Genesis
-
 KAIST Discovers Molecular Switch that Reverses Cancerous Transformation at the Critical Moment of Transition
< (From left) PhD student Seoyoon D. Jeong, (bottom) Professor Kwang-Hyun Cho, (top) Dr. Dongkwan Shin, Dr. Jeong-Ryeol Gong >
Professor Kwang-Hyun Cho’s research team has recently been highlighted for their work on developing an original technology for cancer reversal treatment that does not kill cancer cells but only changes their characteristics to reverse them to a state similar to normal cells. This time, they have succeeded in revealing for the first time that a molecular switch that can induce cancer reversal at the moment when normal cells change into cancer cells is hidden in the genetic network.
KAIST (President Kwang-Hyung Lee) announced on the 5th of February that Professor Kwang-Hyun Cho's research team of the Department of Bio and Brain Engineering has succeeded in developing a fundamental technology to capture the critical transition phenomenon at the moment when normal cells change into cancer cells and analyze it to discover a molecular switch that can revert cancer cells back into normal cells.
A critical transition is a phenomenon in which a sudden change in state occurs at a specific point in time, like water changing into steam at 100℃. This critical transition phenomenon also occurs in the process in which normal cells change into cancer cells at a specific point in time due to the accumulation of genetic and epigenetic changes.
The research team discovered that normal cells can enter an unstable critical transition state where normal cells and cancer cells coexist just before they change into cancer cells during tumorigenesis, the production or development of tumors, and analyzed this critical transition state using a systems biology method to develop a cancer reversal molecular switch identification technology that can reverse the cancerization process. They then applied this to colon cancer cells and confirmed through molecular cell experiments that cancer cells can recover the characteristics of normal cells.
This is an original technology that automatically infers a computer model of the genetic network that controls the critical transition of cancer development from single-cell RNA sequencing data, and systematically finds molecular switches for cancer reversion by simulation analysis. It is expected that this technology will be applied to the development of reversion therapies for other cancers in the future.
Professor Kwang-Hyun Cho said, "We have discovered a molecular switch that can revert the fate of cancer cells back to a normal state by capturing the moment of critical transition right before normal cells are changed into an irreversible cancerous state."
< Figure 1. Overall conceptual framework of the technology that automatically constructs a molecular regulatory network from single-cell RNA sequencing data of colon cancer cells to discover molecular switches for cancer reversion through computer simulation analysis. Professor Kwang-Hyun Cho's research team established a fundamental technology for automatic construction of a computer model of a core gene network by analyzing the entire process of tumorigenesis of colon cells turning into cancer cells, and developed an original technology for discovering the molecular switches that can induce cancer cell reversal through attractor landscape analysis. >
He continued, "In particular, this study has revealed in detail, at the genetic network level, what changes occur within cells behind the process of cancer development, which has been considered a mystery until now." He emphasized, "This is the first study to reveal that an important clue that can revert the fate of tumorigenesis is hidden at this very critical moment of change."
< Figure 2. Identification of tumor transition state using single-cell RNA sequencing data from colorectal cancer. Using single-cell RNA sequencing data from colorectal cancer patient-derived organoids for normal and cancerous tissues, a critical transition was identified in which normal and cancerous cells coexist and instability increases (a-d). The critical transition was confirmed to show intermediate levels of major phenotypic features related to cancer or normal tissues that are indicative of the states between the normal and cancerous cells (e). >
The results of this study, conducted by KAIST Dr. Dongkwan Shin (currently at the National Cancer Center), Dr. Jeong-Ryeol Gong, and doctoral student Seoyoon D. Jeong jointly with a research team at Seoul National University that provided the organoids (in vitro cultured tissues) from colon cancer patient, were published as an online paper in the international journal ‘Advanced Science’ published by Wiley on January 22nd.
(Paper title: Attractor landscape analysis reveals a reversion switch in the transition of colorectal tumorigenesis) (DOI: https://doi.org/10.1002/advs.202412503)
< Figure 3. Reconstruction of a dynamic network model for the transition state of colorectal cancer.
A new technology was established to build a gene network computer model that can simulate the dynamic changes between genes by integrating single-cell RNA sequencing data and existing experimental results on gene-to-gene interactions in the critical transition of cancer. (a). Using this technology, a gene network computer model for the critical transition of colorectal cancer was constructed, and the distribution of attractors representing normal and cancer cell phenotypes was investigated through attractor landscape analysis (b-e). >
This study was conducted with the support of the National Research Foundation of Korea under the Ministry of Science and ICT through the Mid-Career Researcher Program and Basic Research Laboratory Program and the Disease-Centered Translational Research Project of the Korea Health Industry Development Institute (KHIDI) of the Ministry of Health and Welfare.
< Figure 4. Quantification of attractor landscapes and discovery of transcription factors for cancer reversibility through perturbation simulation analysis. A methodology for implementing discontinuous attractor landscapes continuously from a computer model of gene networks and quantifying them as cancer scores was introduced (a), and attractor landscapes for the critical transition of colorectal cancer were secured (b-d). By tracking the change patterns of normal and cancer cell attractors through perturbation simulation analysis for each gene, the optimal combination of transcription factors for cancer reversion was discovered (e-h). This was confirmed in various parameter combinations as well (i). >
< Figure 5. Identification and experimental validation of the optimal target gene for cancer reversion. Among the common target genes of the discovered transcription factor combinations, we identified cancer reversing molecular switches that are predicted to suppress cancer cell proliferation and restore the characteristics of normal colon cells (a-d). When inhibitors for the molecular switches were treated to organoids derived from colon cancer patients, it was confirmed that cancer cell proliferation was suppressed and the expression of key genes related to cancer development was inhibited (e-h), and a group of genes related to normal colon epithelium was activated and transformed into a state similar to normal colon cells (i-j). >
< Figure 6. Schematic diagram of the research results. Professor Kwang-Hyun Cho's research team developed an original technology to systematically discover key molecular switches that can induce reversion of colon cancer cells through a systems biology approach using an attractor landscape analysis of a genetic network model for the critical transition at the moment of transformation from normal cells to cancer cells, and verified the reversing effect of actual colon cancer through cellular experiments. >
2025.02.05 View 26467
KAIST Discovers Molecular Switch that Reverses Cancerous Transformation at the Critical Moment of Transition
< (From left) PhD student Seoyoon D. Jeong, (bottom) Professor Kwang-Hyun Cho, (top) Dr. Dongkwan Shin, Dr. Jeong-Ryeol Gong >
Professor Kwang-Hyun Cho’s research team has recently been highlighted for their work on developing an original technology for cancer reversal treatment that does not kill cancer cells but only changes their characteristics to reverse them to a state similar to normal cells. This time, they have succeeded in revealing for the first time that a molecular switch that can induce cancer reversal at the moment when normal cells change into cancer cells is hidden in the genetic network.
KAIST (President Kwang-Hyung Lee) announced on the 5th of February that Professor Kwang-Hyun Cho's research team of the Department of Bio and Brain Engineering has succeeded in developing a fundamental technology to capture the critical transition phenomenon at the moment when normal cells change into cancer cells and analyze it to discover a molecular switch that can revert cancer cells back into normal cells.
A critical transition is a phenomenon in which a sudden change in state occurs at a specific point in time, like water changing into steam at 100℃. This critical transition phenomenon also occurs in the process in which normal cells change into cancer cells at a specific point in time due to the accumulation of genetic and epigenetic changes.
The research team discovered that normal cells can enter an unstable critical transition state where normal cells and cancer cells coexist just before they change into cancer cells during tumorigenesis, the production or development of tumors, and analyzed this critical transition state using a systems biology method to develop a cancer reversal molecular switch identification technology that can reverse the cancerization process. They then applied this to colon cancer cells and confirmed through molecular cell experiments that cancer cells can recover the characteristics of normal cells.
This is an original technology that automatically infers a computer model of the genetic network that controls the critical transition of cancer development from single-cell RNA sequencing data, and systematically finds molecular switches for cancer reversion by simulation analysis. It is expected that this technology will be applied to the development of reversion therapies for other cancers in the future.
Professor Kwang-Hyun Cho said, "We have discovered a molecular switch that can revert the fate of cancer cells back to a normal state by capturing the moment of critical transition right before normal cells are changed into an irreversible cancerous state."
< Figure 1. Overall conceptual framework of the technology that automatically constructs a molecular regulatory network from single-cell RNA sequencing data of colon cancer cells to discover molecular switches for cancer reversion through computer simulation analysis. Professor Kwang-Hyun Cho's research team established a fundamental technology for automatic construction of a computer model of a core gene network by analyzing the entire process of tumorigenesis of colon cells turning into cancer cells, and developed an original technology for discovering the molecular switches that can induce cancer cell reversal through attractor landscape analysis. >
He continued, "In particular, this study has revealed in detail, at the genetic network level, what changes occur within cells behind the process of cancer development, which has been considered a mystery until now." He emphasized, "This is the first study to reveal that an important clue that can revert the fate of tumorigenesis is hidden at this very critical moment of change."
< Figure 2. Identification of tumor transition state using single-cell RNA sequencing data from colorectal cancer. Using single-cell RNA sequencing data from colorectal cancer patient-derived organoids for normal and cancerous tissues, a critical transition was identified in which normal and cancerous cells coexist and instability increases (a-d). The critical transition was confirmed to show intermediate levels of major phenotypic features related to cancer or normal tissues that are indicative of the states between the normal and cancerous cells (e). >
The results of this study, conducted by KAIST Dr. Dongkwan Shin (currently at the National Cancer Center), Dr. Jeong-Ryeol Gong, and doctoral student Seoyoon D. Jeong jointly with a research team at Seoul National University that provided the organoids (in vitro cultured tissues) from colon cancer patient, were published as an online paper in the international journal ‘Advanced Science’ published by Wiley on January 22nd.
(Paper title: Attractor landscape analysis reveals a reversion switch in the transition of colorectal tumorigenesis) (DOI: https://doi.org/10.1002/advs.202412503)
< Figure 3. Reconstruction of a dynamic network model for the transition state of colorectal cancer.
A new technology was established to build a gene network computer model that can simulate the dynamic changes between genes by integrating single-cell RNA sequencing data and existing experimental results on gene-to-gene interactions in the critical transition of cancer. (a). Using this technology, a gene network computer model for the critical transition of colorectal cancer was constructed, and the distribution of attractors representing normal and cancer cell phenotypes was investigated through attractor landscape analysis (b-e). >
This study was conducted with the support of the National Research Foundation of Korea under the Ministry of Science and ICT through the Mid-Career Researcher Program and Basic Research Laboratory Program and the Disease-Centered Translational Research Project of the Korea Health Industry Development Institute (KHIDI) of the Ministry of Health and Welfare.
< Figure 4. Quantification of attractor landscapes and discovery of transcription factors for cancer reversibility through perturbation simulation analysis. A methodology for implementing discontinuous attractor landscapes continuously from a computer model of gene networks and quantifying them as cancer scores was introduced (a), and attractor landscapes for the critical transition of colorectal cancer were secured (b-d). By tracking the change patterns of normal and cancer cell attractors through perturbation simulation analysis for each gene, the optimal combination of transcription factors for cancer reversion was discovered (e-h). This was confirmed in various parameter combinations as well (i). >
< Figure 5. Identification and experimental validation of the optimal target gene for cancer reversion. Among the common target genes of the discovered transcription factor combinations, we identified cancer reversing molecular switches that are predicted to suppress cancer cell proliferation and restore the characteristics of normal colon cells (a-d). When inhibitors for the molecular switches were treated to organoids derived from colon cancer patients, it was confirmed that cancer cell proliferation was suppressed and the expression of key genes related to cancer development was inhibited (e-h), and a group of genes related to normal colon epithelium was activated and transformed into a state similar to normal colon cells (i-j). >
< Figure 6. Schematic diagram of the research results. Professor Kwang-Hyun Cho's research team developed an original technology to systematically discover key molecular switches that can induce reversion of colon cancer cells through a systems biology approach using an attractor landscape analysis of a genetic network model for the critical transition at the moment of transformation from normal cells to cancer cells, and verified the reversing effect of actual colon cancer through cellular experiments. >
2025.02.05 View 26467 -
 KAIST Team Develops Surface-Lighting MicroLED Patch with Significant Melanogenesis Inhibition Effect
A KAIST research team led by Ph.d candidate Jae Hee Lee and Professor Keon Jae Lee from the Department of Materials Science and Engineering has developed a surface-lighting microLED patch for UV-induced melanogenesis inhibition.
Melanin is brown or dark pigments existing in the skin, which can be abnormally synthesized by external UV or stress. Since the excessive melanin leads to skin diseases such as spots and freckles, proper treatment is required to return normal skin condition.
Recently, LED-based photo-stimulators have been released for skin care, however, their therapeutic effect is still controversial. Since conventional LED stimulators cannot conformally attach to the human skin, distance-induced side effects are caused by light loss and high heat transfer. To achieve effective phototreatment, the LED stimulator needs to be irradiated in contact with the human skin surface, enabling proper and uniform light deliver to the dermis with minimal optical loss.
In this work, the research team fabricated skin-attachable surface-lighting microLED (SµLED, 4 × 4 cm2) patch by utilizing a thousand of microLED chips and silica-embedded light diffusion layer. 100 µm-sized LED chips are vertically-interconnected for high flexibility and low heat generation, allowing its long-term operation on the human skin.
< Image 1. The overall concept of SµLED patch. a) SµLED patch operated on the human skin. b) Schematic illustration of SµLED patch structure. c) 4 × 4 cm2-sized SµLED patch. d) Schematic illustration of the advantages of SµLED patch such as efficient light delivery, low heat generation, and surface-lighting irradiation. >
The research team confirmed melanogenesis inhibition by irradiating the SµLED patch and the conventional LED (CLED) on the artificial human skin and mice dorsal skin. The SµLED-treated groups of human cells and mouse tissues showed minimal epidermal photo-toxicity and consistently effective reduction in synthesized melanin, compared to CLED-treated groups. In addition, significant suppression of proteins/catalysts expression involved in melanin synthesis such as MITF (microphthalmia-associated transcription factor), Melan-A and tyrosinase was verified.
< Image 2. The efficacy of melanogenesis inhibition on 3D human skin cells. a). Different irradiation conditions for a-MSH (major factor to stimulate melanin synthesis) treated cells. b) The ratio of pigmented area to total epidermis area. c) Relative variance of melanin level in 1 cm2-sized skin cells. A low variance means that melanin is evenly distributed, and a high variance means that the melanin is irregularly distributed. d) Optical images after in vitro experiments for 12 days. Scale bar, 1cm. e) Histological analysis of 3D skin, showing the greatest reduction in melanin after SµLED irradiation. Scale bar, 20 µm. >
< Image 3. The efficacy of melanogenesis inhibition on mouse dorsal skin. a) Optical images of mice dorsal skin after photo-treatment for 20 days. b) Histological analysis of mice dorsal skin. Less brown color means less expression of protein/catalysis involved in melanin synthesis. Scale bar, 50 µm. >
Prof. Keon Jae Lee said, “Our inorganic-based SµLED patch has outstanding characteristics in light efficiency, reliability, and durability. The SµLED patch is expected to give a great impact on the cosmetic field by reducing side effects and maximizing phototherapeutic effects.” The core technology of cosmetic SµLED has been transferred to Fronics co., Ltd, founded by Prof. Lee. Fronics is building foundry and equipment for mass production of SµLED masks for whole face cover and plans to release the products in March next year.
This paper entitled “Wearable Surface-Lighting Micro-Light-Emitting Diode Patch for Melanogenesis Inhibition” was published in the November 2022 issue of Advanced Healthcare Materials.
2022.11.22 View 13024
KAIST Team Develops Surface-Lighting MicroLED Patch with Significant Melanogenesis Inhibition Effect
A KAIST research team led by Ph.d candidate Jae Hee Lee and Professor Keon Jae Lee from the Department of Materials Science and Engineering has developed a surface-lighting microLED patch for UV-induced melanogenesis inhibition.
Melanin is brown or dark pigments existing in the skin, which can be abnormally synthesized by external UV or stress. Since the excessive melanin leads to skin diseases such as spots and freckles, proper treatment is required to return normal skin condition.
Recently, LED-based photo-stimulators have been released for skin care, however, their therapeutic effect is still controversial. Since conventional LED stimulators cannot conformally attach to the human skin, distance-induced side effects are caused by light loss and high heat transfer. To achieve effective phototreatment, the LED stimulator needs to be irradiated in contact with the human skin surface, enabling proper and uniform light deliver to the dermis with minimal optical loss.
In this work, the research team fabricated skin-attachable surface-lighting microLED (SµLED, 4 × 4 cm2) patch by utilizing a thousand of microLED chips and silica-embedded light diffusion layer. 100 µm-sized LED chips are vertically-interconnected for high flexibility and low heat generation, allowing its long-term operation on the human skin.
< Image 1. The overall concept of SµLED patch. a) SµLED patch operated on the human skin. b) Schematic illustration of SµLED patch structure. c) 4 × 4 cm2-sized SµLED patch. d) Schematic illustration of the advantages of SµLED patch such as efficient light delivery, low heat generation, and surface-lighting irradiation. >
The research team confirmed melanogenesis inhibition by irradiating the SµLED patch and the conventional LED (CLED) on the artificial human skin and mice dorsal skin. The SµLED-treated groups of human cells and mouse tissues showed minimal epidermal photo-toxicity and consistently effective reduction in synthesized melanin, compared to CLED-treated groups. In addition, significant suppression of proteins/catalysts expression involved in melanin synthesis such as MITF (microphthalmia-associated transcription factor), Melan-A and tyrosinase was verified.
< Image 2. The efficacy of melanogenesis inhibition on 3D human skin cells. a). Different irradiation conditions for a-MSH (major factor to stimulate melanin synthesis) treated cells. b) The ratio of pigmented area to total epidermis area. c) Relative variance of melanin level in 1 cm2-sized skin cells. A low variance means that melanin is evenly distributed, and a high variance means that the melanin is irregularly distributed. d) Optical images after in vitro experiments for 12 days. Scale bar, 1cm. e) Histological analysis of 3D skin, showing the greatest reduction in melanin after SµLED irradiation. Scale bar, 20 µm. >
< Image 3. The efficacy of melanogenesis inhibition on mouse dorsal skin. a) Optical images of mice dorsal skin after photo-treatment for 20 days. b) Histological analysis of mice dorsal skin. Less brown color means less expression of protein/catalysis involved in melanin synthesis. Scale bar, 50 µm. >
Prof. Keon Jae Lee said, “Our inorganic-based SµLED patch has outstanding characteristics in light efficiency, reliability, and durability. The SµLED patch is expected to give a great impact on the cosmetic field by reducing side effects and maximizing phototherapeutic effects.” The core technology of cosmetic SµLED has been transferred to Fronics co., Ltd, founded by Prof. Lee. Fronics is building foundry and equipment for mass production of SµLED masks for whole face cover and plans to release the products in March next year.
This paper entitled “Wearable Surface-Lighting Micro-Light-Emitting Diode Patch for Melanogenesis Inhibition” was published in the November 2022 issue of Advanced Healthcare Materials.
2022.11.22 View 13024 -
 Genomic Data Reveals New Insights into Human Embryonic Development
KAIST researchers have used whole-genome sequencing to track the development from a single fertilized-egg to a human body
Genomic scientists at KAIST have revealed new insights into the process of human embryonic development using large-scale, whole-genome sequencing of cells and tissues from adult humans. The study, published in Nature on Aug.25, is the first to analyse somatic mutations in normal tissue across multiple organs within and between humans.
An adult human body comprises trillions of cells of more than 200 types. How a human develops from a single fertilized egg to a fully grown adult is a fundamental question in biomedical science. Due to the ethical challenges of performing studies on human embryos, however, the details of this process remain largely unknown.
To overcome these issues, the research team took a different approach. They analysed genetic mutations in cells taken from adult human post-mortem tissue. Specifically, they identified mutations that occur spontaneously in early developmental cell divisions. These mutations, also called genomic scars, act like unique genetic fingerprints that can be used to trace the embryonic development process.
The study, which looked at 334 single-cell colonies and 379 tissue samples from seven recently deceased human body donors, is the largest single-cell, whole-genome analysis carried out to date. The researchers examined the genomic scars of each individual in order to reconstruct their early embryonic cellular dynamics.
The result revealed several key characteristics of the human embryonic development process. Firstly, mutation rates are higher in the first cell division, but then decrease to approximately one mutation per cell during later cell division. Secondly, early cells contributed unequally to the development of the embryo in all informative donors, for example, at the two-cell stage, one of the cells always left more progeny cells than the other. The ratio of this was different from person to person, implying that the process varies between individuals and is not fully deterministic.
The researchers were also able to deduce the timing of when cells begin to differentiate into individual organ-specific cells. They found that within three days of fertilization, embryonic cells began to be distributed asymmetrically into tissues for the left and right sides of the body, followed by differentiation into three germ layers, and then differentiation into specific tissues and organs.
“It is an impressive scientific achievement that, within 20 years of the completion of human genome project, genomic technology has advanced to the extent that we are now able to accurately identify mutations in a single-cell genome,” said Professor Young Seok Ju from the Graduate School of Medical Science and Engineering at KAIST. “This technology will enable us to track human embryogenesis at even higher resolutions in the future.”
The techniques used in this study could be used to improve our understanding of rare diseases caused by abnormalities in embryonic development, and to design new precision diagnostics and treatments for patients.
The research was completed in collaboration with Kyungpook National University Hospital, the Korea Institute of Science and Technology Information, Catholic University of Korea School of Medicine, Genome Insights Inc, and Immune Square Inc. This work was supported by the Suh Kyungbae Foundation, the Ministry of Health and Welfare of Korea, the National Research Foundastion of Korea.
-PublicationSeongyeol Park, Nanda Mali, Ryul Kim et al. ‘Clonal dynamics in early human embryogenesis inferred from somatic mutation’ Nature Online ahead of print, Aug. 25, 2021 (https://doi.org/10.1038/s41586-021-03786-8)
-ProfileProfessor Young Seok JuLab of Cancer Genomics (https://www.julab.kaist.ac.kr/)Graduate School of Medical Science and EngineeringKAIST
2021.08.31 View 10150
Genomic Data Reveals New Insights into Human Embryonic Development
KAIST researchers have used whole-genome sequencing to track the development from a single fertilized-egg to a human body
Genomic scientists at KAIST have revealed new insights into the process of human embryonic development using large-scale, whole-genome sequencing of cells and tissues from adult humans. The study, published in Nature on Aug.25, is the first to analyse somatic mutations in normal tissue across multiple organs within and between humans.
An adult human body comprises trillions of cells of more than 200 types. How a human develops from a single fertilized egg to a fully grown adult is a fundamental question in biomedical science. Due to the ethical challenges of performing studies on human embryos, however, the details of this process remain largely unknown.
To overcome these issues, the research team took a different approach. They analysed genetic mutations in cells taken from adult human post-mortem tissue. Specifically, they identified mutations that occur spontaneously in early developmental cell divisions. These mutations, also called genomic scars, act like unique genetic fingerprints that can be used to trace the embryonic development process.
The study, which looked at 334 single-cell colonies and 379 tissue samples from seven recently deceased human body donors, is the largest single-cell, whole-genome analysis carried out to date. The researchers examined the genomic scars of each individual in order to reconstruct their early embryonic cellular dynamics.
The result revealed several key characteristics of the human embryonic development process. Firstly, mutation rates are higher in the first cell division, but then decrease to approximately one mutation per cell during later cell division. Secondly, early cells contributed unequally to the development of the embryo in all informative donors, for example, at the two-cell stage, one of the cells always left more progeny cells than the other. The ratio of this was different from person to person, implying that the process varies between individuals and is not fully deterministic.
The researchers were also able to deduce the timing of when cells begin to differentiate into individual organ-specific cells. They found that within three days of fertilization, embryonic cells began to be distributed asymmetrically into tissues for the left and right sides of the body, followed by differentiation into three germ layers, and then differentiation into specific tissues and organs.
“It is an impressive scientific achievement that, within 20 years of the completion of human genome project, genomic technology has advanced to the extent that we are now able to accurately identify mutations in a single-cell genome,” said Professor Young Seok Ju from the Graduate School of Medical Science and Engineering at KAIST. “This technology will enable us to track human embryogenesis at even higher resolutions in the future.”
The techniques used in this study could be used to improve our understanding of rare diseases caused by abnormalities in embryonic development, and to design new precision diagnostics and treatments for patients.
The research was completed in collaboration with Kyungpook National University Hospital, the Korea Institute of Science and Technology Information, Catholic University of Korea School of Medicine, Genome Insights Inc, and Immune Square Inc. This work was supported by the Suh Kyungbae Foundation, the Ministry of Health and Welfare of Korea, the National Research Foundastion of Korea.
-PublicationSeongyeol Park, Nanda Mali, Ryul Kim et al. ‘Clonal dynamics in early human embryogenesis inferred from somatic mutation’ Nature Online ahead of print, Aug. 25, 2021 (https://doi.org/10.1038/s41586-021-03786-8)
-ProfileProfessor Young Seok JuLab of Cancer Genomics (https://www.julab.kaist.ac.kr/)Graduate School of Medical Science and EngineeringKAIST
2021.08.31 View 10150 -
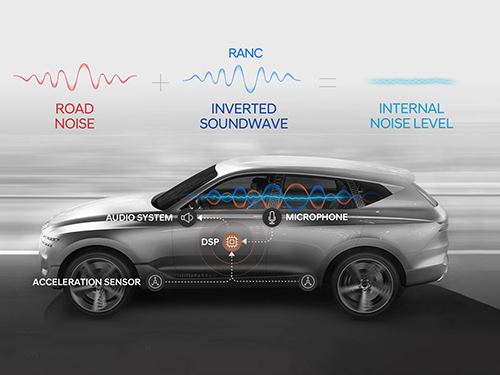 A System Controlling Road Active Noise to Hit the Road
The research team led by Professor Youngjin Park of the Department of Mechanical Engineering has developed a road noise active noise control (RANC) system to be commercialized in partnership with Hyundai Motor Group.
On December 11, Hyundai Motor Group announced the successful development of the RANC system, which significantly reduces the road noise flowing into cars. The carmaker has completed the domestic and American patent applications for the location of sensors and the signal selection method, the core technology of RANC.
RANC is a technology for reducing road noise during driving. This system consists of an acceleration sensor, digital signal processor (the control computer to analyze sound signals), microphone, amplifier, and audio system. To make the system as simple as possible, the audio system utilizes the original audio system embedded in the car instead of a separate system.
The acceleration sensor first calculates the vibration from the road into the car. The location of the sensor is important for accurately identifying the vibration path. The research team was able to find the optimal sensor location through a number of tests.
The System Dynamics and Applied Control Laboratory of Professor Park researched ways to significantly reduce road noise with Hyundai Motor Group for four years from 1993 as a G7 national project and published the results in international journals. In 2002, the researchers published an article titled “Noise Quietens Driving” in Nature, where they announced the first success in reducing road noise in actual cars. The achievement did not lead to commercialization, however, due to the lack of auxiliary technologies at the time, digital amplifiers and DSP for cars for example, and pricing issues.
Since 2013, Professor Park’s research team has participated in one technology transfer and eight university-industry projects. Based on these efforts, the team was able to successfully develop the RANC system with domestic technology in partnership with Hyundai’s NVH Research Lab (Research Fellow, Dr. Gangdeok Lee; Ph.D. in aviation engineering, 1996), Optomech (Founder, Professor Gyeongsu Kim; Ph.D. in mechanical engineering, 1999), ARE (CEO Hyeonseok Kim; Ph.D. in mechanical engineering, 1998), WeAcom, and BurnYoung.
Professor Park’s team led the project by performing theory-based research during the commercialization stage in collaboration with Hyundai Motor Group.
For the commercialization of the RANC system, Hyundai Motor Group is planning to collaborate with the global car audio company Harman to increase the degree of completion and apply the RANC system to the GV 80, the first SUV model of the Genesis brand.
“I am very delighted as an engineer to see the research I worked on from my early days at KAIST be commercialized after 20 years,” noted Professor Park. “I am thrilled to make a contribution to such commercialization with my students in my lab.”
2019.12.27 View 13173
A System Controlling Road Active Noise to Hit the Road
The research team led by Professor Youngjin Park of the Department of Mechanical Engineering has developed a road noise active noise control (RANC) system to be commercialized in partnership with Hyundai Motor Group.
On December 11, Hyundai Motor Group announced the successful development of the RANC system, which significantly reduces the road noise flowing into cars. The carmaker has completed the domestic and American patent applications for the location of sensors and the signal selection method, the core technology of RANC.
RANC is a technology for reducing road noise during driving. This system consists of an acceleration sensor, digital signal processor (the control computer to analyze sound signals), microphone, amplifier, and audio system. To make the system as simple as possible, the audio system utilizes the original audio system embedded in the car instead of a separate system.
The acceleration sensor first calculates the vibration from the road into the car. The location of the sensor is important for accurately identifying the vibration path. The research team was able to find the optimal sensor location through a number of tests.
The System Dynamics and Applied Control Laboratory of Professor Park researched ways to significantly reduce road noise with Hyundai Motor Group for four years from 1993 as a G7 national project and published the results in international journals. In 2002, the researchers published an article titled “Noise Quietens Driving” in Nature, where they announced the first success in reducing road noise in actual cars. The achievement did not lead to commercialization, however, due to the lack of auxiliary technologies at the time, digital amplifiers and DSP for cars for example, and pricing issues.
Since 2013, Professor Park’s research team has participated in one technology transfer and eight university-industry projects. Based on these efforts, the team was able to successfully develop the RANC system with domestic technology in partnership with Hyundai’s NVH Research Lab (Research Fellow, Dr. Gangdeok Lee; Ph.D. in aviation engineering, 1996), Optomech (Founder, Professor Gyeongsu Kim; Ph.D. in mechanical engineering, 1999), ARE (CEO Hyeonseok Kim; Ph.D. in mechanical engineering, 1998), WeAcom, and BurnYoung.
Professor Park’s team led the project by performing theory-based research during the commercialization stage in collaboration with Hyundai Motor Group.
For the commercialization of the RANC system, Hyundai Motor Group is planning to collaborate with the global car audio company Harman to increase the degree of completion and apply the RANC system to the GV 80, the first SUV model of the Genesis brand.
“I am very delighted as an engineer to see the research I worked on from my early days at KAIST be commercialized after 20 years,” noted Professor Park. “I am thrilled to make a contribution to such commercialization with my students in my lab.”
2019.12.27 View 13173 -
 Education Innovation Day Reaffirms Rewarding of Excellence
Professors Tae-Eog Lee and Il-Chul Moon from the Department of Industrial & Systems Engineering received the Linkgenesis Best Teacher Award and the Soo-Young Lee Teaching Innovation Award on May 10. They were each awarded with 10 million KRW in prize money during the Education Innovation Day ceremony held at the Chung Kun-mo conference hall.
The award was endowed by KAIST Alumni Scholarship Chairman Hyung-Kyu Lim and KAIST Foundation Chairman Soo-Young Lee to support the innovation initiative and acknowledge faculty members who made significant contributions to educational innovation and benefited the general public though their innovations.
“KAIST’s vision for excellence and commitment to innovation is a game changer. Educational innovation is one of five pillars of Vision 2031, and it is our priority to foster critical and creative thinking students,” said President Sung-Chul Shin at the ceremony. All the awardees made presentation on their innovative projects and shared their ideas on better pedagogical methodology for next generation.
Professor Lee, dean of the KAIST Academy and the head of the Center for Excellence in Learning & Teaching was recognized for his contribution to enhancing educational quality through innovative learning and teaching methodology development. He has set up an Education 3.0 Initiative, an online education platform for flipped learning at KAIST.
Professor Moon also upgraded the online education platform to the 4.0 version and extended KAIST’s massive online courses through KOOC framework. This open platform offers more than 62 courses, with more than 170 thousand users registered since 2014.
Professor Song-Hong Park from the Department of Bio and Brain Engineering and Professor Jae-Woo Lee from the Department of Chemical and Biomolecular Engineering also won the Excellence Award.
2019.05.10 View 10106
Education Innovation Day Reaffirms Rewarding of Excellence
Professors Tae-Eog Lee and Il-Chul Moon from the Department of Industrial & Systems Engineering received the Linkgenesis Best Teacher Award and the Soo-Young Lee Teaching Innovation Award on May 10. They were each awarded with 10 million KRW in prize money during the Education Innovation Day ceremony held at the Chung Kun-mo conference hall.
The award was endowed by KAIST Alumni Scholarship Chairman Hyung-Kyu Lim and KAIST Foundation Chairman Soo-Young Lee to support the innovation initiative and acknowledge faculty members who made significant contributions to educational innovation and benefited the general public though their innovations.
“KAIST’s vision for excellence and commitment to innovation is a game changer. Educational innovation is one of five pillars of Vision 2031, and it is our priority to foster critical and creative thinking students,” said President Sung-Chul Shin at the ceremony. All the awardees made presentation on their innovative projects and shared their ideas on better pedagogical methodology for next generation.
Professor Lee, dean of the KAIST Academy and the head of the Center for Excellence in Learning & Teaching was recognized for his contribution to enhancing educational quality through innovative learning and teaching methodology development. He has set up an Education 3.0 Initiative, an online education platform for flipped learning at KAIST.
Professor Moon also upgraded the online education platform to the 4.0 version and extended KAIST’s massive online courses through KOOC framework. This open platform offers more than 62 courses, with more than 170 thousand users registered since 2014.
Professor Song-Hong Park from the Department of Bio and Brain Engineering and Professor Jae-Woo Lee from the Department of Chemical and Biomolecular Engineering also won the Excellence Award.
2019.05.10 View 10106 -
 Professor Gou Young Koh, 2018 Laureate of Ho-Am Prize
Distinguished Professor Gou Young Koh from the Graduate School of Medical Science and Engineering was appointed a 2018 laureate in medicine of the Ho-Am Prize by the Ho-Am Foundation. Professor Koh is a renowned expert in the field of tumor angiogenesis by exploring the hidden nature of capillary and lymphatic vessels in human organs.
He was recognized for demonstrating the effective reduction of tumor progression and metastasis via tumor vessel normalization. This counterintuitive study result is regarded as a stepping stone for a drug discovery to prevent microvascular diseases.
Besides Professor Koh, Professor Hee Oh from Yale University (Science), Professor Nam-Gyu Park from Sungkyunkwan University (Engineering), Opera Singer Kwangchul Youn (The Arts) and Sister Carla Kang (Community Service) received awards.
The Ho-Am Prize is presented to individuals who have contributed to academics, the arts, and social development, or furthered the welfare of humanity, and commemorates the noble spirit of public service espoused by the late Chairman Byung-chull Lee, who used the pen name Ho-Am.
It was established in 1990 by Kun-Hee Lee, the chairman of Samsung. Awards have been presented to 143 individuals worth a total of 24.4 billion KRW.
2018.04.11 View 9425
Professor Gou Young Koh, 2018 Laureate of Ho-Am Prize
Distinguished Professor Gou Young Koh from the Graduate School of Medical Science and Engineering was appointed a 2018 laureate in medicine of the Ho-Am Prize by the Ho-Am Foundation. Professor Koh is a renowned expert in the field of tumor angiogenesis by exploring the hidden nature of capillary and lymphatic vessels in human organs.
He was recognized for demonstrating the effective reduction of tumor progression and metastasis via tumor vessel normalization. This counterintuitive study result is regarded as a stepping stone for a drug discovery to prevent microvascular diseases.
Besides Professor Koh, Professor Hee Oh from Yale University (Science), Professor Nam-Gyu Park from Sungkyunkwan University (Engineering), Opera Singer Kwangchul Youn (The Arts) and Sister Carla Kang (Community Service) received awards.
The Ho-Am Prize is presented to individuals who have contributed to academics, the arts, and social development, or furthered the welfare of humanity, and commemorates the noble spirit of public service espoused by the late Chairman Byung-chull Lee, who used the pen name Ho-Am.
It was established in 1990 by Kun-Hee Lee, the chairman of Samsung. Awards have been presented to 143 individuals worth a total of 24.4 billion KRW.
2018.04.11 View 9425 -
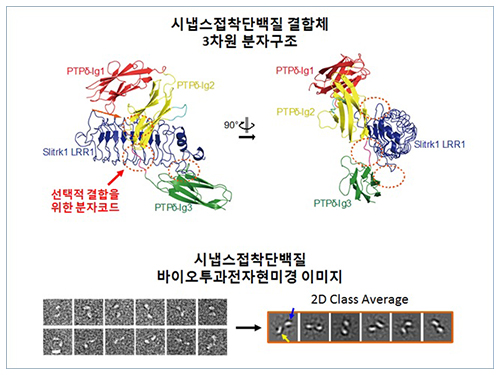 Structure of Neuron-Connecting Synaptic Adhesion Molecules Discovered
A research team has found the three-dimensional structure of synaptic adhesion molecules, which orchestrate synaptogenesis. The research findings also propose the mechanism of synapses in its initial formation. Some brain diseases such as obsessive compulsive disorder (OCD) or bipolar disorders arise from a malfunction of synapses. The team expects the findings to be applied in investigating pathogenesis and developing medicines for such diseases.
The research was conducted by a Master’s candidate Kee Hun Kim, Professor Ji Won Um from Yonsei University, and Professor Beom Seok Park from Eulji University under the guidance of Professor Homin Kim from the Graduate School of Medical Science and Engineering, KAIST, and Professor Jaewon Ko from Yonsei University. Sponsored by the Ministry of Science, ICT and Future Planning and the National Research Foundation of Korea, the research findings were published online in the November 14th issue of Nature Communications.
A protein that exists in the neuronal transmembrane, Slitrk, interacts with the presynaptic leukocyte common antigen-related receptor protein tyrosine phosphatases (LAR-RPTPs) and forms a protein complex. It is involved in the development of synapses in the initial stage, and balances excitatory and inhibitory signals of neurons.
It is known that a disorder in those two proteins cause a malfunction of synapses, resulting in neuropsychosis such as autism, epilepsy, OCD, and bipolar disorders. However, because the structure as well as synaptogenic function of these proteins were not understood, the development of cures could not progress.
The research team discovered the three-dimensional structure of two synaptic adhesion molecules like Slitrk and LAR-RPTPs and identified the regions of interaction through protein crystallography and transmission electron microscopy (TEM). Furthermore, they found that the formation of the synapse is induced after the combination of two synaptic adhesion molecules develops a cluster.
Professor Kim said, “The research findings will serve as a basis of understanding the pathogenesis of brain diseases which arises from a malfunction of synaptic adhesion molecules. In particular, this is a good example in which collaboration between structural biology and neurobiology has led to a fruitful result.” Professor Ko commented that “this will give new directions to synaptic formation-related-researches by revealing the molecular mechanism of synaptic adhesion molecules.”
Figure 1: Overview of the PTPd Ig1–3/Slitrk1 LRR1 complex.
Figure 2: Representative negative-stained electron microscopy images of Slitrk1 Full ectodomain (yellow arrows indicate the horseshoe-shaped LRR domains). The typical horseshoe-shaped structures and the randomness of the relative positions of each LRR domain can be observed from the two-dimensional class averages displayed in the orange box.
Figure 3: Model of the two-step presynaptic differentiation process mediated by the biding of Slitrks to LAR-RPTPs and subsequent lateral assembly of trans-synaptic LAR-RPTPs/Slitrik complexes.
2014.11.28 View 13132
Structure of Neuron-Connecting Synaptic Adhesion Molecules Discovered
A research team has found the three-dimensional structure of synaptic adhesion molecules, which orchestrate synaptogenesis. The research findings also propose the mechanism of synapses in its initial formation. Some brain diseases such as obsessive compulsive disorder (OCD) or bipolar disorders arise from a malfunction of synapses. The team expects the findings to be applied in investigating pathogenesis and developing medicines for such diseases.
The research was conducted by a Master’s candidate Kee Hun Kim, Professor Ji Won Um from Yonsei University, and Professor Beom Seok Park from Eulji University under the guidance of Professor Homin Kim from the Graduate School of Medical Science and Engineering, KAIST, and Professor Jaewon Ko from Yonsei University. Sponsored by the Ministry of Science, ICT and Future Planning and the National Research Foundation of Korea, the research findings were published online in the November 14th issue of Nature Communications.
A protein that exists in the neuronal transmembrane, Slitrk, interacts with the presynaptic leukocyte common antigen-related receptor protein tyrosine phosphatases (LAR-RPTPs) and forms a protein complex. It is involved in the development of synapses in the initial stage, and balances excitatory and inhibitory signals of neurons.
It is known that a disorder in those two proteins cause a malfunction of synapses, resulting in neuropsychosis such as autism, epilepsy, OCD, and bipolar disorders. However, because the structure as well as synaptogenic function of these proteins were not understood, the development of cures could not progress.
The research team discovered the three-dimensional structure of two synaptic adhesion molecules like Slitrk and LAR-RPTPs and identified the regions of interaction through protein crystallography and transmission electron microscopy (TEM). Furthermore, they found that the formation of the synapse is induced after the combination of two synaptic adhesion molecules develops a cluster.
Professor Kim said, “The research findings will serve as a basis of understanding the pathogenesis of brain diseases which arises from a malfunction of synaptic adhesion molecules. In particular, this is a good example in which collaboration between structural biology and neurobiology has led to a fruitful result.” Professor Ko commented that “this will give new directions to synaptic formation-related-researches by revealing the molecular mechanism of synaptic adhesion molecules.”
Figure 1: Overview of the PTPd Ig1–3/Slitrk1 LRR1 complex.
Figure 2: Representative negative-stained electron microscopy images of Slitrk1 Full ectodomain (yellow arrows indicate the horseshoe-shaped LRR domains). The typical horseshoe-shaped structures and the randomness of the relative positions of each LRR domain can be observed from the two-dimensional class averages displayed in the orange box.
Figure 3: Model of the two-step presynaptic differentiation process mediated by the biding of Slitrks to LAR-RPTPs and subsequent lateral assembly of trans-synaptic LAR-RPTPs/Slitrik complexes.
2014.11.28 View 13132 -
 The Journal of Clinical Investigation: Researchers Uncover the Secret Lymphatic Identity of the Schlemm's Canal
The Journal of Clinical Investigation (JCI), a peer-reviewed, top-tier medical journal published by the American Society for Clinical Investigation, carried a commentary entitled “Schlemm’s Canal: More Than Meets the Eye, Lymphatics in Disguise” in the July 25, 2014 issue.
In the commentary, the authors compared a research paper (“Lymphatic regular PROX1 determines Schlemm’s canal integrity and identity”) by Professor Gou-Young Koh of the Graduate School of Medical Science and Engineering at KAIST with research work from the University of Helsinki (article entitled “The Schlemm’s canal is a VEGF-C/VEGFR-3 responsive lymphatic-like vessel”).
The JCI released a press statement dated July 25, 2014 on its commentary. It mentioned that glaucoma, one of the leading causes of blindness worldwide, elevates eye pressure owing to poor drainage of aqueous humor. A specialized structure called “Schlemm’s canal” funnels aqueous humor from the eye back into circulation, which is critical to prevent pressure buildup in the eye. The article discussed the role of Schlemm’s canal in the context of lymphatic vascular characteristics by reviewing two research group’s papers back-to-back.
For the full text of the press release, please visit the link below:
Press Release from the Journal of Clinical Investigation, July 25, 2014
“Researchers uncover the secret lymphatic identity of the Schlemm’s canal”
http://www.eurekalert.org/pub_releases/2014-07/joci-rut072414.php
2014.07.28 View 9209
The Journal of Clinical Investigation: Researchers Uncover the Secret Lymphatic Identity of the Schlemm's Canal
The Journal of Clinical Investigation (JCI), a peer-reviewed, top-tier medical journal published by the American Society for Clinical Investigation, carried a commentary entitled “Schlemm’s Canal: More Than Meets the Eye, Lymphatics in Disguise” in the July 25, 2014 issue.
In the commentary, the authors compared a research paper (“Lymphatic regular PROX1 determines Schlemm’s canal integrity and identity”) by Professor Gou-Young Koh of the Graduate School of Medical Science and Engineering at KAIST with research work from the University of Helsinki (article entitled “The Schlemm’s canal is a VEGF-C/VEGFR-3 responsive lymphatic-like vessel”).
The JCI released a press statement dated July 25, 2014 on its commentary. It mentioned that glaucoma, one of the leading causes of blindness worldwide, elevates eye pressure owing to poor drainage of aqueous humor. A specialized structure called “Schlemm’s canal” funnels aqueous humor from the eye back into circulation, which is critical to prevent pressure buildup in the eye. The article discussed the role of Schlemm’s canal in the context of lymphatic vascular characteristics by reviewing two research group’s papers back-to-back.
For the full text of the press release, please visit the link below:
Press Release from the Journal of Clinical Investigation, July 25, 2014
“Researchers uncover the secret lymphatic identity of the Schlemm’s canal”
http://www.eurekalert.org/pub_releases/2014-07/joci-rut072414.php
2014.07.28 View 9209