http%3A%2F%2Fmbel.kaist.ac.kr
-
 Distinguished Professor Sang Yup Lee Honored with Charles D. Scott Award
Vice President for Research Sang Yup Lee received the 2021 Charles D. Scott Award from the Society for Industrial Microbiology and Biotechnology. Distinguished Professor Lee from the Department of Chemical and Biomolecular Engineering at KAIST is the first Asian awardee.
The Charles D. Scott Award, initiated in 1995, recognizes individuals who have made significant contributions to enable and further the use of biotechnology to produce fuels and chemicals. The award is named in honor of Dr. Charles D. Scott, who founded the Symposium on Biomaterials, Fuels, and Chemicals and chaired the conference for its first ten years.
Professor Lee has pioneered systems metabolic engineering and developed various micro-organisms capable of producing a wide range of fuels, chemicals, materials, and natural compounds, many of them for the first time. Some of the breakthroughs include the microbial production of gasoline, diacids, diamines, PLA and PLGA polymers, and several natural products.
More recently, his team has developed a microbial strain capable of the mass production of succinic acid, a monomer for manufacturing polyester, with the highest production efficiency to date, as well as a Corynebacterium glutamicum strain capable of producing high-level glutaric acid. They also engineered for the first time a bacterium capable of producing carminic acid, a natural red colorant that is widely used for food and cosmetics.
Professor Lee is one of the Highly Cited Researchers (HCR), ranked in the top 1% by citations in their field by Clarivate Analytics for four consecutive years from 2017. He is the first Korean fellow ever elected into the National Academy of Inventors in the US and one of 13 scholars elected as an International Member of both the National Academy of Sciences and the National Academy of Engineering in the USA.
The awards ceremony will take place during the Symposium on Biomaterials, Fuels, and Chemicals held online from April 26.
2021.04.27 View 10096
Distinguished Professor Sang Yup Lee Honored with Charles D. Scott Award
Vice President for Research Sang Yup Lee received the 2021 Charles D. Scott Award from the Society for Industrial Microbiology and Biotechnology. Distinguished Professor Lee from the Department of Chemical and Biomolecular Engineering at KAIST is the first Asian awardee.
The Charles D. Scott Award, initiated in 1995, recognizes individuals who have made significant contributions to enable and further the use of biotechnology to produce fuels and chemicals. The award is named in honor of Dr. Charles D. Scott, who founded the Symposium on Biomaterials, Fuels, and Chemicals and chaired the conference for its first ten years.
Professor Lee has pioneered systems metabolic engineering and developed various micro-organisms capable of producing a wide range of fuels, chemicals, materials, and natural compounds, many of them for the first time. Some of the breakthroughs include the microbial production of gasoline, diacids, diamines, PLA and PLGA polymers, and several natural products.
More recently, his team has developed a microbial strain capable of the mass production of succinic acid, a monomer for manufacturing polyester, with the highest production efficiency to date, as well as a Corynebacterium glutamicum strain capable of producing high-level glutaric acid. They also engineered for the first time a bacterium capable of producing carminic acid, a natural red colorant that is widely used for food and cosmetics.
Professor Lee is one of the Highly Cited Researchers (HCR), ranked in the top 1% by citations in their field by Clarivate Analytics for four consecutive years from 2017. He is the first Korean fellow ever elected into the National Academy of Inventors in the US and one of 13 scholars elected as an International Member of both the National Academy of Sciences and the National Academy of Engineering in the USA.
The awards ceremony will take place during the Symposium on Biomaterials, Fuels, and Chemicals held online from April 26.
2021.04.27 View 10096 -
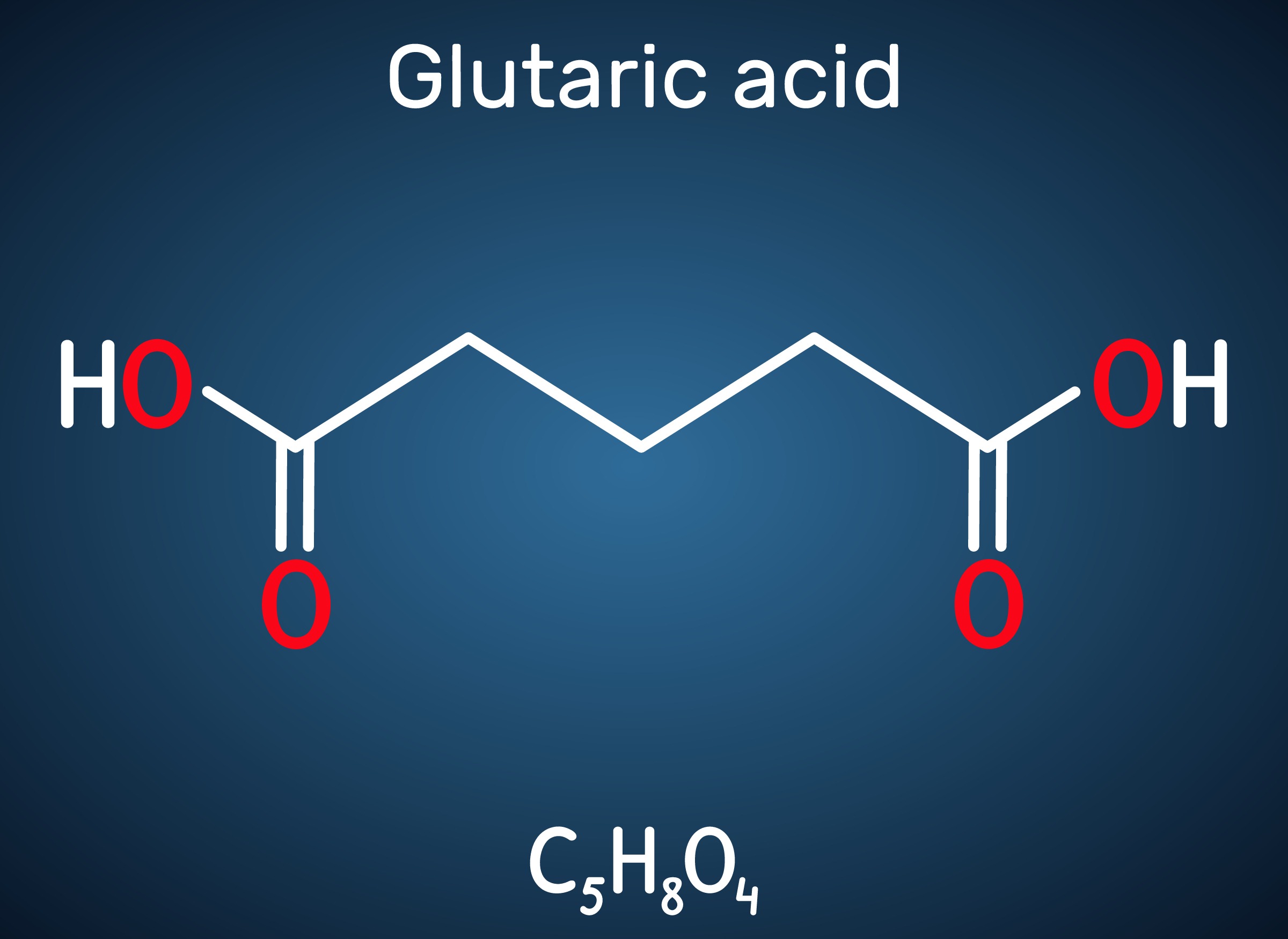 Engineered C. glutamicum Strain Capable of Producing High-Level Glutaric Acid from Glucose
An engineered C. glutamicum strain that can produce the world’s highest titer of glutaric acid was developed by employing systems metabolic engineering strategies
A metabolic engineering research group at KAIST has developed an engineered Corynebacterium glutamicum strain capable of producing high-level glutaric acid without byproducts from glucose. This new strategy will be useful for developing engineered micro-organisms for the bio-based production of value-added chemicals.
Glutaric acid, also known as pentanedioic acid, is a carboxylic acid that is widely used for various applications including the production of polyesters, polyamides, polyurethanes, glutaric anhydride, 1,5-pentanediol, and 5-hydroxyvaleric acid. Glutaric acid has been produced using various petroleum-based chemical methods, relying on non-renewable and toxic starting materials. Thus, various approaches have been taken to biologically produce glutaric acid from renewable resources. Previously, the development of the first glutaric acid producing Escherichia coli by introducing Pseudomonas putida genes was reported by a research group from KAIST, but the titer was low. Glutaric acid production by metabolically engineered Corynebacterium glutamicum has also been reported in several studies, but further improvements in glutaric acid production seemed possible since C. glutamicum has the capability of producing more than 130 g/L of L-lysine.
A research group comprised of Taehee Han, Gi Bae Kim, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering addressed this issue. Their research paper “Glutaric acid production by systems metabolic engineering of an L-lysine-overproducing Corynebacterium glutamicum” was published online in PNAS on November 16, 2020.
This research reports the development of a metabolically engineered C. glutamicum strain capable of efficiently producing glutaric acid, starting from an L-lysine overproducer. The following novel strategies and approaches to achieve high-level glutaric acid production were employed. First, metabolic pathways in C. glutamicum were reconstituted for glutaric acid production by introducing P. putida genes. Then, multi-omics analyses including genome, transcriptome, and fluxome were conducted to understand the phenotype of the L-lysine overproducer strain. In addition to systematic understanding of the host strain, gene manipulation targets were predicted by omics analyses and applied for engineering C. glutamicum, which resulted in the development of an engineered strain capable of efficiently producing glutaric acid. Furthermore, the new glutaric acid exporter was discovered for the first time, which was used to further increase glutaric acid production through enhancing product excretion. Last but not least, culture conditions were optimized for high-level glutaric acid production. As a result, the final engineered strain was able to produce 105.3 g/L glutaric acid, the highest titer ever reported, in 69 hours by fed-batch fermentation.
Professor Sang Yup Lee said, “It is meaningful that we were able to develop a highly efficient glutaric acid producer capable of producing glutaric acid at the world’s highest titer without any byproducts from renewable carbon sources. This will further accelerate the bio-based production of valuable chemicals in pharmaceutical/medical/chemical industries.” This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation and funded by the Ministry of Science and ICT.
-Profile
Distinguished Professor Sang Yup Lee
leesy@kaist.ac.kr
http://mbel.kaist.ac.kr
Department of Chemical and Biomolecular Engineering
KAIST
2020.11.17 View 7707
Engineered C. glutamicum Strain Capable of Producing High-Level Glutaric Acid from Glucose
An engineered C. glutamicum strain that can produce the world’s highest titer of glutaric acid was developed by employing systems metabolic engineering strategies
A metabolic engineering research group at KAIST has developed an engineered Corynebacterium glutamicum strain capable of producing high-level glutaric acid without byproducts from glucose. This new strategy will be useful for developing engineered micro-organisms for the bio-based production of value-added chemicals.
Glutaric acid, also known as pentanedioic acid, is a carboxylic acid that is widely used for various applications including the production of polyesters, polyamides, polyurethanes, glutaric anhydride, 1,5-pentanediol, and 5-hydroxyvaleric acid. Glutaric acid has been produced using various petroleum-based chemical methods, relying on non-renewable and toxic starting materials. Thus, various approaches have been taken to biologically produce glutaric acid from renewable resources. Previously, the development of the first glutaric acid producing Escherichia coli by introducing Pseudomonas putida genes was reported by a research group from KAIST, but the titer was low. Glutaric acid production by metabolically engineered Corynebacterium glutamicum has also been reported in several studies, but further improvements in glutaric acid production seemed possible since C. glutamicum has the capability of producing more than 130 g/L of L-lysine.
A research group comprised of Taehee Han, Gi Bae Kim, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering addressed this issue. Their research paper “Glutaric acid production by systems metabolic engineering of an L-lysine-overproducing Corynebacterium glutamicum” was published online in PNAS on November 16, 2020.
This research reports the development of a metabolically engineered C. glutamicum strain capable of efficiently producing glutaric acid, starting from an L-lysine overproducer. The following novel strategies and approaches to achieve high-level glutaric acid production were employed. First, metabolic pathways in C. glutamicum were reconstituted for glutaric acid production by introducing P. putida genes. Then, multi-omics analyses including genome, transcriptome, and fluxome were conducted to understand the phenotype of the L-lysine overproducer strain. In addition to systematic understanding of the host strain, gene manipulation targets were predicted by omics analyses and applied for engineering C. glutamicum, which resulted in the development of an engineered strain capable of efficiently producing glutaric acid. Furthermore, the new glutaric acid exporter was discovered for the first time, which was used to further increase glutaric acid production through enhancing product excretion. Last but not least, culture conditions were optimized for high-level glutaric acid production. As a result, the final engineered strain was able to produce 105.3 g/L glutaric acid, the highest titer ever reported, in 69 hours by fed-batch fermentation.
Professor Sang Yup Lee said, “It is meaningful that we were able to develop a highly efficient glutaric acid producer capable of producing glutaric acid at the world’s highest titer without any byproducts from renewable carbon sources. This will further accelerate the bio-based production of valuable chemicals in pharmaceutical/medical/chemical industries.” This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation and funded by the Ministry of Science and ICT.
-Profile
Distinguished Professor Sang Yup Lee
leesy@kaist.ac.kr
http://mbel.kaist.ac.kr
Department of Chemical and Biomolecular Engineering
KAIST
2020.11.17 View 7707 -
 E. coli Engineered to Grow on CO₂ and Formic Acid as Sole Carbon Sources
- An E. coli strain that can grow to a relatively high cell density solely on CO₂ and formic acid was developed by employing metabolic engineering. -
Most biorefinery processes have relied on the use of biomass as a raw material for the production of chemicals and materials. Even though the use of CO₂ as a carbon source in biorefineries is desirable, it has not been possible to make common microbial strains such as E. coli grow on CO₂.
Now, a metabolic engineering research group at KAIST has developed a strategy to grow an E. coli strain to higher cell density solely on CO₂ and formic acid. Formic acid is a one carbon carboxylic acid, and can be easily produced from CO₂ using a variety of methods. Since it is easier to store and transport than CO₂, formic acid can be considered a good liquid-form alternative of CO₂.
With support from the C1 Gas Refinery R&D Center and the Ministry of Science and ICT, a research team led by Distinguished Professor Sang Yup Lee stepped up their work to develop an engineered E. coli strain capable of growing up to 11-fold higher cell density than those previously reported, using CO₂ and formic acid as sole carbon sources. This work was published in Nature Microbiology on September 28.
Despite the recent reports by several research groups on the development of E. coli strains capable of growing on CO₂ and formic acid, the maximum cell growth remained too low (optical density of around 1) and thus the production of chemicals from CO₂ and formic acid has been far from realized.
The team previously reported the reconstruction of the tetrahydrofolate cycle and reverse glycine cleavage pathway to construct an engineered E. coli strain that can sustain growth on CO₂ and formic acid. To further enhance the growth, the research team introduced the previously designed synthetic CO₂ and formic acid assimilation pathway, and two formate dehydrogenases.
Metabolic fluxes were also fine-tuned, the gluconeogenic flux enhanced, and the levels of cytochrome bo3 and bd-I ubiquinol oxidase for ATP generation were optimized. This engineered E. coli strain was able to grow to a relatively high OD600 of 7~11, showing promise as a platform strain growing solely on CO₂ and formic acid.
Professor Lee said, “We engineered E. coli that can grow to a higher cell density only using CO₂ and formic acid. We think that this is an important step forward, but this is not the end. The engineered strain we developed still needs further engineering so that it can grow faster to a much higher density.”
Professor Lee’s team is continuing to develop such a strain. “In the future, we would be delighted to see the production of chemicals from an engineered E. coli strain using CO₂ and formic acid as sole carbon sources,” he added.
-Profile:Distinguished Professor Sang Yup Leehttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.09.29 View 11844
E. coli Engineered to Grow on CO₂ and Formic Acid as Sole Carbon Sources
- An E. coli strain that can grow to a relatively high cell density solely on CO₂ and formic acid was developed by employing metabolic engineering. -
Most biorefinery processes have relied on the use of biomass as a raw material for the production of chemicals and materials. Even though the use of CO₂ as a carbon source in biorefineries is desirable, it has not been possible to make common microbial strains such as E. coli grow on CO₂.
Now, a metabolic engineering research group at KAIST has developed a strategy to grow an E. coli strain to higher cell density solely on CO₂ and formic acid. Formic acid is a one carbon carboxylic acid, and can be easily produced from CO₂ using a variety of methods. Since it is easier to store and transport than CO₂, formic acid can be considered a good liquid-form alternative of CO₂.
With support from the C1 Gas Refinery R&D Center and the Ministry of Science and ICT, a research team led by Distinguished Professor Sang Yup Lee stepped up their work to develop an engineered E. coli strain capable of growing up to 11-fold higher cell density than those previously reported, using CO₂ and formic acid as sole carbon sources. This work was published in Nature Microbiology on September 28.
Despite the recent reports by several research groups on the development of E. coli strains capable of growing on CO₂ and formic acid, the maximum cell growth remained too low (optical density of around 1) and thus the production of chemicals from CO₂ and formic acid has been far from realized.
The team previously reported the reconstruction of the tetrahydrofolate cycle and reverse glycine cleavage pathway to construct an engineered E. coli strain that can sustain growth on CO₂ and formic acid. To further enhance the growth, the research team introduced the previously designed synthetic CO₂ and formic acid assimilation pathway, and two formate dehydrogenases.
Metabolic fluxes were also fine-tuned, the gluconeogenic flux enhanced, and the levels of cytochrome bo3 and bd-I ubiquinol oxidase for ATP generation were optimized. This engineered E. coli strain was able to grow to a relatively high OD600 of 7~11, showing promise as a platform strain growing solely on CO₂ and formic acid.
Professor Lee said, “We engineered E. coli that can grow to a higher cell density only using CO₂ and formic acid. We think that this is an important step forward, but this is not the end. The engineered strain we developed still needs further engineering so that it can grow faster to a much higher density.”
Professor Lee’s team is continuing to develop such a strain. “In the future, we would be delighted to see the production of chemicals from an engineered E. coli strain using CO₂ and formic acid as sole carbon sources,” he added.
-Profile:Distinguished Professor Sang Yup Leehttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.09.29 View 11844