF) and the National Research Council of Science and Technology (NST) of Korea, the National Institutes of Health (NIH), the Larry L. Hillblom Foundation, and the Health Fellowship Foundation.
Image credit: Professor Hail Kim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Moon, J. H et al. (2020) ‘Lactation improves pancreatic β cell mass and function through serotonin production.’ Science Translational Medicine, 12, eaay0455. Available online at https://doi.org/10.1126/scitranslmed.aay0455
Profile: Hail Kim, MD, PhD
hailkim@kaist.edu
Associate Professor
Graduate School of Medical Science and Engineering (GSMSE)
Korea Advanced Institute of Science and Technology (KAIST)
Profile: Hak Chul Jang, MD, PhD
janghak@snu.ac.kr
Professor
Division of Endocrinology and Metabolism
Seoul National University Bundang Hospital (SNUBH)
President
Korean Diabetes Association
Profile: Joon Ho Moon, MD, PhD
moonjoonho@gmail.com
Clinical Fellow
Division of Endocrinology and Metabolism
SNUBH
Profile: Hyeongseok Kim, MD, PhD
hskim85kor@gmail.com
Assistant Professor
Chungnam National University (CNU)
Profile: Professor Michael S. German, MD
Michael.German@ucsf.edu
Professor
Diabetes Center
University of California, San Francisco (UCSF)
(END)
2020.04.29 View 18635 -
 KAIST Showcases Advanced Technologies at CES 2020
< President Sung-Chul Shin experiencing cooling gaming headset developed by TEGWAY >
KAIST Pavilion showcased 12 KAIST startups and alumni companies’ technologies at the International Consumer Electronics Show (CES) 2020 held in Las Vegas last month. Especially four companies, TEGWAY, THE.WAVE.TALK, Sherpa Space, and LiBEST won the CES 2020 Innovation Awards presented by the Consumer Technology Association (CTA). The CTA selects the most innovative items from among all submissions.
TEGWAY spinned off by KAIST Professor Byung Jin Cho already made international headlines for their flexible, wearable, and temperature immersive thermoelectric device. The device was selected as one of the top ten most promising digital technologies by the Netexplo Forum in 2015, and has been expanded into VR, AR, and games.
THE.WAVE.TALK has developed their first home appliance product in collaboration with ID+IM Design Laboratory of KAIST in which Professor Sang-Min Bae heads as creative director. Their real-time bacteria analysis with smart IoT sensor won the home appliances section.
Sherpa Space and LiBEST are the alumni companies. Sherpa Space’s lighting for plants won the sustainability, eco-design, and smart energy section, and LiBEST’s full-range flexible battery won the section for technology for a better world.
KAIST’s Alumni Association, Development Foundation, and the Office of University-Industry Cooperation (OUIC) made every effort to present KAIST technologies to the global market. President Sung-Chul Shin led the delegation comprising of 70 faculty, researchers, and young entrepreneurs. The KAIST Alumni Association fully funded the traveling costs of 30 alumni entrepreneurs and students, establishing scholarship for the CES participation. Ten young entrepreneurs were selected through the KAIST Startup Awards, and 20 current students preparing to start their own companies were selected via recommendation from the respective departments.
Associate Vice President of the OUIC Kyung Cheol Choi said in excitement, “We received many offers for joint research and investment from leading companies around the world,” adding, “We will continue doing our best to generate global value by developing the innovative technologies obtained from education and research into businesses.”
The KAIST pavilion at CES 2020 showcased:
1. flexible thermoelectric device ThermoReal and cooling gaming headset from TEGWAY,
2. wearable flexible battery from LiBEST,
3. applications such as conductive transparent electrode film and transparent heating film from J-Micro,
4. on-device AI solution based on deep learning model compression technology from Nota,
5. portable high resolution brain imaging device from OBELAB,
6. real-time bacteria analysis technology from THE.WAVE.TALK,
7. conversation-based AI-1 radio service platform from Timecode Archive,
8. light source solutions for different stages in a plant’s life cycle from Sherpa Space,
9. skin attached micro-LED patch and flexible piezoelectric acoustic sensor from FRONICS,
10. real-time cardiovascular measurement device from Healthrian,
11. block chain based mobile research documentation system from ReDWit, and
12. student-developed comprehensive healthcare device using a smart mirror.
(END)
2020.01.13 View 11632
KAIST Showcases Advanced Technologies at CES 2020
< President Sung-Chul Shin experiencing cooling gaming headset developed by TEGWAY >
KAIST Pavilion showcased 12 KAIST startups and alumni companies’ technologies at the International Consumer Electronics Show (CES) 2020 held in Las Vegas last month. Especially four companies, TEGWAY, THE.WAVE.TALK, Sherpa Space, and LiBEST won the CES 2020 Innovation Awards presented by the Consumer Technology Association (CTA). The CTA selects the most innovative items from among all submissions.
TEGWAY spinned off by KAIST Professor Byung Jin Cho already made international headlines for their flexible, wearable, and temperature immersive thermoelectric device. The device was selected as one of the top ten most promising digital technologies by the Netexplo Forum in 2015, and has been expanded into VR, AR, and games.
THE.WAVE.TALK has developed their first home appliance product in collaboration with ID+IM Design Laboratory of KAIST in which Professor Sang-Min Bae heads as creative director. Their real-time bacteria analysis with smart IoT sensor won the home appliances section.
Sherpa Space and LiBEST are the alumni companies. Sherpa Space’s lighting for plants won the sustainability, eco-design, and smart energy section, and LiBEST’s full-range flexible battery won the section for technology for a better world.
KAIST’s Alumni Association, Development Foundation, and the Office of University-Industry Cooperation (OUIC) made every effort to present KAIST technologies to the global market. President Sung-Chul Shin led the delegation comprising of 70 faculty, researchers, and young entrepreneurs. The KAIST Alumni Association fully funded the traveling costs of 30 alumni entrepreneurs and students, establishing scholarship for the CES participation. Ten young entrepreneurs were selected through the KAIST Startup Awards, and 20 current students preparing to start their own companies were selected via recommendation from the respective departments.
Associate Vice President of the OUIC Kyung Cheol Choi said in excitement, “We received many offers for joint research and investment from leading companies around the world,” adding, “We will continue doing our best to generate global value by developing the innovative technologies obtained from education and research into businesses.”
The KAIST pavilion at CES 2020 showcased:
1. flexible thermoelectric device ThermoReal and cooling gaming headset from TEGWAY,
2. wearable flexible battery from LiBEST,
3. applications such as conductive transparent electrode film and transparent heating film from J-Micro,
4. on-device AI solution based on deep learning model compression technology from Nota,
5. portable high resolution brain imaging device from OBELAB,
6. real-time bacteria analysis technology from THE.WAVE.TALK,
7. conversation-based AI-1 radio service platform from Timecode Archive,
8. light source solutions for different stages in a plant’s life cycle from Sherpa Space,
9. skin attached micro-LED patch and flexible piezoelectric acoustic sensor from FRONICS,
10. real-time cardiovascular measurement device from Healthrian,
11. block chain based mobile research documentation system from ReDWit, and
12. student-developed comprehensive healthcare device using a smart mirror.
(END)
2020.01.13 View 11632 -
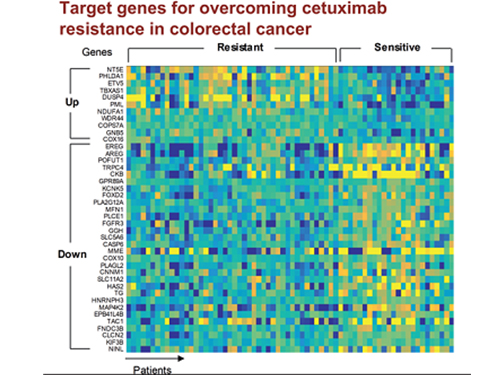 5 Biomarkers for Overcoming Colorectal Cancer Drug Resistance Identified
< Professor Kwang-Hyun Cho's Team >
KAIST researchers have identified five biomarkers that will help them address resistance to cancer-targeting therapeutics. This new treatment strategy will bring us one step closer to precision medicine for patients who showed resistance.
Colorectal cancer is one of the most common types of cancer worldwide. The number of patients has surpassed 1 million, and its five-year survival rate significantly drops to about 20 percent when metastasized. In Korea, the surge of colorectal cancer has been the highest in the last 10 years due to increasing Westernized dietary patterns and obesity. It is expected that the number and mortality rates of colorectal cancer patients will increase sharply as the nation is rapidly facing an increase in its aging population.
Recently, anticancer agents targeting only specific molecules of colon cancer cells have been developed. Unlike conventional anticancer medications, these selectively treat only specific target factors, so they can significantly reduce some of the side-effects of anticancer therapy while enhancing drug efficacy.
Cetuximab is the most well-known FDA approved anticancer medication. It is a biomarker that predicts drug reactivity and utilizes the presence of the ‘KRAS’ gene mutation. Cetuximab is prescribed to patients who don’t carry the KRAS gene mutation.
However, even in patients without the KRAS gene mutation, the response rate of Cetuximab is only about fifty percent, and there is also resistance to drugs after targeted chemotherapy. Compared with conventional chemotherapy alone, the life expectancy only lasts five months on average.
In research featured in the FEBS Journal as the cover paper for the April 7 edition, the KAIST research team led by Professor Kwang-Hyun Cho at the Department of Bio and Brain Engineering presented five additional biomarkers that could increase Cetuximab responsiveness using systems biology approach that combines genomic data analysis, mathematical modeling, and cell experiments. The experimental inhibition of newly discovered biomarkers DUSP4, ETV5, GNB5, NT5E, and PHLDA1 in colorectal cancer cells has been shown to overcome Cetuximab resistance in KRAS-normal genes. The research team confirmed that when suppressing GNB5, one of the new biomarkers, it was shown to overcome resistance to Cetuximab regardless of having a mutation in the KRAS gene.
Professor Cho said, “There has not been an example of colorectal cancer treatment involving regulation of the GNB5 gene.” He continued, “Identifying the principle of drug resistance in cancer cells through systems biology and discovering new biomarkers that could be a new molecular target to overcome drug resistance suggest real potential to actualize precision medicine.”
This study was supported by the National Research Foundation of Korea (NRF) and funded by the Ministry of Science and ICT (2017R1A2A1A17069642 and 2015M3A9A7067220).
Image 1. The cover of FEBS Journal for April 2019
2019.05.27 View 59096
5 Biomarkers for Overcoming Colorectal Cancer Drug Resistance Identified
< Professor Kwang-Hyun Cho's Team >
KAIST researchers have identified five biomarkers that will help them address resistance to cancer-targeting therapeutics. This new treatment strategy will bring us one step closer to precision medicine for patients who showed resistance.
Colorectal cancer is one of the most common types of cancer worldwide. The number of patients has surpassed 1 million, and its five-year survival rate significantly drops to about 20 percent when metastasized. In Korea, the surge of colorectal cancer has been the highest in the last 10 years due to increasing Westernized dietary patterns and obesity. It is expected that the number and mortality rates of colorectal cancer patients will increase sharply as the nation is rapidly facing an increase in its aging population.
Recently, anticancer agents targeting only specific molecules of colon cancer cells have been developed. Unlike conventional anticancer medications, these selectively treat only specific target factors, so they can significantly reduce some of the side-effects of anticancer therapy while enhancing drug efficacy.
Cetuximab is the most well-known FDA approved anticancer medication. It is a biomarker that predicts drug reactivity and utilizes the presence of the ‘KRAS’ gene mutation. Cetuximab is prescribed to patients who don’t carry the KRAS gene mutation.
However, even in patients without the KRAS gene mutation, the response rate of Cetuximab is only about fifty percent, and there is also resistance to drugs after targeted chemotherapy. Compared with conventional chemotherapy alone, the life expectancy only lasts five months on average.
In research featured in the FEBS Journal as the cover paper for the April 7 edition, the KAIST research team led by Professor Kwang-Hyun Cho at the Department of Bio and Brain Engineering presented five additional biomarkers that could increase Cetuximab responsiveness using systems biology approach that combines genomic data analysis, mathematical modeling, and cell experiments. The experimental inhibition of newly discovered biomarkers DUSP4, ETV5, GNB5, NT5E, and PHLDA1 in colorectal cancer cells has been shown to overcome Cetuximab resistance in KRAS-normal genes. The research team confirmed that when suppressing GNB5, one of the new biomarkers, it was shown to overcome resistance to Cetuximab regardless of having a mutation in the KRAS gene.
Professor Cho said, “There has not been an example of colorectal cancer treatment involving regulation of the GNB5 gene.” He continued, “Identifying the principle of drug resistance in cancer cells through systems biology and discovering new biomarkers that could be a new molecular target to overcome drug resistance suggest real potential to actualize precision medicine.”
This study was supported by the National Research Foundation of Korea (NRF) and funded by the Ministry of Science and ICT (2017R1A2A1A17069642 and 2015M3A9A7067220).
Image 1. The cover of FEBS Journal for April 2019
2019.05.27 View 59096 -
 The 1st Korea Toray Science and Technology Awardee, Prof. Sukbok Chang
(Distinguished Professor Sukbok Chang from the Department of Chemistry)
The Korea Toray Science Foundation (KTSF) awarded the first Korea Toray Science Technology Award in basic science to Distinguished Professor Sukbok Chang from the Department of Chemistry on September 19.
KTSF was established in January 2018, and its award goes to researchers who have significantly contributed to the development of chemistry and materials research with funds to support research projects.
Distinguished Professor Chang has devoted himself in organocatalysis research; in particular, his work on catalysts for effective lactam formation, which was an intricate problem, received great attention.
The award ceremony will take place in The Federation of Korean Industries Hall on October 31. KTFS board members, judges, and the CEO of Toray Industries Akihiro Nikkaku will attend the ceremony. Also, Dr. Ryoji Noyori, the Nobel Laureate in Chemistry, will give a talk on the role of chemistry and creative challenges as a researcher.
2018.10.04 View 8382
The 1st Korea Toray Science and Technology Awardee, Prof. Sukbok Chang
(Distinguished Professor Sukbok Chang from the Department of Chemistry)
The Korea Toray Science Foundation (KTSF) awarded the first Korea Toray Science Technology Award in basic science to Distinguished Professor Sukbok Chang from the Department of Chemistry on September 19.
KTSF was established in January 2018, and its award goes to researchers who have significantly contributed to the development of chemistry and materials research with funds to support research projects.
Distinguished Professor Chang has devoted himself in organocatalysis research; in particular, his work on catalysts for effective lactam formation, which was an intricate problem, received great attention.
The award ceremony will take place in The Federation of Korean Industries Hall on October 31. KTFS board members, judges, and the CEO of Toray Industries Akihiro Nikkaku will attend the ceremony. Also, Dr. Ryoji Noyori, the Nobel Laureate in Chemistry, will give a talk on the role of chemistry and creative challenges as a researcher.
2018.10.04 View 8382 -
 Doctoral Student Receives the Best Paper Award from the International Metabolic Engineering Conference 2016
So Young Choi, a Ph.D. candidate at the Department of Chemical and Biomolecular Engineering at KAIST, received the Student and Young Investigator Poster Award at the 11th International Metabolic Engineering Conference held in Awaji, Japan on June 26-30.
Choi received the award for her research on one-step fermentative production of Poly(lactate-co-glycolate) (PLGA) from carbohydrates in Escherichia coli, which was published in the April 2016 issue of Nature Biotechnology.
In her paper, she presented a novel technology to synthesize PLGA, a non-natural copolymer, through a biological production process. Because of its biodegradability, non-toxicity, and biocompatibility, PLGA is widely used in biomedical and therapeutic applications, including surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Employing a metabolic engineering approach, Choi manipulated the metabolic pathway of an Escherichia coli bacterium to convert glucose and xylose into the biosynthesis of PLGA within the cell. Previously, PLGA could be obtained only through chemical synthesis.
Choi said, “I’m thrilled to receive an award from a flagship conference of my research field. Mindful of this recognition, I will continue my research to produce meaningful results, thereby contributing to the development of science and technology in Korea.”
The International Metabolic Engineering Conference is a leading professional gathering where state-of-the-art developments and achievements made in the field of metabolic engineering are shared. With the participation of about 400 professionals from all around the world, the conference participants discussed this year’s theme of “Design, Synthesis and System Integration for Metabolic Engineering.”
2016.07.07 View 11041
Doctoral Student Receives the Best Paper Award from the International Metabolic Engineering Conference 2016
So Young Choi, a Ph.D. candidate at the Department of Chemical and Biomolecular Engineering at KAIST, received the Student and Young Investigator Poster Award at the 11th International Metabolic Engineering Conference held in Awaji, Japan on June 26-30.
Choi received the award for her research on one-step fermentative production of Poly(lactate-co-glycolate) (PLGA) from carbohydrates in Escherichia coli, which was published in the April 2016 issue of Nature Biotechnology.
In her paper, she presented a novel technology to synthesize PLGA, a non-natural copolymer, through a biological production process. Because of its biodegradability, non-toxicity, and biocompatibility, PLGA is widely used in biomedical and therapeutic applications, including surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Employing a metabolic engineering approach, Choi manipulated the metabolic pathway of an Escherichia coli bacterium to convert glucose and xylose into the biosynthesis of PLGA within the cell. Previously, PLGA could be obtained only through chemical synthesis.
Choi said, “I’m thrilled to receive an award from a flagship conference of my research field. Mindful of this recognition, I will continue my research to produce meaningful results, thereby contributing to the development of science and technology in Korea.”
The International Metabolic Engineering Conference is a leading professional gathering where state-of-the-art developments and achievements made in the field of metabolic engineering are shared. With the participation of about 400 professionals from all around the world, the conference participants discussed this year’s theme of “Design, Synthesis and System Integration for Metabolic Engineering.”
2016.07.07 View 11041 -
 Professor Jeong Ho Lee Receives the 2015 Pediatric Epilepsies Research Award
The award identifies leading scientists worldwide and funds their cutting-edge research in epilepsy.
The Citizen United for Research in Epilepsy (CURE) announced on September 7, 2015, that Jeong Ho Lee, a professor of the Graduate School of Medical Science and Engineering at KAIST, will be awarded the 2015 Pediatric Epilepsies Research Award.
The Pediatric Epilepsies Research Award is given annually to a researcher who has conducted novel, innovative research projects that address severe, intractable pediatric epilepsies as well as collaborative, interdisciplinary projects that explore new approaches to find a treatment for pediatric epilepsies.
Lee was recognized for his leading study in the field of intractable epilepsy. He is the first Korean who has ever received this award, securing a research grant of USD 250,000 for two years.
Lee has conducted research on brain somatic mutations as the novel cause of childhood intractable epilepsy. Pediatric epilepsies account for approximately 70% of all cases of epilepsy.
Established in 1998, CURE is a non-profit American organization based in Chicago, Illinois, which is committed to funding research and various initiatives that will lead to breakthroughs to cure epilepsy.
Since its inception, CURE has been at the forefront of epilepsy research, raising more than USD 32 million to support researchers and scientists worldwide. It has also awarded more than 180 cutting-edge projects in 13 countries.
2015.09.09 View 11152
Professor Jeong Ho Lee Receives the 2015 Pediatric Epilepsies Research Award
The award identifies leading scientists worldwide and funds their cutting-edge research in epilepsy.
The Citizen United for Research in Epilepsy (CURE) announced on September 7, 2015, that Jeong Ho Lee, a professor of the Graduate School of Medical Science and Engineering at KAIST, will be awarded the 2015 Pediatric Epilepsies Research Award.
The Pediatric Epilepsies Research Award is given annually to a researcher who has conducted novel, innovative research projects that address severe, intractable pediatric epilepsies as well as collaborative, interdisciplinary projects that explore new approaches to find a treatment for pediatric epilepsies.
Lee was recognized for his leading study in the field of intractable epilepsy. He is the first Korean who has ever received this award, securing a research grant of USD 250,000 for two years.
Lee has conducted research on brain somatic mutations as the novel cause of childhood intractable epilepsy. Pediatric epilepsies account for approximately 70% of all cases of epilepsy.
Established in 1998, CURE is a non-profit American organization based in Chicago, Illinois, which is committed to funding research and various initiatives that will lead to breakthroughs to cure epilepsy.
Since its inception, CURE has been at the forefront of epilepsy research, raising more than USD 32 million to support researchers and scientists worldwide. It has also awarded more than 180 cutting-edge projects in 13 countries.
2015.09.09 View 11152 -
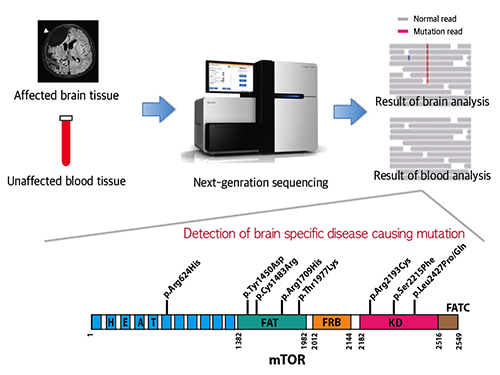 Mutations Occurring Only in Brain Responsible for Intractable Epilepsy Identified
KAIST researchers have discovered that brain somatic mutations in MTOR gene induce intractable epilepsy and suggest a precision medicine to treat epileptic seizures.
Epilepsy is a brain disorder which afflicts more than 50 million people worldwide. Many epilepsy patients can control their symptoms through medication, but about 30% suffer from intractable epilepsy and are unable to manage the disease with drugs. Intractable epilepsy causes multiple seizures, permanent mental, physical, and developmental disabilities, and even death. Therefore, surgical removal of the affected area from the brain has been practiced as a treatment for patients with medically refractory seizures, but this too fails to provide a complete solution because only 60% of the patients who undergo surgery are rendered free of seizures.
A Korean research team led by Professor Jeong Ho Lee of the Graduate School of Medical Science and Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and Professor Dong-Seok Kim of Epilepsy Research Center at Yonsei University College of Medicine has recently identified brain somatic mutations in the gene of mechanistic target of rapamycin (MTOR) as the cause of focal cortical dysplasia type II (FCDII), one of the most important and common inducers to intractable epilepsy, particularly in children. They propose a targeted therapy to lessen epileptic seizures by suppressing the activation of mTOR kinase, a signaling protein in the brain. Their research results were published online in Nature Medicine on March 23, 2015.
FCDII contributes to the abnormal developments of the cerebral cortex, ranging from cortical disruption to severe forms of cortical dyslamination, balloon cells, and dysplastic neurons. The research team studied 77 FCDII patients with intractable epilepsy who had received a surgery to remove the affected regions from the brain. The researchers used various deep sequencing technologies to conduct comparative DNA analysis of the samples obtained from the patients’ brain and blood, or saliva. They reported that about 16% of the studied patients had somatic mutations in their brain. Such mutations, however, did not take place in their blood or saliva DNA.
Professor Jeong Ho Lee of KAIST said, “This is an important finding. Unlike our previous belief that genetic mutations causing intractable epilepsy exist anywhere in the human body including blood, specific gene mutations incurred only in the brain can lead to intractable epilepsy. From our animal models, we could see how a small fraction of mutations carrying neurons in the brain could affect its entire function.”
The research team recapitulated the pathogenesis of intractable epilepsy by inducing the focal cortical expression of mutated mTOR in the mouse brain via electroporation method and observed as the mouse develop epileptic symptoms. They then treated these mice with the drug called “rapamycin” to inhibit the activity of mTOR protein and observed that it suppressed the development of epileptic seizures with cytomegalic neurons.
“Our study offers the first evidence that brain-somatic activating mutations in MTOR cause FCDII and identifies mTOR as a treatment target for intractable epilepsy,” said co-author Dr. Dong-Seok Kim, a neurosurgeon at Yonsei Medical Center with the country’s largest surgical experiences in treating patients with this condition.
The research paper is titled “Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy.” (Digital Object Identifier #: 10.1038/nm.3824)
Picture 1: A schematic image to show how to detect brain specific mutation using next-generation sequencing technology with blood-brain paired sample. Simple comparison of non-overlapping mutations between affected and unaffected tissues is able to detect brain specific mutations.
Picture 2: A schematic image to show how to generate focal cortical dysplasia mouse model. This mouse model open the new window of drug screening for seizure patients.
Picture 3: Targeted medicine can rescue the focal cortical dysplasia symptoms including cytomegalic neuron & intractable epilepsy.
2015.03.25 View 14215
Mutations Occurring Only in Brain Responsible for Intractable Epilepsy Identified
KAIST researchers have discovered that brain somatic mutations in MTOR gene induce intractable epilepsy and suggest a precision medicine to treat epileptic seizures.
Epilepsy is a brain disorder which afflicts more than 50 million people worldwide. Many epilepsy patients can control their symptoms through medication, but about 30% suffer from intractable epilepsy and are unable to manage the disease with drugs. Intractable epilepsy causes multiple seizures, permanent mental, physical, and developmental disabilities, and even death. Therefore, surgical removal of the affected area from the brain has been practiced as a treatment for patients with medically refractory seizures, but this too fails to provide a complete solution because only 60% of the patients who undergo surgery are rendered free of seizures.
A Korean research team led by Professor Jeong Ho Lee of the Graduate School of Medical Science and Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and Professor Dong-Seok Kim of Epilepsy Research Center at Yonsei University College of Medicine has recently identified brain somatic mutations in the gene of mechanistic target of rapamycin (MTOR) as the cause of focal cortical dysplasia type II (FCDII), one of the most important and common inducers to intractable epilepsy, particularly in children. They propose a targeted therapy to lessen epileptic seizures by suppressing the activation of mTOR kinase, a signaling protein in the brain. Their research results were published online in Nature Medicine on March 23, 2015.
FCDII contributes to the abnormal developments of the cerebral cortex, ranging from cortical disruption to severe forms of cortical dyslamination, balloon cells, and dysplastic neurons. The research team studied 77 FCDII patients with intractable epilepsy who had received a surgery to remove the affected regions from the brain. The researchers used various deep sequencing technologies to conduct comparative DNA analysis of the samples obtained from the patients’ brain and blood, or saliva. They reported that about 16% of the studied patients had somatic mutations in their brain. Such mutations, however, did not take place in their blood or saliva DNA.
Professor Jeong Ho Lee of KAIST said, “This is an important finding. Unlike our previous belief that genetic mutations causing intractable epilepsy exist anywhere in the human body including blood, specific gene mutations incurred only in the brain can lead to intractable epilepsy. From our animal models, we could see how a small fraction of mutations carrying neurons in the brain could affect its entire function.”
The research team recapitulated the pathogenesis of intractable epilepsy by inducing the focal cortical expression of mutated mTOR in the mouse brain via electroporation method and observed as the mouse develop epileptic symptoms. They then treated these mice with the drug called “rapamycin” to inhibit the activity of mTOR protein and observed that it suppressed the development of epileptic seizures with cytomegalic neurons.
“Our study offers the first evidence that brain-somatic activating mutations in MTOR cause FCDII and identifies mTOR as a treatment target for intractable epilepsy,” said co-author Dr. Dong-Seok Kim, a neurosurgeon at Yonsei Medical Center with the country’s largest surgical experiences in treating patients with this condition.
The research paper is titled “Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy.” (Digital Object Identifier #: 10.1038/nm.3824)
Picture 1: A schematic image to show how to detect brain specific mutation using next-generation sequencing technology with blood-brain paired sample. Simple comparison of non-overlapping mutations between affected and unaffected tissues is able to detect brain specific mutations.
Picture 2: A schematic image to show how to generate focal cortical dysplasia mouse model. This mouse model open the new window of drug screening for seizure patients.
Picture 3: Targeted medicine can rescue the focal cortical dysplasia symptoms including cytomegalic neuron & intractable epilepsy.
2015.03.25 View 14215 -
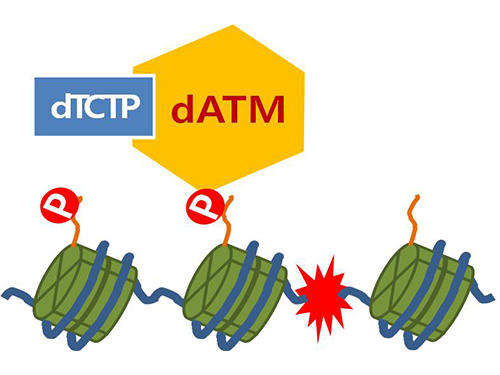 Mechanism in regulation of cancer-related key enzyme, ATM, for DNA damage and repair revealed
Professor Kwang-Wook Choi
A research team led by Professor Kwang-Wook Choi and Dr. Seong-Tae Hong from the Department of Biological Sciences at KAIST has successfully investigated the operational mechanism of the protein Ataxia Telangiectasia Mutated (ATM), an essential protein to the function of a crucial key enzyme that repairs the damaged DNA which stores biometric information. The results were published on December 19th Nature Communications online edition.
All organisms, including humans, constantly strive to protect the information within their DNA from damages posed by a number of factors, such as carbonized materials in our daily food intake, radioactive materials such as radon emitting from the cement of buildings or ultraviolet of the sunlight, which could be a trigger for cancer.
In order to keep the DNA information safe, the organisms are always carrying out complex and sophisticated DNA repair work, which involves the crucial DNA damage repair protein ATM. Consequently, a faulty ATM leads to higher risks of cancer.
Until now, academia predicted that the Translationally Controlled Tumor Protein (TCTP) will play an important role in regulating the function of ATM. However, since most of main research regarding TCTP has only been conducted in cultured cells, it was unable to identify exactly what mechanisms TCTP employs to control ATM.
The KAIST research team identified that TCTP can combine with ATM or increase the enzymatic activity of ATM. In addition, Drosophilia, one of the most widely used model organisms for molecular genetics, has been used to identify that TCTP and ATM play a very important role in repairing the DNA damaged by radiation. This information has allowed the researchers to establish TCTP’s essential function in maintaining the DNA information in cell cultures and even in higher organisms, and to provide specific and important clues to the regulation of ATM by TCTP.
Professor Kwang-Wook Choi said, “Our research is a good example that basic research using Drosophilia can make important contributions to understanding the process of diseases, such as cancer, and to developing adequate treatment.”
The research has been funded by the Ministry of Science, ICT and Future Planning, Republic of Korea, and the National Research Foundation of Korea.
Figure 1. When the amount of TCTP protein is reduced, cells of the Drosophila's eye are abnormally deformed by radiation. Scale bars = 200mm
Figure 2. When the amount of TCTP protein is reduced, the chromosomes of Drosophilia are easily broken by radiation. Scale bars = 10 mm.
Figure 3. When gene expressions of TCTP and ATM are reduced, large defects occur in the normal development of the eye. (Left: normal Drosophilia's eye, right: development-deficient eye)
Figure 4. ATM marks the position of the broken DNA, with TCTP helping to facilitate this reaction. DNA (blue line) within the cell nucleus is coiled around the histone protein (green cylinder). When DNA is broken, ATM protein attaches a phosphate group (P). Multiple DNA repair protein recognizes the phosphate as a signal that requires repair and gathers at the site.
2014.01.07 View 13377
Mechanism in regulation of cancer-related key enzyme, ATM, for DNA damage and repair revealed
Professor Kwang-Wook Choi
A research team led by Professor Kwang-Wook Choi and Dr. Seong-Tae Hong from the Department of Biological Sciences at KAIST has successfully investigated the operational mechanism of the protein Ataxia Telangiectasia Mutated (ATM), an essential protein to the function of a crucial key enzyme that repairs the damaged DNA which stores biometric information. The results were published on December 19th Nature Communications online edition.
All organisms, including humans, constantly strive to protect the information within their DNA from damages posed by a number of factors, such as carbonized materials in our daily food intake, radioactive materials such as radon emitting from the cement of buildings or ultraviolet of the sunlight, which could be a trigger for cancer.
In order to keep the DNA information safe, the organisms are always carrying out complex and sophisticated DNA repair work, which involves the crucial DNA damage repair protein ATM. Consequently, a faulty ATM leads to higher risks of cancer.
Until now, academia predicted that the Translationally Controlled Tumor Protein (TCTP) will play an important role in regulating the function of ATM. However, since most of main research regarding TCTP has only been conducted in cultured cells, it was unable to identify exactly what mechanisms TCTP employs to control ATM.
The KAIST research team identified that TCTP can combine with ATM or increase the enzymatic activity of ATM. In addition, Drosophilia, one of the most widely used model organisms for molecular genetics, has been used to identify that TCTP and ATM play a very important role in repairing the DNA damaged by radiation. This information has allowed the researchers to establish TCTP’s essential function in maintaining the DNA information in cell cultures and even in higher organisms, and to provide specific and important clues to the regulation of ATM by TCTP.
Professor Kwang-Wook Choi said, “Our research is a good example that basic research using Drosophilia can make important contributions to understanding the process of diseases, such as cancer, and to developing adequate treatment.”
The research has been funded by the Ministry of Science, ICT and Future Planning, Republic of Korea, and the National Research Foundation of Korea.
Figure 1. When the amount of TCTP protein is reduced, cells of the Drosophila's eye are abnormally deformed by radiation. Scale bars = 200mm
Figure 2. When the amount of TCTP protein is reduced, the chromosomes of Drosophilia are easily broken by radiation. Scale bars = 10 mm.
Figure 3. When gene expressions of TCTP and ATM are reduced, large defects occur in the normal development of the eye. (Left: normal Drosophilia's eye, right: development-deficient eye)
Figure 4. ATM marks the position of the broken DNA, with TCTP helping to facilitate this reaction. DNA (blue line) within the cell nucleus is coiled around the histone protein (green cylinder). When DNA is broken, ATM protein attaches a phosphate group (P). Multiple DNA repair protein recognizes the phosphate as a signal that requires repair and gathers at the site.
2014.01.07 View 13377 -
 2010 International Presidential Forum was held successfully.
On October 11th, the 2010 International Presidential Forum on “The Role of the Research University in an S&T Dominated Era: Expectation & Delivery” was held successfully at the Westin Chosun Hotel in Seoul. The third International Presidential Forum to be held, participants of the 2010 Presidential Forum engaged in an in-depth discussion about the direction that research universities should take in the 21st Century.
On its opening, President Nam Pyo Suh delivered a congratulatory message saying, “This forum is a meaningful gathering where research universities will suggest role models and find ways research universities can contribute to the progress of mankind in this century.”
Following, Lee Ki Jun, CEO of the Korean Federation of Science and Technology Societies said, “The common goal of the world’s research universities is to solve the problems mankind is facing together. I believe that the discussion we will hold today at the forum will point to the future direction of research universities.”
“To produce next generation engineers meeting global standards, exchange and dual degree programs between universities must be strengthened,” said Lars Pallesen, President of the Technical University of Denmark. “Research universities must support the exchange between students beyond cultural and national borders to adapt to the global market.”
Ichiro Okura, Vice President of Tokyo Institute of Technology, presented on the “Asian Science and Technology Pioneering Institutes of Research and Education, ASPIRE.” ASPIRE is a community created by the coalition between science and technology universities in the Far East. Its purpose is to contribute to sustainable global growth by educating high-quality human resources and lead Asia’s technology innovation based on science and technology development.
“For research universities to solve today’s global issues, universities must create new ideas by performing fundamental studies and developing innovative technology. The financial resources of universities must be focused with choices based on results,” remarked President Suh.
Zaini Ujang, Vice-President of the Universiti Teknologi Malaysia stated that “the Malaysian government is planning on converting from a ‘labor-intensive economy’ to an ‘innovative leading economy’ with the goal of joining the advanced countries by 2020. In today’s science and technology era where innovative technology is necessary, research universities have an important role of developing the knowledge environmental system to lead the world economy.” Vice-President Ujang then explained what strategies Malaysian research universities devised in the innovative leading economy era to create research universities that bring creativity and innovation.
Tod A. Laursen, President of KUSTAR, said that “KUSTAR has a leading role in bringing science and technology and manpower necessary in converting the oil-centered economy of UAE to a knowledge-based economy. KUSTAR will continuously strengthen international cooperation to become not only the best engineering university in the Arab region but in the world.”
At this year’s forum, thirty international presidents and vice presidents from 24 universities in 15 countries including Georgia Tech, Technical University of Denmark, Technion-Israel Institute of Technology, University of Queensland, Tokyo University, Nanyang Technological University, University Teknologi Malaysia and Hong Kong Institute of Science and Technology along with forty national figures such as the presidents of Hanyang University and Handong Global University, governmental bureaucrats and representatives from national business and institutions participated.
2010.10.20 View 16592
2010 International Presidential Forum was held successfully.
On October 11th, the 2010 International Presidential Forum on “The Role of the Research University in an S&T Dominated Era: Expectation & Delivery” was held successfully at the Westin Chosun Hotel in Seoul. The third International Presidential Forum to be held, participants of the 2010 Presidential Forum engaged in an in-depth discussion about the direction that research universities should take in the 21st Century.
On its opening, President Nam Pyo Suh delivered a congratulatory message saying, “This forum is a meaningful gathering where research universities will suggest role models and find ways research universities can contribute to the progress of mankind in this century.”
Following, Lee Ki Jun, CEO of the Korean Federation of Science and Technology Societies said, “The common goal of the world’s research universities is to solve the problems mankind is facing together. I believe that the discussion we will hold today at the forum will point to the future direction of research universities.”
“To produce next generation engineers meeting global standards, exchange and dual degree programs between universities must be strengthened,” said Lars Pallesen, President of the Technical University of Denmark. “Research universities must support the exchange between students beyond cultural and national borders to adapt to the global market.”
Ichiro Okura, Vice President of Tokyo Institute of Technology, presented on the “Asian Science and Technology Pioneering Institutes of Research and Education, ASPIRE.” ASPIRE is a community created by the coalition between science and technology universities in the Far East. Its purpose is to contribute to sustainable global growth by educating high-quality human resources and lead Asia’s technology innovation based on science and technology development.
“For research universities to solve today’s global issues, universities must create new ideas by performing fundamental studies and developing innovative technology. The financial resources of universities must be focused with choices based on results,” remarked President Suh.
Zaini Ujang, Vice-President of the Universiti Teknologi Malaysia stated that “the Malaysian government is planning on converting from a ‘labor-intensive economy’ to an ‘innovative leading economy’ with the goal of joining the advanced countries by 2020. In today’s science and technology era where innovative technology is necessary, research universities have an important role of developing the knowledge environmental system to lead the world economy.” Vice-President Ujang then explained what strategies Malaysian research universities devised in the innovative leading economy era to create research universities that bring creativity and innovation.
Tod A. Laursen, President of KUSTAR, said that “KUSTAR has a leading role in bringing science and technology and manpower necessary in converting the oil-centered economy of UAE to a knowledge-based economy. KUSTAR will continuously strengthen international cooperation to become not only the best engineering university in the Arab region but in the world.”
At this year’s forum, thirty international presidents and vice presidents from 24 universities in 15 countries including Georgia Tech, Technical University of Denmark, Technion-Israel Institute of Technology, University of Queensland, Tokyo University, Nanyang Technological University, University Teknologi Malaysia and Hong Kong Institute of Science and Technology along with forty national figures such as the presidents of Hanyang University and Handong Global University, governmental bureaucrats and representatives from national business and institutions participated.
2010.10.20 View 16592