energy+storage
-
 KAIST Develops Sodium Battery Capable of Rapid Charging in Just a Few Seconds
Sodium (Na), which is over 500 times more abundant than lithium (Li), has recently garnered significant attention for its potential in sodium-ion battery technologies. However, existing sodium-ion batteries face fundamental limitations, including lower power output, constrained storage properties, and longer charging times, necessitating the development of next-generation energy storage materials.
On the 11th of April, KAIST (represented by President Kwang Hyung Lee) announced that a research team led by Professor Jeung Ku Kang from the Department of Materials Science and Engineering had developed a high-energy, high-power hybrid sodium-ion battery capable of rapid charging.
The innovative hybrid energy storage system integrates anode materials typically used in batteries with cathodes suitable for supercapacitors. This combination allows the device to achieve both high storage capacities and rapid charge-discharge rates, positioning it as a viable next-generation alternative to lithium-ion batteries.
However, the development of a hybrid battery with high energy and high power density requires an improvement to the slow energy storage rate of battery-type anodes as well as the enhancement of the relatively low capacity of supercapacitor-type cathode materials.
< Figure 1. Schematic synthetic procedures of high-capacity/high-rate anode and cathode materials for a sodium-ion hybrid energy storages (SIHES) and their proposed energy storage mechanisms. Synthetic procedures for (a) ultrafine iron sulfide-embedded S-doped carbon/graphene (FS/C/G) anode and (b) zeolitic imidazolate framework-derived porous carbon (ZDPC) cathode materials. (c) Proposed energy storage mechanisms of Na+ ions in FS/C/G anode and ClO-4 ions in ZDPC cathode for an SIHES. >
To account for this, Professor Kang's team utilized two distinct metal-organic frameworks for the optimized synthesis of hybrid batteries. This approach led to the development of an anode material with improved kinetics through the inclusion of fine active materials in porous carbon derived from metal-organic frameworks. Additionally, a high-capacity cathode material was synthesized, and the combination of the cathode and anode materials allowed for the development of a sodium-ion storage system optimizing the balance and minimizing the disparities in energy storage rates between the electrodes.
The assembled full cell, comprising the newly developed anode and cathode, forms a high-performance hybrid sodium-ion energy storage device. This device surpasses the energy density of commercial lithium-ion batteries and exhibits the characteristics of supercapacitors' power density. It is expected to be suitable for rapid charging applications ranging from electric vehicles to smart electronic devices and aerospace technologies.
< Figure 2. Electrochemical characterizations of FS/C/G-20//ZDPC SIHES full cells (left). Ragone plots for FS/C/G-20//ZDPC (this work) and other previously reported sodium-ion electrochemical energy storage devices (right). >
Professor Kang noted that the hybrid sodium-ion energy storage device, capable of rapid charging and achieving an energy density of 247 Wh/kg and a power density of 34,748 W/kg, represents a breakthrough in overcoming the current limitations of energy storage systems. He anticipates broader applications across various electronic devices, including electric vehicles.
This research, co-authored by KAIST doctoral candidates Jong Hui Choi and Dong Won Kim, was published in the international journal Energy Storage Materials on March 29 with the title "Low-crystallinity conductive multivalence iron sulfide-embedded S-doped anode and high-surface-area O-doped cathode of 3D porous N-rich graphitic carbon frameworks for high-performance sodium-ion hybrid energy storages."
The study was conducted with support from the Ministry of Science and ICT and the National Research Foundation of Korea through the Nanomaterial Technology Development Project.
2024.04.18 View 18487
KAIST Develops Sodium Battery Capable of Rapid Charging in Just a Few Seconds
Sodium (Na), which is over 500 times more abundant than lithium (Li), has recently garnered significant attention for its potential in sodium-ion battery technologies. However, existing sodium-ion batteries face fundamental limitations, including lower power output, constrained storage properties, and longer charging times, necessitating the development of next-generation energy storage materials.
On the 11th of April, KAIST (represented by President Kwang Hyung Lee) announced that a research team led by Professor Jeung Ku Kang from the Department of Materials Science and Engineering had developed a high-energy, high-power hybrid sodium-ion battery capable of rapid charging.
The innovative hybrid energy storage system integrates anode materials typically used in batteries with cathodes suitable for supercapacitors. This combination allows the device to achieve both high storage capacities and rapid charge-discharge rates, positioning it as a viable next-generation alternative to lithium-ion batteries.
However, the development of a hybrid battery with high energy and high power density requires an improvement to the slow energy storage rate of battery-type anodes as well as the enhancement of the relatively low capacity of supercapacitor-type cathode materials.
< Figure 1. Schematic synthetic procedures of high-capacity/high-rate anode and cathode materials for a sodium-ion hybrid energy storages (SIHES) and their proposed energy storage mechanisms. Synthetic procedures for (a) ultrafine iron sulfide-embedded S-doped carbon/graphene (FS/C/G) anode and (b) zeolitic imidazolate framework-derived porous carbon (ZDPC) cathode materials. (c) Proposed energy storage mechanisms of Na+ ions in FS/C/G anode and ClO-4 ions in ZDPC cathode for an SIHES. >
To account for this, Professor Kang's team utilized two distinct metal-organic frameworks for the optimized synthesis of hybrid batteries. This approach led to the development of an anode material with improved kinetics through the inclusion of fine active materials in porous carbon derived from metal-organic frameworks. Additionally, a high-capacity cathode material was synthesized, and the combination of the cathode and anode materials allowed for the development of a sodium-ion storage system optimizing the balance and minimizing the disparities in energy storage rates between the electrodes.
The assembled full cell, comprising the newly developed anode and cathode, forms a high-performance hybrid sodium-ion energy storage device. This device surpasses the energy density of commercial lithium-ion batteries and exhibits the characteristics of supercapacitors' power density. It is expected to be suitable for rapid charging applications ranging from electric vehicles to smart electronic devices and aerospace technologies.
< Figure 2. Electrochemical characterizations of FS/C/G-20//ZDPC SIHES full cells (left). Ragone plots for FS/C/G-20//ZDPC (this work) and other previously reported sodium-ion electrochemical energy storage devices (right). >
Professor Kang noted that the hybrid sodium-ion energy storage device, capable of rapid charging and achieving an energy density of 247 Wh/kg and a power density of 34,748 W/kg, represents a breakthrough in overcoming the current limitations of energy storage systems. He anticipates broader applications across various electronic devices, including electric vehicles.
This research, co-authored by KAIST doctoral candidates Jong Hui Choi and Dong Won Kim, was published in the international journal Energy Storage Materials on March 29 with the title "Low-crystallinity conductive multivalence iron sulfide-embedded S-doped anode and high-surface-area O-doped cathode of 3D porous N-rich graphitic carbon frameworks for high-performance sodium-ion hybrid energy storages."
The study was conducted with support from the Ministry of Science and ICT and the National Research Foundation of Korea through the Nanomaterial Technology Development Project.
2024.04.18 View 18487 -
 A KAIST Research Team Develops a Smart Color-Changing Flexible Battery with Ultra-high Efficiency
With the rapid growth of the smart and wearable electronic devices market, smart next-generation energy storage systems that have energy storage functions as well as additional color-changing properties are receiving a great deal of attention. However, existing electrochromic devices have low electrical conductivity, leading to low efficiency in electron and ion mobility, and low storage capacities. Such batteries have therefore been limited to use in flexible and wearable devices.
On August 21, a joint research team led by Professor Il-Doo Kim from the KAIST Department of Materials Science and Engineering (DMSE) and Professor Tae Gwang Yun from the Myongji University Department of Materials Science and Engineering announced the development of a smart electrochromic Zn-ion battery that can visually represent its charging and discharging processes using an electrochromic polymer anode incorporated with a “π-bridge spacer”, which increases electron and ion mobility efficiency.
Batteries topped with electrochromic properties are groundbreaking inventions that can visually represent their charged and discharged states using colors, and can be used as display devices that cut down energy consumption for indoor cooling by controlling solar absorbance. The research team successfully built a flexible and electrochromic smart Zn-ion battery that can maintain its excellent electrochromic and electrochemical properties, even under long-term exposure to the atmosphere and mechanical deformations.
< Figure 1. Electrochromic zinc ion battery whose anode is made of a polymer that turns dark blue when charged and transparent when discharged. >
To maximize the efficiency of electron and ion mobility, the team modelled and synthesized the first π-bridge spacer-incorporated polymer anode in the world. π-bonds can improve the mobility of electrons within a structure to speed up ion movement and maximize ion adsorption efficiency, which improves its energy storage capacity.
In anode-based batteries with a π-bridge spacer, the spacer provides room for quicker ion movement. This allows fast charging, an improved zinc-ion discharging capacity of 110 mAh/g, which is 40% greater than previously reported, and a 30% increase in electrochromic function that switches from dark blue to transparent when the device is charged/discharged. In addition, should the transparent flexible battery technology be applied to smart windows, they would display darker colors during the day while they absorb solar energy, and function as a futuristic energy storage technique that can block out UV radiation and replace curtains.
< Figure 2. A schematic diagram of the structure of the electrochromic polymer with π-π spacer and the operation of a smart flexible battery using this cathode material. >
< Figure 3. (A) Density Functional Theory (DFT) theory-based atomic and electronic structure analysis. (B) Comparison of rate characteristics for polymers with and without π-bridge spacers. (C) Electrochemical performance comparison graph with previously reported zinc ion batteries. The anode material, which has an electron donor-acceptor structure with a built-in π-bridge spacer, shows better electrochemical performance and electrochromic properties than existing zinc ion batteries and electrochromic devices. >
Professor Il-Doo Kim said, “We have developed a polymer incorporated with a π-bridge spacer and successfully built a smart Zn-ion battery with excellent electrochromic efficiency and high energy storage capacity.” He added, “This technique goes beyond the existing concept of batteries that are used simply as energy storage devices, and we expect this technology to be used as a futuristic energy storage system that accelerates innovation in smart batteries and wearable technologies.”
This research, co-first authored by the alums of KAIST Departments of Material Sciences of Engineering, Professor Tae Gwang Yun of Myongji University, Dr. Jiyoung Lee, a post-doctoral associate at Northwestern University, and Professor Han Seul Kim at Chungbuk National University, was published as an inside cover article for Advanced Materials on August 3 under the title, “A π-Bridge Spacer Embedded Electron Donor-Acceptor Polymer for Flexible Electrochromic Zn-Ion Batteries”.
< Figure 4. Advanced Materials Inside Cover (August Issue) >
This research was supported by the Nanomaterial Technology Development Project under the Korean Ministry of Science and ICT, the Nano and Material Technology Development Project under the National Research Foundation of Korea, the Successive Academic Generation Development Project under the Korean Ministry of Education, and the Alchemist Project under the Korean Ministry of Trade, Industry & Energy.
2023.09.01 View 8882
A KAIST Research Team Develops a Smart Color-Changing Flexible Battery with Ultra-high Efficiency
With the rapid growth of the smart and wearable electronic devices market, smart next-generation energy storage systems that have energy storage functions as well as additional color-changing properties are receiving a great deal of attention. However, existing electrochromic devices have low electrical conductivity, leading to low efficiency in electron and ion mobility, and low storage capacities. Such batteries have therefore been limited to use in flexible and wearable devices.
On August 21, a joint research team led by Professor Il-Doo Kim from the KAIST Department of Materials Science and Engineering (DMSE) and Professor Tae Gwang Yun from the Myongji University Department of Materials Science and Engineering announced the development of a smart electrochromic Zn-ion battery that can visually represent its charging and discharging processes using an electrochromic polymer anode incorporated with a “π-bridge spacer”, which increases electron and ion mobility efficiency.
Batteries topped with electrochromic properties are groundbreaking inventions that can visually represent their charged and discharged states using colors, and can be used as display devices that cut down energy consumption for indoor cooling by controlling solar absorbance. The research team successfully built a flexible and electrochromic smart Zn-ion battery that can maintain its excellent electrochromic and electrochemical properties, even under long-term exposure to the atmosphere and mechanical deformations.
< Figure 1. Electrochromic zinc ion battery whose anode is made of a polymer that turns dark blue when charged and transparent when discharged. >
To maximize the efficiency of electron and ion mobility, the team modelled and synthesized the first π-bridge spacer-incorporated polymer anode in the world. π-bonds can improve the mobility of electrons within a structure to speed up ion movement and maximize ion adsorption efficiency, which improves its energy storage capacity.
In anode-based batteries with a π-bridge spacer, the spacer provides room for quicker ion movement. This allows fast charging, an improved zinc-ion discharging capacity of 110 mAh/g, which is 40% greater than previously reported, and a 30% increase in electrochromic function that switches from dark blue to transparent when the device is charged/discharged. In addition, should the transparent flexible battery technology be applied to smart windows, they would display darker colors during the day while they absorb solar energy, and function as a futuristic energy storage technique that can block out UV radiation and replace curtains.
< Figure 2. A schematic diagram of the structure of the electrochromic polymer with π-π spacer and the operation of a smart flexible battery using this cathode material. >
< Figure 3. (A) Density Functional Theory (DFT) theory-based atomic and electronic structure analysis. (B) Comparison of rate characteristics for polymers with and without π-bridge spacers. (C) Electrochemical performance comparison graph with previously reported zinc ion batteries. The anode material, which has an electron donor-acceptor structure with a built-in π-bridge spacer, shows better electrochemical performance and electrochromic properties than existing zinc ion batteries and electrochromic devices. >
Professor Il-Doo Kim said, “We have developed a polymer incorporated with a π-bridge spacer and successfully built a smart Zn-ion battery with excellent electrochromic efficiency and high energy storage capacity.” He added, “This technique goes beyond the existing concept of batteries that are used simply as energy storage devices, and we expect this technology to be used as a futuristic energy storage system that accelerates innovation in smart batteries and wearable technologies.”
This research, co-first authored by the alums of KAIST Departments of Material Sciences of Engineering, Professor Tae Gwang Yun of Myongji University, Dr. Jiyoung Lee, a post-doctoral associate at Northwestern University, and Professor Han Seul Kim at Chungbuk National University, was published as an inside cover article for Advanced Materials on August 3 under the title, “A π-Bridge Spacer Embedded Electron Donor-Acceptor Polymer for Flexible Electrochromic Zn-Ion Batteries”.
< Figure 4. Advanced Materials Inside Cover (August Issue) >
This research was supported by the Nanomaterial Technology Development Project under the Korean Ministry of Science and ICT, the Nano and Material Technology Development Project under the National Research Foundation of Korea, the Successive Academic Generation Development Project under the Korean Ministry of Education, and the Alchemist Project under the Korean Ministry of Trade, Industry & Energy.
2023.09.01 View 8882 -
 Researchers Report Longest-lived Aqueous Flow Batteries
New technology to overcome the life limit of next-generation water-cell batteries
A research team led by Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering has developed water-based zinc/bromine redox flow batteries (ZBBs) with the best life expectancy among all the redox flow batteries reported by identifying and solving the deterioration issue with zinc electrodes.
Professor Kim, head of the Advanced Battery Center at KAIST's Nano-fusion Research Institute, said, "We presented a new technology to overcome the life limit of next-generation water-cell batteries. Not only is it cheaper than conventional lithium-ion batteries, but it can contribute to the expansion of renewable energy and the safe supply of energy storage systems that can run with more than 80 percent energy efficiency."
ZBBs were found to have stable life spans of more than 5,000 cycles, even at a high current density of 100 mA/cm2. It was also confirmed that it represented the highest output and life expectancy compared to Redox flow batteries (RFBs) reported worldwide, which use other redox couples such as zinc-bromine, zinc-iodine, zinc-iron, and vanadium.
Recently, more attention has been focused on energy storage system (ESS) that can improve energy utilization efficiency by storing new and late-night power in large quantities and supplying it to the grid if necessary to supplement the intermittent nature of renewable energy and meet peak power demand.
However, lithium-ion batteries (LIBs), which are currently the core technology of ESSs, have been criticized for not being suitable for ESSs, which store large amounts of electricity due to their inherent risk of ignition and fire. In fact, a total of 33 cases of ESSs using LIBs in Korea had fire accidents, and 35% of all ESS facilities were shut down. This is estimated to have resulted in more than 700 billion won in losses.
As a result, water-based RFBs have drawn great attention. In particular, ZBBs that use ultra-low-cost bromide (ZnBr2) as an active material have been developed for ESSs since the 1970s, with their advantages of high cell voltage, high energy density, and low price compared to other RFBs. Until now, however, the commercialization of ZBBs has been delayed due to the short life span caused by the zinc electrodes. In particular, the uneven "dendrite" growth behavior of zinc metals during the charging and discharging process leads to internal short circuits in the battery which shorten its life.
The research team noted that self-aggregation occurs through the surface diffusion of zinc nuclei on the carbon electrode surface with low surface energy, and determined that self-aggregation was the main cause of zinc dendrite formation through quantum mechanics-based computer simulations and transmission electron microscopy. Furthermore, it was found that the surface diffusion of the zinc nuclei was inhibited in certain carbon fault structures so that dendrites were not produced.
Single vacancy defect, where one carbon atom is removed, exchanges zinc nuclei and electrons, and is strongly coupled, thus inhibiting surface diffusion and enabling uniform nuclear production/growth. The research team applied carbon electrodes with high density fault structure to ZBBs, achieving life characteristics of more than 5,000 cycles at a high charge current density (100 mA/cm2), which is 30 times that of LIBs.
This ESS technology, which can supply eco-friendly electric energy such as renewable energy to the private sector through technology that can drive safe and cheap redox flow batteries for long life, is expected to draw attention once again.
Publication:
Ju-Hyuk Lee, Riyul Kim, Soohyun Kim, Jiyun Heo, Hyeokjin Kwon, Jung Hoon Yang, and Hee-Tak Kim. 2020. Dendrite-free Zn electrodeposition triggered by interatomic orbital hybridization of Zn and single vacancy carbon defects for aqueous Zn-based flow batteries. Energy and Environmental Science, 2020, 13, 2839-2848.
Link to download the full-text paper:http://xlink.rsc.org/?DOI=D0EE00723D
Profile: Prof. Hee-Tak Kimheetak.kim@kaist.ac.krhttp://eed.kaist.ac.krAssociate ProfessorDepartment of Chemical & Biomolecular EngineeringKAIST
2020.12.16 View 14785
Researchers Report Longest-lived Aqueous Flow Batteries
New technology to overcome the life limit of next-generation water-cell batteries
A research team led by Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering has developed water-based zinc/bromine redox flow batteries (ZBBs) with the best life expectancy among all the redox flow batteries reported by identifying and solving the deterioration issue with zinc electrodes.
Professor Kim, head of the Advanced Battery Center at KAIST's Nano-fusion Research Institute, said, "We presented a new technology to overcome the life limit of next-generation water-cell batteries. Not only is it cheaper than conventional lithium-ion batteries, but it can contribute to the expansion of renewable energy and the safe supply of energy storage systems that can run with more than 80 percent energy efficiency."
ZBBs were found to have stable life spans of more than 5,000 cycles, even at a high current density of 100 mA/cm2. It was also confirmed that it represented the highest output and life expectancy compared to Redox flow batteries (RFBs) reported worldwide, which use other redox couples such as zinc-bromine, zinc-iodine, zinc-iron, and vanadium.
Recently, more attention has been focused on energy storage system (ESS) that can improve energy utilization efficiency by storing new and late-night power in large quantities and supplying it to the grid if necessary to supplement the intermittent nature of renewable energy and meet peak power demand.
However, lithium-ion batteries (LIBs), which are currently the core technology of ESSs, have been criticized for not being suitable for ESSs, which store large amounts of electricity due to their inherent risk of ignition and fire. In fact, a total of 33 cases of ESSs using LIBs in Korea had fire accidents, and 35% of all ESS facilities were shut down. This is estimated to have resulted in more than 700 billion won in losses.
As a result, water-based RFBs have drawn great attention. In particular, ZBBs that use ultra-low-cost bromide (ZnBr2) as an active material have been developed for ESSs since the 1970s, with their advantages of high cell voltage, high energy density, and low price compared to other RFBs. Until now, however, the commercialization of ZBBs has been delayed due to the short life span caused by the zinc electrodes. In particular, the uneven "dendrite" growth behavior of zinc metals during the charging and discharging process leads to internal short circuits in the battery which shorten its life.
The research team noted that self-aggregation occurs through the surface diffusion of zinc nuclei on the carbon electrode surface with low surface energy, and determined that self-aggregation was the main cause of zinc dendrite formation through quantum mechanics-based computer simulations and transmission electron microscopy. Furthermore, it was found that the surface diffusion of the zinc nuclei was inhibited in certain carbon fault structures so that dendrites were not produced.
Single vacancy defect, where one carbon atom is removed, exchanges zinc nuclei and electrons, and is strongly coupled, thus inhibiting surface diffusion and enabling uniform nuclear production/growth. The research team applied carbon electrodes with high density fault structure to ZBBs, achieving life characteristics of more than 5,000 cycles at a high charge current density (100 mA/cm2), which is 30 times that of LIBs.
This ESS technology, which can supply eco-friendly electric energy such as renewable energy to the private sector through technology that can drive safe and cheap redox flow batteries for long life, is expected to draw attention once again.
Publication:
Ju-Hyuk Lee, Riyul Kim, Soohyun Kim, Jiyun Heo, Hyeokjin Kwon, Jung Hoon Yang, and Hee-Tak Kim. 2020. Dendrite-free Zn electrodeposition triggered by interatomic orbital hybridization of Zn and single vacancy carbon defects for aqueous Zn-based flow batteries. Energy and Environmental Science, 2020, 13, 2839-2848.
Link to download the full-text paper:http://xlink.rsc.org/?DOI=D0EE00723D
Profile: Prof. Hee-Tak Kimheetak.kim@kaist.ac.krhttp://eed.kaist.ac.krAssociate ProfessorDepartment of Chemical & Biomolecular EngineeringKAIST
2020.12.16 View 14785 -
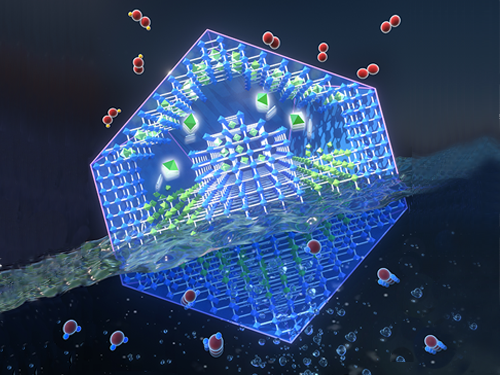 Energy Storage Using Oxygen to Boost Battery Performance
Researchers have presented a novel electrode material for advanced energy storage device that is directly charged with oxygen from the air. Professor Jeung Ku Kang’s team synthesized and preserved the sub-nanometric particles of atomic cluster sizes at high mass loadings within metal-organic frameworks (MOF) by controlling the behavior of reactants at the molecular level. This new strategy ensures high performance for lithium-oxygen batteries, acclaimed as a next-generation energy storage technology and widely used in electric vehicles.
Lithium-oxygen batteries in principle can generate ten times higher energy densities than conventional lithium-ion batteries, but they suffer from very poor cyclability. One of the methods to improve cycle stability is to reduce the overpotential of electrocatalysts in cathode electrodes. When the size of an electrocatalyst material is reduced to the atomic level, the increased surface energy leads to increased activity while significantly accelerating the material’s agglomeration.
As a solution to this challenge, Professor Kang from the Department of Materials Science and Engineering aimed to maintain the improved activity by stabilizing atomic-scale sized electrocatalysts into the sub-nanometric spaces. This is a novel strategy for simultaneously producing and stabilizing atomic-level electrocatalysts within metal-organic frameworks (MOFs).
Metal-organic frameworks continuously assemble metal ions and organic linkers.
The team controlled hydrogen affinities between water molecules to separate them and transfer the isolated water molecules one by one through the sub-nanometric pores of MOFs. The transferred water molecules reacted with cobalt ions to form di-nuclear cobalt hydroxide under precisely controlled synthetic conditions, then the atomic-level cobalt hydroxide is stabilized inside the sub-nanometric pores.
The di-nuclear cobalt hydroxide that is stabilized in the sub-nanometric pores of metal-organic frameworks (MOFs) reduced the overpotential by 63.9% and showed ten-fold improvements in the life cycle.
Professor Kang said, “Simultaneously generating and stabilizing atomic-level electrocatalysts within MOFs can diversify materials according to numerous combinations of metal and organic linkers. It can expand not only the development of electrocatalysts, but also various research fields such as photocatalysts, medicine, the environment, and petrochemicals.”
This study was reported in Advanced Science (Title: Autogenous Production and Stabilization of Highly Loaded Sub-Nanometric Particles within Multishell Hollow Metal-Organic Frameworks and Their Utilization for High Performance in Li-O2 Batteries).
This research was mainly supported by the Global Frontier R&D Program of the Ministry of Science, ICT & Planning (Grant No. 2013M3A6B1078884) funded by the Ministry of Science, ICT & Future Planning, and the National Research Foundation of Korea (Grant No. 2019M3E6A1104196).
Profile:Professor Jeung Ku Kang
jeungku@kaist.ac.kr
http://nanosf.kaist.ac.kr/
Nano Materials Simulation and Fabrication Laboratory
Department of Materials Science and Engineering
KAIST
2020.06.15 View 14209
Energy Storage Using Oxygen to Boost Battery Performance
Researchers have presented a novel electrode material for advanced energy storage device that is directly charged with oxygen from the air. Professor Jeung Ku Kang’s team synthesized and preserved the sub-nanometric particles of atomic cluster sizes at high mass loadings within metal-organic frameworks (MOF) by controlling the behavior of reactants at the molecular level. This new strategy ensures high performance for lithium-oxygen batteries, acclaimed as a next-generation energy storage technology and widely used in electric vehicles.
Lithium-oxygen batteries in principle can generate ten times higher energy densities than conventional lithium-ion batteries, but they suffer from very poor cyclability. One of the methods to improve cycle stability is to reduce the overpotential of electrocatalysts in cathode electrodes. When the size of an electrocatalyst material is reduced to the atomic level, the increased surface energy leads to increased activity while significantly accelerating the material’s agglomeration.
As a solution to this challenge, Professor Kang from the Department of Materials Science and Engineering aimed to maintain the improved activity by stabilizing atomic-scale sized electrocatalysts into the sub-nanometric spaces. This is a novel strategy for simultaneously producing and stabilizing atomic-level electrocatalysts within metal-organic frameworks (MOFs).
Metal-organic frameworks continuously assemble metal ions and organic linkers.
The team controlled hydrogen affinities between water molecules to separate them and transfer the isolated water molecules one by one through the sub-nanometric pores of MOFs. The transferred water molecules reacted with cobalt ions to form di-nuclear cobalt hydroxide under precisely controlled synthetic conditions, then the atomic-level cobalt hydroxide is stabilized inside the sub-nanometric pores.
The di-nuclear cobalt hydroxide that is stabilized in the sub-nanometric pores of metal-organic frameworks (MOFs) reduced the overpotential by 63.9% and showed ten-fold improvements in the life cycle.
Professor Kang said, “Simultaneously generating and stabilizing atomic-level electrocatalysts within MOFs can diversify materials according to numerous combinations of metal and organic linkers. It can expand not only the development of electrocatalysts, but also various research fields such as photocatalysts, medicine, the environment, and petrochemicals.”
This study was reported in Advanced Science (Title: Autogenous Production and Stabilization of Highly Loaded Sub-Nanometric Particles within Multishell Hollow Metal-Organic Frameworks and Their Utilization for High Performance in Li-O2 Batteries).
This research was mainly supported by the Global Frontier R&D Program of the Ministry of Science, ICT & Planning (Grant No. 2013M3A6B1078884) funded by the Ministry of Science, ICT & Future Planning, and the National Research Foundation of Korea (Grant No. 2019M3E6A1104196).
Profile:Professor Jeung Ku Kang
jeungku@kaist.ac.kr
http://nanosf.kaist.ac.kr/
Nano Materials Simulation and Fabrication Laboratory
Department of Materials Science and Engineering
KAIST
2020.06.15 View 14209 -
 Hydrogen-Natural Gas Hydrates Harvested by Natural Gas
A hydrogen-natural gas blend (HNGB) can be a game changer only if it can be stored safely and used as a sustainable clean energy resource. A recent study has suggested a new strategy for stably storing hydrogen, using natural gas as a stabilizer. The research proposed a practical gas phase modulator based synthesis of HNGB without generating chemical waste after dissociation for the immediate service.
The research team of Professor Jae Woo Lee from the Department of Chemical and Biomolecular Engineering in collaboration with the Gwangju Institute of Science and Technology (GIST) demonstrated that the natural gas modulator based synthesis leads to significantly reduced synthesis pressure simultaneously with the formation of hydrogen clusters in the confined nanoporous cages of clathrate hydrates. This approach minimizes the environmental impact and reduces operation costs since clathrate hydrates do not generate any chemical waste in both the synthesis and decomposition processes.
For the efficient storage and transportation of hydrogen, numerous materials have been investigated. Among others, clathrate hydrates offer distinct benefits. Clathrate hydrates are nanoporous inclusion compounds composed of a 3D network of polyhedral cages made of hydrogen-bonded ‘host’ water molecules and captured ‘guest’ gas or liquid molecules.
In this study, the research team used two gases, methane and ethane, which have lower equilibrium conditions compared to hydrogen as thermodynamic stabilizers. As a result, they succeeded in stably storing the hydrogen-natural gas compound in hydrates. According to the composition ratio of methane and ethane, structure I or II hydrates can be formed, both of which can stably store hydrogen-natural gas in low-pressure conditions.
The research team found that two hydrogen molecules are stored in small cages in tuned structure I hydrates, while up to three hydrogen molecules can be stored in both small and large cages in tuned structure II hydrates. Hydrates can store gas up to about 170-times its volume and the natural gas used as thermodynamic stabilizers in this study can also be used as an energy source.
The research team developed technology to produce hydrates from ice, produced hydrogen-natural gas hydrates by substitution, and successfully observed that the tuning phenomenon only occurs when hydrogen is involved in hydrate formation from the start for both structures of hydrates.
They expect that the findings can be applied to not only an energy-efficient gas storage material, but also a smart platform to utilize hydrogen natural gas blends, which can serve as a new alternative energy source with targeted hydrogen contents by designing synthetic pathways of mixed gas hydrates.
The research was published online in Energy Storage Materials on June 6, with the title ‘One-step formation of hydrogen clusters in clathrate hydrates stabilized via natural gas blending’.
Professor Lee said, “HNGB will utilize the existing natural gas infrastructure for transportation, so it is very likely that we can commercialize this hydrate system. We are investigating the kinetic performance through a follow-up strategy to increase the volume of gas storage.
This study was funded by the National Research Foundation of Korea and BK21 plus program.
(Figure1. Schematics showing the storage method for hydrogen in a natural gas hydrate using a substitution method and storage method directly from ice to a hydrogen-natural gas hydrate.)
(Figure 2. Artificially synthesized and dissociated hydrogen-natural gas hydrates. The Raman spectra of tuned sI and sII hydrate showing the hydrogen clusters in each cage.)
2019.06.21 View 42734
Hydrogen-Natural Gas Hydrates Harvested by Natural Gas
A hydrogen-natural gas blend (HNGB) can be a game changer only if it can be stored safely and used as a sustainable clean energy resource. A recent study has suggested a new strategy for stably storing hydrogen, using natural gas as a stabilizer. The research proposed a practical gas phase modulator based synthesis of HNGB without generating chemical waste after dissociation for the immediate service.
The research team of Professor Jae Woo Lee from the Department of Chemical and Biomolecular Engineering in collaboration with the Gwangju Institute of Science and Technology (GIST) demonstrated that the natural gas modulator based synthesis leads to significantly reduced synthesis pressure simultaneously with the formation of hydrogen clusters in the confined nanoporous cages of clathrate hydrates. This approach minimizes the environmental impact and reduces operation costs since clathrate hydrates do not generate any chemical waste in both the synthesis and decomposition processes.
For the efficient storage and transportation of hydrogen, numerous materials have been investigated. Among others, clathrate hydrates offer distinct benefits. Clathrate hydrates are nanoporous inclusion compounds composed of a 3D network of polyhedral cages made of hydrogen-bonded ‘host’ water molecules and captured ‘guest’ gas or liquid molecules.
In this study, the research team used two gases, methane and ethane, which have lower equilibrium conditions compared to hydrogen as thermodynamic stabilizers. As a result, they succeeded in stably storing the hydrogen-natural gas compound in hydrates. According to the composition ratio of methane and ethane, structure I or II hydrates can be formed, both of which can stably store hydrogen-natural gas in low-pressure conditions.
The research team found that two hydrogen molecules are stored in small cages in tuned structure I hydrates, while up to three hydrogen molecules can be stored in both small and large cages in tuned structure II hydrates. Hydrates can store gas up to about 170-times its volume and the natural gas used as thermodynamic stabilizers in this study can also be used as an energy source.
The research team developed technology to produce hydrates from ice, produced hydrogen-natural gas hydrates by substitution, and successfully observed that the tuning phenomenon only occurs when hydrogen is involved in hydrate formation from the start for both structures of hydrates.
They expect that the findings can be applied to not only an energy-efficient gas storage material, but also a smart platform to utilize hydrogen natural gas blends, which can serve as a new alternative energy source with targeted hydrogen contents by designing synthetic pathways of mixed gas hydrates.
The research was published online in Energy Storage Materials on June 6, with the title ‘One-step formation of hydrogen clusters in clathrate hydrates stabilized via natural gas blending’.
Professor Lee said, “HNGB will utilize the existing natural gas infrastructure for transportation, so it is very likely that we can commercialize this hydrate system. We are investigating the kinetic performance through a follow-up strategy to increase the volume of gas storage.
This study was funded by the National Research Foundation of Korea and BK21 plus program.
(Figure1. Schematics showing the storage method for hydrogen in a natural gas hydrate using a substitution method and storage method directly from ice to a hydrogen-natural gas hydrate.)
(Figure 2. Artificially synthesized and dissociated hydrogen-natural gas hydrates. The Raman spectra of tuned sI and sII hydrate showing the hydrogen clusters in each cage.)
2019.06.21 View 42734 -
 Dr. Steven Chu Talks on Sustainable Energy Policy at KAIST
Nobel Laureate in physics and former US Energy Secretary Steven Chu called for concerted efforts to develop a more sustainable energy policy and portfolio at a lecture held at KAIST and a forum in Seoul on November 23.
A policy with an energy mix including nuclear power and renewable energy could be ideal for retaining a stable energy supply given Korea’s very limited geographical conditions, Chu said during the Future Energy Forum in Seoul. He also held a lecture at KAIST’s Daejeon campus on “Climate Change, the Importance of Science and Policy in Achieving a Sustainable Future.”
He said that unlike the United States, Korea and Japan have geographical limitations for generating enough renewable energy. "Wind speeds of more than 10 meters per second would allow wind power generation, but, South Korea's southernmost wind speed in Jeju is less than 8 meters per second, and the amount of sunshine is lower than in the Middle East. It is ideal to combine renewable energy with nuclear power plants," he said.
Chu also stressed the role of science in achieving a sustainable future, citing many cases in foreign countries. For instance, Germany once decided to do away with nuclear power. However, their initial plan does not directly raise energy efficiency and the proportion of fossil fuels has led to an increase in the environmental issue of fine particular matter as well as carbon dioxide emission increases.
He said that in the long term, renewable energy will emerge as major alternative resources, stressing the role of science in achieving a sustainable future. Without this alternative, we will eventually burn more fossil fuels and pollute the air.
Chu also said that nuclear waste and safe plant operation will be a big concern, but it is technologically viable since Korea has already proven its prowess in nuclear power plant building and safety technology.
Chu added, "Research in chemical energy storage through novel electrochemistry may lead to solutions, but for the next half century we will need additional energy-on-demand and carbon-free sources of energy from proven technologies."
"While science, innovation and technology will no doubt lead to better solutions, sound government policies are needed to advance the transition to carbon-free energy needed to achieve a more sustainable world," he said.
After serving as the US Secretary of Energy for four years from 2009 to 2013, Professor Chu returned to Stanford University, and currently holds a position of the William R. Kenan, Jr. Professor of Physics as well as Professor in the Department of Molecular and Cellular Physiology.
Professor Chu is known for his research at Bell Labs and Stanford University regarding the cooling and trapping of atoms with laser light, for which he won the Nobel Prize in Physics in 1997.
2017.11.24 View 5491
Dr. Steven Chu Talks on Sustainable Energy Policy at KAIST
Nobel Laureate in physics and former US Energy Secretary Steven Chu called for concerted efforts to develop a more sustainable energy policy and portfolio at a lecture held at KAIST and a forum in Seoul on November 23.
A policy with an energy mix including nuclear power and renewable energy could be ideal for retaining a stable energy supply given Korea’s very limited geographical conditions, Chu said during the Future Energy Forum in Seoul. He also held a lecture at KAIST’s Daejeon campus on “Climate Change, the Importance of Science and Policy in Achieving a Sustainable Future.”
He said that unlike the United States, Korea and Japan have geographical limitations for generating enough renewable energy. "Wind speeds of more than 10 meters per second would allow wind power generation, but, South Korea's southernmost wind speed in Jeju is less than 8 meters per second, and the amount of sunshine is lower than in the Middle East. It is ideal to combine renewable energy with nuclear power plants," he said.
Chu also stressed the role of science in achieving a sustainable future, citing many cases in foreign countries. For instance, Germany once decided to do away with nuclear power. However, their initial plan does not directly raise energy efficiency and the proportion of fossil fuels has led to an increase in the environmental issue of fine particular matter as well as carbon dioxide emission increases.
He said that in the long term, renewable energy will emerge as major alternative resources, stressing the role of science in achieving a sustainable future. Without this alternative, we will eventually burn more fossil fuels and pollute the air.
Chu also said that nuclear waste and safe plant operation will be a big concern, but it is technologically viable since Korea has already proven its prowess in nuclear power plant building and safety technology.
Chu added, "Research in chemical energy storage through novel electrochemistry may lead to solutions, but for the next half century we will need additional energy-on-demand and carbon-free sources of energy from proven technologies."
"While science, innovation and technology will no doubt lead to better solutions, sound government policies are needed to advance the transition to carbon-free energy needed to achieve a more sustainable world," he said.
After serving as the US Secretary of Energy for four years from 2009 to 2013, Professor Chu returned to Stanford University, and currently holds a position of the William R. Kenan, Jr. Professor of Physics as well as Professor in the Department of Molecular and Cellular Physiology.
Professor Chu is known for his research at Bell Labs and Stanford University regarding the cooling and trapping of atoms with laser light, for which he won the Nobel Prize in Physics in 1997.
2017.11.24 View 5491