Biomolecular+Engineering+Laboratory
-
 KAIST research team develops clathrin assembly for targeted protein delivery to cancer cells
In order to effectively treat cancer without additional side effects, we need a way to deliver drugs specifically to tumor cells. Protein assemblies have been widely used for drug delivery in the field of cancer treatment, but to use them for drug delivery they must first be functionalized, meaning they must be bound to the protein that recognizes the target tumor cell and deliver a drug that kills it. However, the functionalization process of protein assemblies is very complex, inefficient, and limited to small-sized chemical drugs, which limits their real-life applicability.
On March 14, a KAIST research team led by Professor Hak-Sung Kim from the KAIST Department of Biological Sciences reported the development of a clathrin assembly that can specifically deliver drugs to cancer cells.
Clathrin assemblies transport materials efficiently through endocytosis in living organisms. They are formed by the self-assembly of triskelion units, which are composed of three heavy chains bonded with three light chains. Inspired by this mechanism, the research team designed a clathrin chain to facilitate the functionalization of tumor cell recognition proteins and toxin proteins in order to deliver drugs specifically to tumor cells. From this, the team created a new type of clathrin assembly.
Figure 1. (Upper) Schematic diagram of the development of a new clathrin assembly that simultaneously functionalizes two types of proteins (cancer cell recognition protein and toxin protein) on heavy and light chains of clathrin in a one-pot reaction (bottom, left) Electron microscopy image of clathrin assembly: formation of an assembly with a diameter of about 28 nanometers (bottom, right) Cancer cell killing effect of CLA: CLA functionalized with epidermal growth factor receptor (EGFR) recognition protein and toxin protein kills only the cancer cells that overexpress EGFR.
The newly developed clathrin assembly requires a one-pot reaction, meaning both the toxin and tumor-recognition proteins can be functionalized simultaneously and show high efficiency. As a result, this technique is expected to be used in a wide variety of applications in the fields of biology and medicine including drug delivery, vaccine development, and diagnosing illnesses.
In this research, an epidermal growth factor receptor (EGFR), a common tumor marker, was used as the recognition protein, allowing drug delivery only to tumor cells. The clathrin assemblies that were functionalized to recognize EGFR showed a bonding strength 900-times stronger than it normally would due to the avidity effect. Based on this finding, the research team confirmed that treatment with toxin-functionalized clathrin assembly led to effective cell death for tumor cells, while it showed no such effect on healthy cells.
This research by Dr. Hong-Sik Kim and his colleagues was published in Small volume 19, issue 8 on February 22 under the title, "Construction and Functionalization of a Clathrin Assembly for a Targeted Protein Delivery", and it was selected as the cover paper.
Figure 2. Cover Paper: This study was published in the international journal 'Small' on February 22nd, Volume 19, No. 8, and was selected as the cover paper.
First author Dr. Hong-Sik Kim said, “Clathrin is difficult to functionalize, and since it is extracted from mammals, realistic applications have been limited.” He added, “But the new clathrin assembly we designed for this research can be functionalized with two different types of proteins through a single-step reaction, and can be produced from E. coli, meaning it can become an applicable protein assembly technology for a wide range of biomedical fields.”
This research was funded by the Global Ph.D. Fellowship and the Mid-career Researcher Grant of the National Research Foundation.
2023.03.22 View 8514
KAIST research team develops clathrin assembly for targeted protein delivery to cancer cells
In order to effectively treat cancer without additional side effects, we need a way to deliver drugs specifically to tumor cells. Protein assemblies have been widely used for drug delivery in the field of cancer treatment, but to use them for drug delivery they must first be functionalized, meaning they must be bound to the protein that recognizes the target tumor cell and deliver a drug that kills it. However, the functionalization process of protein assemblies is very complex, inefficient, and limited to small-sized chemical drugs, which limits their real-life applicability.
On March 14, a KAIST research team led by Professor Hak-Sung Kim from the KAIST Department of Biological Sciences reported the development of a clathrin assembly that can specifically deliver drugs to cancer cells.
Clathrin assemblies transport materials efficiently through endocytosis in living organisms. They are formed by the self-assembly of triskelion units, which are composed of three heavy chains bonded with three light chains. Inspired by this mechanism, the research team designed a clathrin chain to facilitate the functionalization of tumor cell recognition proteins and toxin proteins in order to deliver drugs specifically to tumor cells. From this, the team created a new type of clathrin assembly.
Figure 1. (Upper) Schematic diagram of the development of a new clathrin assembly that simultaneously functionalizes two types of proteins (cancer cell recognition protein and toxin protein) on heavy and light chains of clathrin in a one-pot reaction (bottom, left) Electron microscopy image of clathrin assembly: formation of an assembly with a diameter of about 28 nanometers (bottom, right) Cancer cell killing effect of CLA: CLA functionalized with epidermal growth factor receptor (EGFR) recognition protein and toxin protein kills only the cancer cells that overexpress EGFR.
The newly developed clathrin assembly requires a one-pot reaction, meaning both the toxin and tumor-recognition proteins can be functionalized simultaneously and show high efficiency. As a result, this technique is expected to be used in a wide variety of applications in the fields of biology and medicine including drug delivery, vaccine development, and diagnosing illnesses.
In this research, an epidermal growth factor receptor (EGFR), a common tumor marker, was used as the recognition protein, allowing drug delivery only to tumor cells. The clathrin assemblies that were functionalized to recognize EGFR showed a bonding strength 900-times stronger than it normally would due to the avidity effect. Based on this finding, the research team confirmed that treatment with toxin-functionalized clathrin assembly led to effective cell death for tumor cells, while it showed no such effect on healthy cells.
This research by Dr. Hong-Sik Kim and his colleagues was published in Small volume 19, issue 8 on February 22 under the title, "Construction and Functionalization of a Clathrin Assembly for a Targeted Protein Delivery", and it was selected as the cover paper.
Figure 2. Cover Paper: This study was published in the international journal 'Small' on February 22nd, Volume 19, No. 8, and was selected as the cover paper.
First author Dr. Hong-Sik Kim said, “Clathrin is difficult to functionalize, and since it is extracted from mammals, realistic applications have been limited.” He added, “But the new clathrin assembly we designed for this research can be functionalized with two different types of proteins through a single-step reaction, and can be produced from E. coli, meaning it can become an applicable protein assembly technology for a wide range of biomedical fields.”
This research was funded by the Global Ph.D. Fellowship and the Mid-career Researcher Grant of the National Research Foundation.
2023.03.22 View 8514 -
 Nanoparticle Cluster Manufacturing Technique Using DNA Binding Protein Developed
Professor Hak-Sung Kim of the Department of Biological Sciences at KAIST and Yiseul Ryu, a doctoral candidate, used the Zinc Finger protein that specifically binds to target DNA sequence to develop a new manufacturing technique for size-controllable magnetic Nanoparticle Clusters (NPCs). Their research results were published in Angewandte Chemie International Edition online on 25 November 2014.
NPCs are structures consisting of magnetic nanoparticles, gold nanoparticles, and quantum dots, each of which are smaller than 100 nm (10-9m). NPCs have a distinctive property of collectivity not seen in single nanoparticles.
Specifically NPCS differ in physical and optical properties such as Plasmon coupling absorbance, energy transfers between particles, electron transfers, and conductivity. Therefore, NPCs can be employed in biological and medical research as well as the development of nanoelectric and nanoplasmon devices.
To make use of these novel properties, the size and the composition of the cluster must be exquisitely controlled. However, previous techniques relied on chemical binding which required complex steps, making it difficult to control the size and composition of NPCs.
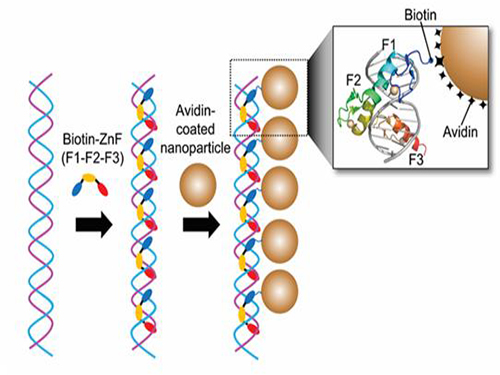
Professor Kim’s team used Zinc Finger, a DNA binding protein, to develop a NPCs manufacturing technique to create clusters of the desired size easily. The Zinc Finger protein contains a zinc ion and specifically recognizes DNA sequence upon binding, which allows the exquisite control of the size and the cluster composition. The technique is also bio-friendly.
Professor Kim’s team created linear structure of different sizes of NPCs using Zinc Finger proteins and three DNA sequences of different lengths. The NPCs they produced confirmed their ability to control the size and structure of the cluster by using different DNA lengths.
The NPCs showed tripled T2 relaxation rates compared to the existing MRI contrast media (Feridex) and effectively transported to targeted cells. The research findings show the potential use of NPCs in biological and medical fields such as MRI contrast media, fluorescence imaging, and drug transport.
The research used the specific binding property of protein and DNA to develop a new method to create an inorganic nanoparticle’s supramolecular assembly. The technique can be used and applied extensively in other nanoparticles for future research in diagnosis, imaging, and drug and gene delivery.
Figure 1. A Mimetic Diagram of NPCs Manufacturing Technique Using DNA Binding Protein Zinc Finger
Figure 2. Transmission Electron Microscopy Images showing different sizes of NPCs depending on the length of the DNA
2014.12.04 View 15415
Nanoparticle Cluster Manufacturing Technique Using DNA Binding Protein Developed
Professor Hak-Sung Kim of the Department of Biological Sciences at KAIST and Yiseul Ryu, a doctoral candidate, used the Zinc Finger protein that specifically binds to target DNA sequence to develop a new manufacturing technique for size-controllable magnetic Nanoparticle Clusters (NPCs). Their research results were published in Angewandte Chemie International Edition online on 25 November 2014.
NPCs are structures consisting of magnetic nanoparticles, gold nanoparticles, and quantum dots, each of which are smaller than 100 nm (10-9m). NPCs have a distinctive property of collectivity not seen in single nanoparticles.
Specifically NPCS differ in physical and optical properties such as Plasmon coupling absorbance, energy transfers between particles, electron transfers, and conductivity. Therefore, NPCs can be employed in biological and medical research as well as the development of nanoelectric and nanoplasmon devices.
To make use of these novel properties, the size and the composition of the cluster must be exquisitely controlled. However, previous techniques relied on chemical binding which required complex steps, making it difficult to control the size and composition of NPCs.
Professor Kim’s team used Zinc Finger, a DNA binding protein, to develop a NPCs manufacturing technique to create clusters of the desired size easily. The Zinc Finger protein contains a zinc ion and specifically recognizes DNA sequence upon binding, which allows the exquisite control of the size and the cluster composition. The technique is also bio-friendly.
Professor Kim’s team created linear structure of different sizes of NPCs using Zinc Finger proteins and three DNA sequences of different lengths. The NPCs they produced confirmed their ability to control the size and structure of the cluster by using different DNA lengths.
The NPCs showed tripled T2 relaxation rates compared to the existing MRI contrast media (Feridex) and effectively transported to targeted cells. The research findings show the potential use of NPCs in biological and medical fields such as MRI contrast media, fluorescence imaging, and drug transport.
The research used the specific binding property of protein and DNA to develop a new method to create an inorganic nanoparticle’s supramolecular assembly. The technique can be used and applied extensively in other nanoparticles for future research in diagnosis, imaging, and drug and gene delivery.
Figure 1. A Mimetic Diagram of NPCs Manufacturing Technique Using DNA Binding Protein Zinc Finger
Figure 2. Transmission Electron Microscopy Images showing different sizes of NPCs depending on the length of the DNA
2014.12.04 View 15415