AR
-
 KAIST Takes the Lead in Developing Core Technologies for Generative AI National R&D Project
<Professor Sanghoo Park from Department of Nuclear and Quantum Engineering>
KAIST announced on the 15th of August that Professor Sanghoo Park of the Department of Nuclear and Quantum Engineering has won two consecutive awards for early-career researchers at two of the world's most prestigious plasma academic conferences.
Professor Park was selected as a recipient of the Early Career Award (ECA) at the Gaseous Electronics Conference (GEC), hosted by the American Physical Society, on August 4. He was also honored with the Young Investigator Award, presented by the International Plasma Chemistry Society (IPCS), on June 19.
The American Physical Society's GEC Early Career Award is given to only one person worldwide every two years, based on a comprehensive evaluation of research excellence, academic influence, and contributions to the field of plasma. The award will be presented at GEC 2025, which will be held at COEX in Seoul from October 13 to 17.
Established in 1948, the GEC is a leading academic conference in the plasma field with a 77-year history of showcasing key research achievements in all areas of plasma, including physics, chemistry, diagnostics, and application technologies. Recently, advanced application research such as eco-friendly chemical processes, next-generation semiconductors, and atomic layer and ultra-low-temperature etching technology for HBM processes have been gaining attention.
To commemorate the award, Professor Park will give an invited lecture at GEC 2025 on the topic of "Deep-Learning-Based Spectroscopic Data Analysis for Advancing Plasma Spectroscopy." In his lecture, he will use case studies to demonstrate a method that allows even non-specialists to easily and quickly perform spectroscopic data analysis—which is essential for spectroscopy, a key analytical method in modern science including plasma diagnostics—by using deep learning technology.
<Award Ceremony at IPCS (Professor Sanghoo Park on the far left)>
Professor Park also won the Young Investigator Award from the IPCS at the 26th International Symposium on Plasma Chemistry (ISPC 26), which was held in Minneapolis, USA, from June 15 to 20.
First held in 1973, the ISPC (International Symposium on Plasma Chemistry) is a representative international conference in the field of plasma chemistry, held biennially. It covers a wide range of topics, from basic plasma chemical reaction principles to applications in semiconductor processes, green energy, environmental science, and biotechnology. Researchers from industry, academia, and research institutions worldwide share their latest findings at each event. The Young Investigator Award is given to a scientist who has obtained their doctorate within the last 10 years and has demonstrated outstanding achievements in the field.
Professor Park was recognized for his leading research achievements in using plasma-liquid interactions and real-time optical diagnostic technology to environmentally fix nitrogen from the air and precisely control the quantity and types of reactive chemical species that are beneficial to the human body and the environment.
< Photo of a certificate>
Professor Sanghoo Park stated, "It is very meaningful to receive the Young Investigator Award representing Korea at the GEC event, which is being held in Korea for the first time in its history." He added, "I am happy that my consistent interest in and achievements in fundamental plasma science have been recognized, and it is even more significant that the efforts of the KAIST research team have been acknowledged by the world's top conferences."
2025.08.16 View 43
KAIST Takes the Lead in Developing Core Technologies for Generative AI National R&D Project
<Professor Sanghoo Park from Department of Nuclear and Quantum Engineering>
KAIST announced on the 15th of August that Professor Sanghoo Park of the Department of Nuclear and Quantum Engineering has won two consecutive awards for early-career researchers at two of the world's most prestigious plasma academic conferences.
Professor Park was selected as a recipient of the Early Career Award (ECA) at the Gaseous Electronics Conference (GEC), hosted by the American Physical Society, on August 4. He was also honored with the Young Investigator Award, presented by the International Plasma Chemistry Society (IPCS), on June 19.
The American Physical Society's GEC Early Career Award is given to only one person worldwide every two years, based on a comprehensive evaluation of research excellence, academic influence, and contributions to the field of plasma. The award will be presented at GEC 2025, which will be held at COEX in Seoul from October 13 to 17.
Established in 1948, the GEC is a leading academic conference in the plasma field with a 77-year history of showcasing key research achievements in all areas of plasma, including physics, chemistry, diagnostics, and application technologies. Recently, advanced application research such as eco-friendly chemical processes, next-generation semiconductors, and atomic layer and ultra-low-temperature etching technology for HBM processes have been gaining attention.
To commemorate the award, Professor Park will give an invited lecture at GEC 2025 on the topic of "Deep-Learning-Based Spectroscopic Data Analysis for Advancing Plasma Spectroscopy." In his lecture, he will use case studies to demonstrate a method that allows even non-specialists to easily and quickly perform spectroscopic data analysis—which is essential for spectroscopy, a key analytical method in modern science including plasma diagnostics—by using deep learning technology.
<Award Ceremony at IPCS (Professor Sanghoo Park on the far left)>
Professor Park also won the Young Investigator Award from the IPCS at the 26th International Symposium on Plasma Chemistry (ISPC 26), which was held in Minneapolis, USA, from June 15 to 20.
First held in 1973, the ISPC (International Symposium on Plasma Chemistry) is a representative international conference in the field of plasma chemistry, held biennially. It covers a wide range of topics, from basic plasma chemical reaction principles to applications in semiconductor processes, green energy, environmental science, and biotechnology. Researchers from industry, academia, and research institutions worldwide share their latest findings at each event. The Young Investigator Award is given to a scientist who has obtained their doctorate within the last 10 years and has demonstrated outstanding achievements in the field.
Professor Park was recognized for his leading research achievements in using plasma-liquid interactions and real-time optical diagnostic technology to environmentally fix nitrogen from the air and precisely control the quantity and types of reactive chemical species that are beneficial to the human body and the environment.
< Photo of a certificate>
Professor Sanghoo Park stated, "It is very meaningful to receive the Young Investigator Award representing Korea at the GEC event, which is being held in Korea for the first time in its history." He added, "I am happy that my consistent interest in and achievements in fundamental plasma science have been recognized, and it is even more significant that the efforts of the KAIST research team have been acknowledged by the world's top conferences."
2025.08.16 View 43 -
 Professor Mikyoung Lim from Mathematical Sciences to Deliver Keynote at International Conference on Applied Inverse Problems
<Professor Mikyoung Lim from KAIST Department of Mathematical Sciences>
Professor Mikyoung Lim from KAIST Department of Mathematical Sciences gave a plenary talk on "Research on Inverse Problems based on Geometric Function Theory" at AIP 2025 (12th Applied Inverse Problems Conference). AIP is one of the leading international conferences in applied mathematics, organized biennially by the Inverse Problems International Association (IPIA). This year's conference was held from July 28 to August 1 in Rio de Janeiro, Brazil, and consisted of plenary talks, over 40 mini-symposia, and poster sessions. The IPIA began in 2007 and was re-established in 2022 as a non-profit international academic organization officially registered in Germany. At that time, Professor Lim served as an executive committee member for the re-establishment.
During the lecture, Professor Lim's research team introduced a new geometric solution and its applications to boundary value problems for electric/elastic equations, which they have been working on for the past 10 years. In particular, they presented a method for reconstructing partial differential equation boundary value problems into matrix equations and applying them to inverse problems using geometric function theory, a classical theory of complex analysis. A representative achievement was the formalization of the relationship between conformal mappings for simply connected domains in a plane and the measured values of solutions to equations of inhomogeneous conductors into a closed-form expression.
This research led to the plenary talk, as it was recognized for pioneering a new methodology for inverse problem research by connecting geometric function theory and layer potential theory.
2025.08.14 View 63
Professor Mikyoung Lim from Mathematical Sciences to Deliver Keynote at International Conference on Applied Inverse Problems
<Professor Mikyoung Lim from KAIST Department of Mathematical Sciences>
Professor Mikyoung Lim from KAIST Department of Mathematical Sciences gave a plenary talk on "Research on Inverse Problems based on Geometric Function Theory" at AIP 2025 (12th Applied Inverse Problems Conference). AIP is one of the leading international conferences in applied mathematics, organized biennially by the Inverse Problems International Association (IPIA). This year's conference was held from July 28 to August 1 in Rio de Janeiro, Brazil, and consisted of plenary talks, over 40 mini-symposia, and poster sessions. The IPIA began in 2007 and was re-established in 2022 as a non-profit international academic organization officially registered in Germany. At that time, Professor Lim served as an executive committee member for the re-establishment.
During the lecture, Professor Lim's research team introduced a new geometric solution and its applications to boundary value problems for electric/elastic equations, which they have been working on for the past 10 years. In particular, they presented a method for reconstructing partial differential equation boundary value problems into matrix equations and applying them to inverse problems using geometric function theory, a classical theory of complex analysis. A representative achievement was the formalization of the relationship between conformal mappings for simply connected domains in a plane and the measured values of solutions to equations of inhomogeneous conductors into a closed-form expression.
This research led to the plenary talk, as it was recognized for pioneering a new methodology for inverse problem research by connecting geometric function theory and layer potential theory.
2025.08.14 View 63 -
 KAIST Develops AI That Automatically Designs Optimal Drug Candidates for Cancer-Targeting Mutations
< (From left) Ph.D candidate Wonho Zhung, Ph.D cadidate Joongwon Lee , Prof. Woo Young Kim , Ph.D candidate Jisu Seo >
Traditional drug development methods involve identifying a target protin (e.g., a cancer cell receptor) that causes disease, and then searching through countless molecular candidates (potential drugs) that could bind to that protein and block its function. This process is costly, time-consuming, and has a low success rate. KAIST researchers have developed an AI model that, using only information about the target protein, can design optimal drug candidates without any prior molecular data—opening up new possibilities for drug discovery.
KAIST (President Kwang Hyung Lee) announced on the 10th that a research team led by Professor Woo Youn Kim in the Department of Chemistry has developed an AI model named BInD (Bond and Interaction-generating Diffusion model), which can design and optimize drug candidate molecules tailored to a protein’s structure alone—without needing prior information about binding molecules. The model also predicts the binding mechanism (non-covalent interactions) between the drug and the target protein.
The core innovation of this technology lies in its “simultaneous design” approach. Previous AI models either focused on generating molecules or separately evaluating whether the generated molecule could bind to the target protein. In contrast, this new model considers the binding mechanism between the molecule and the protein during the generation process, enabling comprehensive design in one step. Since it pre-accounts for critical factors in protein-ligand binding, it has a much higher likelihood of generating effective and stable molecules. The generation process visually demonstrates how types and positions of atoms, covalent bonds, and interactions are created simultaneously to fit the protein’s binding site.
<Figure 1. Schematic of the diffusion model developed by the research team, which generates molecular structures and non-covalent interactions based on protein structures. Starting from a noise distribution, the model gradually removes noise (via reverse diffusion) to restore the atom positions, types, covalent bond types, and interaction types, thereby generating molecules. Interacting patterns are extracted from prior knowledge of known binding molecules or proteins, and through an inpainting technique, these patterns are kept fixed during the reverse diffusion process to guide the molecular generation.>
Moreover, this model is designed to meet multiple essential drug design criteria simultaneously—such as target binding affinity, drug-like properties, and structural stability. Traditional models often optimized for only one or two goals at the expense of others, but this new model balances various objectives, significantly enhancing its practical applicability.
The research team explained that the AI operates based on a “diffusion model”—a generative approach where a structure becomes increasingly refined from a random state. This is the same type of model used in AlphaFold 3, the 2024 Nobel Chemistry Prize-winning tool for protein-ligand structure generation, which has already demonstrated high efficiency.
Unlike AlphaFold 3, which provides spatial coordinates for atom positions, this study introduced a knowledge-based guide grounded in actual chemical laws—such as bond lengths and protein-ligand distances—enabling more chemically realistic structure generation.
<Figure 2. (Left) Target protein and the original bound molecule; (Right) Examples of molecules designed using the model developed in this study. The values for protein binding affinity (Vina), drug-likeness (QED), and synthetic accessibility (SA) are shown at the bottom.>
Additionally, the team applied an optimization strategy where outstanding binding patterns from prior results are reused. This allowed the model to generate even better drug candidates without additional training. Notably, the AI successfully produced molecules that selectively bind to the mutated residues of EGFR, a cancer-related target protein.
This study is also meaningful because it advances beyond the team’s previous research, which required prior input about the molecular conditions for the interaction pattern of protein binding.
Professor Woo Youn Kim commented that “the newly developed AI can learn and understand the key features required for strong binding to a target protein, and design optimal drug candidate molecules—even without any prior input. This could significantly shift the paradigm of drug development.” He added, “Since this technology generates molecular structures based on principles of chemical interactions, it is expected to enable faster and more reliable drug development.”
Joongwon Lee and Wonho Zhung, PhD students in the Department of Chemistry, participated as co-first authors of this study. The research results were published in the international journal Advanced Science (IF = 14.1) on July 11.
● Paper Title: BInD: Bond and Interaction-Generating Diffusion Model for Multi-Objective Structure-Based Drug Design
● DOI: 10.1002/advs.202502702
This research was supported by the National Research Foundation of Korea and the Ministry of Health and Welfare.
2025.08.12 View 233
KAIST Develops AI That Automatically Designs Optimal Drug Candidates for Cancer-Targeting Mutations
< (From left) Ph.D candidate Wonho Zhung, Ph.D cadidate Joongwon Lee , Prof. Woo Young Kim , Ph.D candidate Jisu Seo >
Traditional drug development methods involve identifying a target protin (e.g., a cancer cell receptor) that causes disease, and then searching through countless molecular candidates (potential drugs) that could bind to that protein and block its function. This process is costly, time-consuming, and has a low success rate. KAIST researchers have developed an AI model that, using only information about the target protein, can design optimal drug candidates without any prior molecular data—opening up new possibilities for drug discovery.
KAIST (President Kwang Hyung Lee) announced on the 10th that a research team led by Professor Woo Youn Kim in the Department of Chemistry has developed an AI model named BInD (Bond and Interaction-generating Diffusion model), which can design and optimize drug candidate molecules tailored to a protein’s structure alone—without needing prior information about binding molecules. The model also predicts the binding mechanism (non-covalent interactions) between the drug and the target protein.
The core innovation of this technology lies in its “simultaneous design” approach. Previous AI models either focused on generating molecules or separately evaluating whether the generated molecule could bind to the target protein. In contrast, this new model considers the binding mechanism between the molecule and the protein during the generation process, enabling comprehensive design in one step. Since it pre-accounts for critical factors in protein-ligand binding, it has a much higher likelihood of generating effective and stable molecules. The generation process visually demonstrates how types and positions of atoms, covalent bonds, and interactions are created simultaneously to fit the protein’s binding site.
<Figure 1. Schematic of the diffusion model developed by the research team, which generates molecular structures and non-covalent interactions based on protein structures. Starting from a noise distribution, the model gradually removes noise (via reverse diffusion) to restore the atom positions, types, covalent bond types, and interaction types, thereby generating molecules. Interacting patterns are extracted from prior knowledge of known binding molecules or proteins, and through an inpainting technique, these patterns are kept fixed during the reverse diffusion process to guide the molecular generation.>
Moreover, this model is designed to meet multiple essential drug design criteria simultaneously—such as target binding affinity, drug-like properties, and structural stability. Traditional models often optimized for only one or two goals at the expense of others, but this new model balances various objectives, significantly enhancing its practical applicability.
The research team explained that the AI operates based on a “diffusion model”—a generative approach where a structure becomes increasingly refined from a random state. This is the same type of model used in AlphaFold 3, the 2024 Nobel Chemistry Prize-winning tool for protein-ligand structure generation, which has already demonstrated high efficiency.
Unlike AlphaFold 3, which provides spatial coordinates for atom positions, this study introduced a knowledge-based guide grounded in actual chemical laws—such as bond lengths and protein-ligand distances—enabling more chemically realistic structure generation.
<Figure 2. (Left) Target protein and the original bound molecule; (Right) Examples of molecules designed using the model developed in this study. The values for protein binding affinity (Vina), drug-likeness (QED), and synthetic accessibility (SA) are shown at the bottom.>
Additionally, the team applied an optimization strategy where outstanding binding patterns from prior results are reused. This allowed the model to generate even better drug candidates without additional training. Notably, the AI successfully produced molecules that selectively bind to the mutated residues of EGFR, a cancer-related target protein.
This study is also meaningful because it advances beyond the team’s previous research, which required prior input about the molecular conditions for the interaction pattern of protein binding.
Professor Woo Youn Kim commented that “the newly developed AI can learn and understand the key features required for strong binding to a target protein, and design optimal drug candidate molecules—even without any prior input. This could significantly shift the paradigm of drug development.” He added, “Since this technology generates molecular structures based on principles of chemical interactions, it is expected to enable faster and more reliable drug development.”
Joongwon Lee and Wonho Zhung, PhD students in the Department of Chemistry, participated as co-first authors of this study. The research results were published in the international journal Advanced Science (IF = 14.1) on July 11.
● Paper Title: BInD: Bond and Interaction-Generating Diffusion Model for Multi-Objective Structure-Based Drug Design
● DOI: 10.1002/advs.202502702
This research was supported by the National Research Foundation of Korea and the Ministry of Health and Welfare.
2025.08.12 View 233 -
 KAIST Develops Bioelectrosynthesis Platform for Switch-Like Precision Control of Cell Signaling
<(From left)Professor Jimin Park, Ph.D candidate Myeongeun Lee, Ph.D cadidate Jaewoong Lee,Professor Jihan Kim>
Cells use various signaling molecules to regulate the nervous, immune, and vascular systems. Among these, nitric oxide (NO) and ammonia (NH₃) play important roles, but their chemical instability and gaseous nature make them difficult to generate or control externally. A KAIST research team has developed a platform that generates specific signaling molecules in situ from a single precursor under an applied electrical signal, enabling switch-like, precise spatiotemporal control of cellular responses. This approach could provide a foundation for future medical technologies such as electroceuticals, electrogenetics, and personalized cell therapies.
KAIST (President Kwang Hyung Lee) announced on August 11 that a research team led by Professor Jimin Park from the Department of Chemical and Biomolecular Engineering, in collaboration with Professor Jihan Kim's group, has developed a 'Bioelectrosynthesis Platform' capable of producing either nitric oxide or ammonia on demand using only an electrical signal. The platform allows control over the timing, spatial range, and duration of cell responses.
Inspired by enzymes involved in nitrite reduction, the researchers implemented an electrochemical strategy that selectively produces nitric oxide or ammonia from a single precursor, nitrite (NO₂⁻). By changing the catalyst, the team generated ammonia or nitric oxide from nitrite using a copper-molybdenum-sulfur catalyst (Cu2MoS4) and an iron-incorporated catalyst (FeCuMS4), respectively.
Through electrochemical measurements and computer simulations, the team revealed that Fe sites in the FeCuMoS4 catalyst bind nitric oxide intermediates more strongly, shifting product selectivity toward nitric oxide. Under the same electrical conditions, the Fe-containing catalyst preferentially produces nitric oxide, whereas the Cu2MoS4 catalyst favors ammonia production.
<Figure 1. Schematic diagram of a bio-electrosynthesis platform that synthesizes a desired signaling substance with an electrical signal (left) and the results of precise cell control using it (right)>
The research team demonstrated biological functionality by using the platform to activate ion channels in human cells. Specifically, electrochemically produced nitric oxide activated TRPV1 channels (responsive to heat and chemical stimuli), while electrochemically produced ammonia induced intracellular alkalinization and activated OTOP1 proton channels. By tuning the applied voltage and electrolysis duration, the team modulated the onset time, spatial extent, and termination of cellular responses, which effectively turned cellular signaling on and off like a switch.
<Figure 2. Experimental results showing the change in the production ratio of nitric oxide and ammonia signaling substances according to the type of catalyst (left) and computational simulation results showing the strong bond between iron and nitric oxide (right)>
Professor Jimin Park said, "This work is significant because it enables precise cellular control by selectively producing signaling molecules with electricity. We believe it has strong potential for applications in electroceutical technologies targeting the nervous system or metabolic disorders."
Myeongeun Lee and Jaewoong Lee, Ph.D. students in the Department of Chemical and Biomolecular Engineering at KAIST, served as the co-first authors. Professor Jihan Kim is a co-author. The paper was published online in 'Angewandte Chemie International Edition' on July 8, 2025 (DOI: 10.1002/ange.202508192).
Reference: https://doi.org/10.1002/ange.202508192
Authors: Myeongeun Lee†, Jaewoong Lee†, Yongha Kim, Changho Lee, Sang Yeon Oh, Prof. Jihan Kim, Prof. Jimin Park*
†These authors contributed equally. *Corresponding author.
2025.08.12 View 147
KAIST Develops Bioelectrosynthesis Platform for Switch-Like Precision Control of Cell Signaling
<(From left)Professor Jimin Park, Ph.D candidate Myeongeun Lee, Ph.D cadidate Jaewoong Lee,Professor Jihan Kim>
Cells use various signaling molecules to regulate the nervous, immune, and vascular systems. Among these, nitric oxide (NO) and ammonia (NH₃) play important roles, but their chemical instability and gaseous nature make them difficult to generate or control externally. A KAIST research team has developed a platform that generates specific signaling molecules in situ from a single precursor under an applied electrical signal, enabling switch-like, precise spatiotemporal control of cellular responses. This approach could provide a foundation for future medical technologies such as electroceuticals, electrogenetics, and personalized cell therapies.
KAIST (President Kwang Hyung Lee) announced on August 11 that a research team led by Professor Jimin Park from the Department of Chemical and Biomolecular Engineering, in collaboration with Professor Jihan Kim's group, has developed a 'Bioelectrosynthesis Platform' capable of producing either nitric oxide or ammonia on demand using only an electrical signal. The platform allows control over the timing, spatial range, and duration of cell responses.
Inspired by enzymes involved in nitrite reduction, the researchers implemented an electrochemical strategy that selectively produces nitric oxide or ammonia from a single precursor, nitrite (NO₂⁻). By changing the catalyst, the team generated ammonia or nitric oxide from nitrite using a copper-molybdenum-sulfur catalyst (Cu2MoS4) and an iron-incorporated catalyst (FeCuMS4), respectively.
Through electrochemical measurements and computer simulations, the team revealed that Fe sites in the FeCuMoS4 catalyst bind nitric oxide intermediates more strongly, shifting product selectivity toward nitric oxide. Under the same electrical conditions, the Fe-containing catalyst preferentially produces nitric oxide, whereas the Cu2MoS4 catalyst favors ammonia production.
<Figure 1. Schematic diagram of a bio-electrosynthesis platform that synthesizes a desired signaling substance with an electrical signal (left) and the results of precise cell control using it (right)>
The research team demonstrated biological functionality by using the platform to activate ion channels in human cells. Specifically, electrochemically produced nitric oxide activated TRPV1 channels (responsive to heat and chemical stimuli), while electrochemically produced ammonia induced intracellular alkalinization and activated OTOP1 proton channels. By tuning the applied voltage and electrolysis duration, the team modulated the onset time, spatial extent, and termination of cellular responses, which effectively turned cellular signaling on and off like a switch.
<Figure 2. Experimental results showing the change in the production ratio of nitric oxide and ammonia signaling substances according to the type of catalyst (left) and computational simulation results showing the strong bond between iron and nitric oxide (right)>
Professor Jimin Park said, "This work is significant because it enables precise cellular control by selectively producing signaling molecules with electricity. We believe it has strong potential for applications in electroceutical technologies targeting the nervous system or metabolic disorders."
Myeongeun Lee and Jaewoong Lee, Ph.D. students in the Department of Chemical and Biomolecular Engineering at KAIST, served as the co-first authors. Professor Jihan Kim is a co-author. The paper was published online in 'Angewandte Chemie International Edition' on July 8, 2025 (DOI: 10.1002/ange.202508192).
Reference: https://doi.org/10.1002/ange.202508192
Authors: Myeongeun Lee†, Jaewoong Lee†, Yongha Kim, Changho Lee, Sang Yeon Oh, Prof. Jihan Kim, Prof. Jimin Park*
†These authors contributed equally. *Corresponding author.
2025.08.12 View 147 -
 'Team Atlanta', in which KAIST Professor Insu Yun research team participated, won the DARPA AI Cyber Challenge in the US, with a prize of 5.5 billion KRW
<Photo1. Group Photo of Team Atlanta>
Team Atlanta, led by Professor Insu Yun of the Department of Electrical and Electronic Engineering at KAIST and Tae-soo Kim, an executive from Samsung Research, along with researchers from POSTECH and Georgia Tech, won the final championship at the AI Cyber Challenge (AIxCC) hosted by the Defense Advanced Research Projects Agency (DARPA). The final was held at the world's largest hacking conference, DEF CON 33, in Las Vegas on August 8 (local time).
With this achievement, the team won a prize of $4 million (approximately 5.5 billion KRW), demonstrating the excellence of their AI-based autonomous cyber defense technology on the global stage.
<Photo2.Championship Commemorative:On the left and right are tournament officials. From the second person, Professor Tae-soo Kim(Samsung Research / Georgia Tech), Researcher Hyeong-seok Han (Samsung Research America), and Professor Insu Yun (KAIST)>
The AI Cyber Challenge is a two-year global competition co-hosted by DARPA and the Advanced Research Projects Agency for Health (ARPA-H). It challenges contestants to automatically analyze, detect, and fix software vulnerabilities using AI-based Cyber Reasoning Systems (CRS). The total prize money for the competition is $29.5 million, with the winning team receiving $4 million.
In the final, Team Atlanta scored a total of 392.76 points, a difference of over 170 points from the second-place team, Trail of Bits, securing a dominant victory. The CRS developed by Team Atlanta successfully and automatically detected various types of vulnerabilities and patched a significant number of them in real time.
Among the 7 finalist teams, an average of 77% of the 70 intentionally injected vulnerabilities were found, and 61% of them were patched. The teams also found 18 additional unknown vulnerabilities in real software, proving the potential of AI security technology.
All CRS technologies, including those of the winning team, will be provided as open-source and are expected to be used to strengthen the security of core infrastructure such as hospitals, water, and power systems.
<Photo3. Final Scoreboard: An overwhelming victory with over 170 points>
Professor Insu Yun of KAIST, a member of Team Atlanta, stated, "I am very happy to have achieved such a great result. This is a remarkable achievement that shows Korea's cyber security research has reached the highest level in the world, and it was meaningful to show the capabilities of Korean researchers on the world stage. I will continue to conduct research to protect the digital safety of the nation and global society through the fusion of AI and security technology."
KAIST President Kwang-hyung Lee stated, "This victory is another example that proves KAIST is a world-leading institution in the field of future cyber security and AI convergence. We will continue to provide full support to our researchers so they can compete and produce results on the world stage."
<Photo4. Results Announcement>
2025.08.10 View 343
'Team Atlanta', in which KAIST Professor Insu Yun research team participated, won the DARPA AI Cyber Challenge in the US, with a prize of 5.5 billion KRW
<Photo1. Group Photo of Team Atlanta>
Team Atlanta, led by Professor Insu Yun of the Department of Electrical and Electronic Engineering at KAIST and Tae-soo Kim, an executive from Samsung Research, along with researchers from POSTECH and Georgia Tech, won the final championship at the AI Cyber Challenge (AIxCC) hosted by the Defense Advanced Research Projects Agency (DARPA). The final was held at the world's largest hacking conference, DEF CON 33, in Las Vegas on August 8 (local time).
With this achievement, the team won a prize of $4 million (approximately 5.5 billion KRW), demonstrating the excellence of their AI-based autonomous cyber defense technology on the global stage.
<Photo2.Championship Commemorative:On the left and right are tournament officials. From the second person, Professor Tae-soo Kim(Samsung Research / Georgia Tech), Researcher Hyeong-seok Han (Samsung Research America), and Professor Insu Yun (KAIST)>
The AI Cyber Challenge is a two-year global competition co-hosted by DARPA and the Advanced Research Projects Agency for Health (ARPA-H). It challenges contestants to automatically analyze, detect, and fix software vulnerabilities using AI-based Cyber Reasoning Systems (CRS). The total prize money for the competition is $29.5 million, with the winning team receiving $4 million.
In the final, Team Atlanta scored a total of 392.76 points, a difference of over 170 points from the second-place team, Trail of Bits, securing a dominant victory. The CRS developed by Team Atlanta successfully and automatically detected various types of vulnerabilities and patched a significant number of them in real time.
Among the 7 finalist teams, an average of 77% of the 70 intentionally injected vulnerabilities were found, and 61% of them were patched. The teams also found 18 additional unknown vulnerabilities in real software, proving the potential of AI security technology.
All CRS technologies, including those of the winning team, will be provided as open-source and are expected to be used to strengthen the security of core infrastructure such as hospitals, water, and power systems.
<Photo3. Final Scoreboard: An overwhelming victory with over 170 points>
Professor Insu Yun of KAIST, a member of Team Atlanta, stated, "I am very happy to have achieved such a great result. This is a remarkable achievement that shows Korea's cyber security research has reached the highest level in the world, and it was meaningful to show the capabilities of Korean researchers on the world stage. I will continue to conduct research to protect the digital safety of the nation and global society through the fusion of AI and security technology."
KAIST President Kwang-hyung Lee stated, "This victory is another example that proves KAIST is a world-leading institution in the field of future cyber security and AI convergence. We will continue to provide full support to our researchers so they can compete and produce results on the world stage."
<Photo4. Results Announcement>
2025.08.10 View 343 -
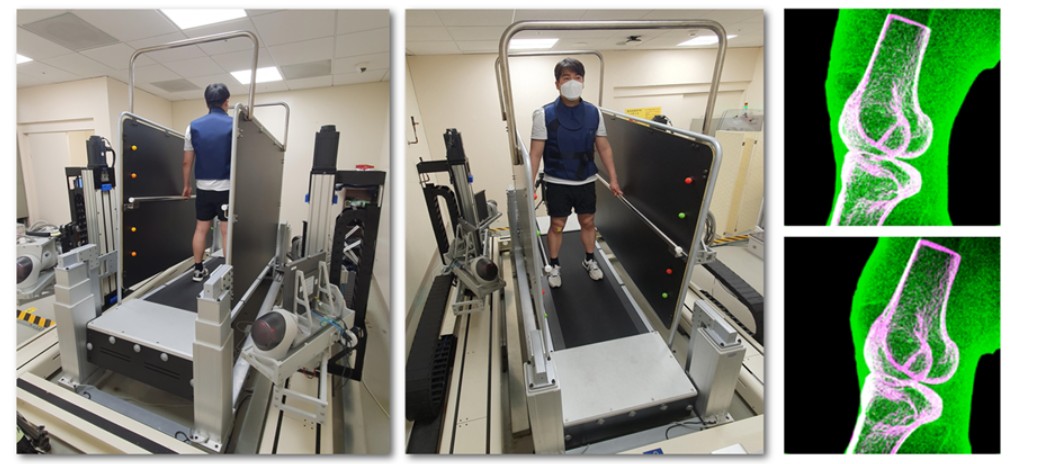 Prof. Seungbum Koo’s Team Receives Clinical Biomechanics Award at the 30th International Society of Biomechanics Conference
<(From Left) Ph.D candidate Jeongseok Oh from KAIST, Dr. Seungwoo Yoon from KAIST, Prof.Joon-Ho Wang from Samsung Medical Center, Prof.Seungbum Koo from KAIST>
Professor Seungbum Koo’s research team received the Clinical Biomechanics Award at the 30th International Society of Biomechanics (ISB) Conference, held in July 2025 in Stockholm, Sweden. The Plenary Lecture was delivered by first author and Ph.D. candidate Jeongseok Oh. This research was conducted in collaboration with Professor Joon-Ho Wang’s team at Samsung Medical Center.
Residual Translational and Rotational Kinematics After Combined ACL and Anterolateral Ligament Reconstruction During Walking
Jeongseok Oh, Seungwoo Yoon, Joon-Ho Wang, Seungbum Koo
The study analyzed gait-related knee joint motion using high-speed biplane X-ray imaging and three-dimensional kinematic reconstruction in 10 healthy individuals and 10 patients who underwent ACL reconstruction with ALL augmentation. The patient group showed excessive anterior translation and internal rotation, suggesting incomplete restoration of normal joint kinematics post-surgery. These findings provide mechanistic insight into the early onset of knee osteoarthritis often reported in this population.'
The ISB conference, held biennially for over 60 years, is the largest international biomechanics meeting. This year, it hosted 1,600 researchers from 46 countries and featured over 1,400 presentations. The Clinical Biomechanics Award is given to one outstanding study selected from five top-rated abstracts invited for full manuscript review. The winning paper is published in Clinical Biomechanics, and the award includes a monetary prize and a Plenary Lecture opportunity.
From 2019 to 2023, Koo and Wang’s teams developed a system with support from the Samsung Future Technology Development Program to track knee motion in real time during treadmill walking, using high-speed biplane X-rays and custom three-dimensional reconstruction software. This system, along with proprietary software that precisely reconstructs the three-dimensional motion of joints, was approved for clinical trials by the Ministry of Food and Drug Safety and installed at Samsung Medical Center. It is being used to quantitatively analyze abnormal joint motion patterns in patients with knee ligament injuries and those who have undergone knee surgery.
Additionally, Jeongseok Oh was named one of five finalists for the David Winter Young Investigator Award, presenting his work during the award session. This award recognizes promising young researchers in biomechanics worldwide.
2025.08.10 View 191
Prof. Seungbum Koo’s Team Receives Clinical Biomechanics Award at the 30th International Society of Biomechanics Conference
<(From Left) Ph.D candidate Jeongseok Oh from KAIST, Dr. Seungwoo Yoon from KAIST, Prof.Joon-Ho Wang from Samsung Medical Center, Prof.Seungbum Koo from KAIST>
Professor Seungbum Koo’s research team received the Clinical Biomechanics Award at the 30th International Society of Biomechanics (ISB) Conference, held in July 2025 in Stockholm, Sweden. The Plenary Lecture was delivered by first author and Ph.D. candidate Jeongseok Oh. This research was conducted in collaboration with Professor Joon-Ho Wang’s team at Samsung Medical Center.
Residual Translational and Rotational Kinematics After Combined ACL and Anterolateral Ligament Reconstruction During Walking
Jeongseok Oh, Seungwoo Yoon, Joon-Ho Wang, Seungbum Koo
The study analyzed gait-related knee joint motion using high-speed biplane X-ray imaging and three-dimensional kinematic reconstruction in 10 healthy individuals and 10 patients who underwent ACL reconstruction with ALL augmentation. The patient group showed excessive anterior translation and internal rotation, suggesting incomplete restoration of normal joint kinematics post-surgery. These findings provide mechanistic insight into the early onset of knee osteoarthritis often reported in this population.'
The ISB conference, held biennially for over 60 years, is the largest international biomechanics meeting. This year, it hosted 1,600 researchers from 46 countries and featured over 1,400 presentations. The Clinical Biomechanics Award is given to one outstanding study selected from five top-rated abstracts invited for full manuscript review. The winning paper is published in Clinical Biomechanics, and the award includes a monetary prize and a Plenary Lecture opportunity.
From 2019 to 2023, Koo and Wang’s teams developed a system with support from the Samsung Future Technology Development Program to track knee motion in real time during treadmill walking, using high-speed biplane X-rays and custom three-dimensional reconstruction software. This system, along with proprietary software that precisely reconstructs the three-dimensional motion of joints, was approved for clinical trials by the Ministry of Food and Drug Safety and installed at Samsung Medical Center. It is being used to quantitatively analyze abnormal joint motion patterns in patients with knee ligament injuries and those who have undergone knee surgery.
Additionally, Jeongseok Oh was named one of five finalists for the David Winter Young Investigator Award, presenting his work during the award session. This award recognizes promising young researchers in biomechanics worldwide.
2025.08.10 View 191 -
 KAIST’s Wearable Robot Design Wins ‘2025 Red Dot Award Best of the Best’
<Professor Hyunjoon Park, M.S candidate Eun-ju Kang, Prospective M.S candidate Jae-seong Kim, undergraduate student Min-su Kim>
A team led by Professor Hyunjoon Park from the Department of Industrial Design won the ‘Best of the Best’ award at the 2025 Red Dot Design Awards, one of the world's top three design awards, for their 'Angel Robotics WSF1 VISION Concept.'
The design for the next-generation wearable robot for people with paraplegia successfully implements functionality, aesthetics, and social inclusion. This latest achievement follows the team's iF Design Award win for the WalkON Suit F1 prototype, which also won a gold medal at the Cybathlon last year. This marks consecutive wins at top-tier international design awards.
KAIST (President Kwang-hyung Lee) announced on the 8th of August that Move Lab, a research team led by Professor Hyunjoon Park from the Department of Industrial Design, won the 'Best of the Best' award in the Design Concept-Professional category at the prestigious '2025 Red Dot Design Awards' for their next-generation wearable robot design, the ‘Angel Robotics WSF1 VISION Concept.’
The German 'Red Dot Design Awards' is one of the world's most well-known design competitions. It is considered one of the world's top three design awards along with Germany’s iF Design Awards and America’s IDEA. The ‘Best of the Best’ award is given to the best design in a category and is awarded only to a very select few of the top designs (within the top 1%) among all Red Dot Award winners.
Professor Hyunjoon Park’s team was honored with the ‘Best of the Best’ award for a user-friendly follow-up development of the ‘WalkON Suit F1 prototype,’ which won a gold medal at the 2024 Cybathlon and an iF Design Award in 2025.
<Figure 1. WSF1 Vision Concept Main Image>
This award-winning design is the result of industry-academic cooperation with Angel Robotics Inc., founded by Professor Kyoungchul Kong from the KAIST Department of Mechanical Engineering. It is a concept design that proposes a next-generation wearable robot (an ultra-personal mobility device) that can be used by people with paraplegia in their daily lives.
The research team focused on transforming Angel Robotics Inc.'s advanced engineering platform into an intuitive and emotional, user-centric experience, implementing a design solution that simultaneously possesses functionality, aesthetics, and social inclusion.
<Figure 2. WSF1 Vision Concept Full Exterior (Front View)>
The WSF1 VISION Concept includes innovative features implemented in Professor Kyoungchul Kong’s Exo Lab, such as:
An autonomous access function where the robot finds the user on its own.
A front-loading mechanism designed for the user to put it on alone while seated.
Multi-directional walking functionality realized through 12 powerful torque actuators and the latest control algorithms.
AI vision technology, along with a multi-visual display system that provides navigation and omnidirectional vision.
This provides users with a safer and more convenient mobility experience.
The strong yet elegant silhouette was achieved through a design process that pursued perfection in proportion, surfaces, and details not seen in existing wearable robots. In particular, the fabric cover that wraps around the entire thigh from the robot's hip joint is a stylish element that respects the wearer's self-esteem and individuality, like fashionable athletic wear. It also acts as a device for the wearer to psychologically feel safe in interacting with the robot and blending in with the general public. This presents a new aesthetic for wearable robots where function and form are harmonized.
<Figure 3. WSF1 Vision Concept's Operating Principle. It walks autonomously and is worn from the front while the user is seated.>
KAIST Professor Hyunjoon Park said of the award, "We are focusing on using technology, aesthetics, and human-centered innovation to present advanced technical solutions as easy, enjoyable, and cool experiences for users. Based on Angel Robotics Inc.'s vision of 'recreating human ability with technology,' the WSF1 VISION Concept aimed to break away from the traditional framework of wearable robots and deliver a design experience that adds dignity, independence, and new style to the user's life."
<Figure 4. WSF1 Vision Concept Detail Image>
A physical model of the WSF1 VISION Concept is scheduled to be unveiled in the Future Hall of the 2025 Gwangju Design Biennale from August 30 to November 2. The theme is 'Po-yong-ji-deok' (the virtue of inclusion), and it will showcase the role of design language in creating an inclusive future society.
<Figure 5. WSF1 Vision Concept: Image of a Person Wearing and Walking>
2025.08.09 View 136
KAIST’s Wearable Robot Design Wins ‘2025 Red Dot Award Best of the Best’
<Professor Hyunjoon Park, M.S candidate Eun-ju Kang, Prospective M.S candidate Jae-seong Kim, undergraduate student Min-su Kim>
A team led by Professor Hyunjoon Park from the Department of Industrial Design won the ‘Best of the Best’ award at the 2025 Red Dot Design Awards, one of the world's top three design awards, for their 'Angel Robotics WSF1 VISION Concept.'
The design for the next-generation wearable robot for people with paraplegia successfully implements functionality, aesthetics, and social inclusion. This latest achievement follows the team's iF Design Award win for the WalkON Suit F1 prototype, which also won a gold medal at the Cybathlon last year. This marks consecutive wins at top-tier international design awards.
KAIST (President Kwang-hyung Lee) announced on the 8th of August that Move Lab, a research team led by Professor Hyunjoon Park from the Department of Industrial Design, won the 'Best of the Best' award in the Design Concept-Professional category at the prestigious '2025 Red Dot Design Awards' for their next-generation wearable robot design, the ‘Angel Robotics WSF1 VISION Concept.’
The German 'Red Dot Design Awards' is one of the world's most well-known design competitions. It is considered one of the world's top three design awards along with Germany’s iF Design Awards and America’s IDEA. The ‘Best of the Best’ award is given to the best design in a category and is awarded only to a very select few of the top designs (within the top 1%) among all Red Dot Award winners.
Professor Hyunjoon Park’s team was honored with the ‘Best of the Best’ award for a user-friendly follow-up development of the ‘WalkON Suit F1 prototype,’ which won a gold medal at the 2024 Cybathlon and an iF Design Award in 2025.
<Figure 1. WSF1 Vision Concept Main Image>
This award-winning design is the result of industry-academic cooperation with Angel Robotics Inc., founded by Professor Kyoungchul Kong from the KAIST Department of Mechanical Engineering. It is a concept design that proposes a next-generation wearable robot (an ultra-personal mobility device) that can be used by people with paraplegia in their daily lives.
The research team focused on transforming Angel Robotics Inc.'s advanced engineering platform into an intuitive and emotional, user-centric experience, implementing a design solution that simultaneously possesses functionality, aesthetics, and social inclusion.
<Figure 2. WSF1 Vision Concept Full Exterior (Front View)>
The WSF1 VISION Concept includes innovative features implemented in Professor Kyoungchul Kong’s Exo Lab, such as:
An autonomous access function where the robot finds the user on its own.
A front-loading mechanism designed for the user to put it on alone while seated.
Multi-directional walking functionality realized through 12 powerful torque actuators and the latest control algorithms.
AI vision technology, along with a multi-visual display system that provides navigation and omnidirectional vision.
This provides users with a safer and more convenient mobility experience.
The strong yet elegant silhouette was achieved through a design process that pursued perfection in proportion, surfaces, and details not seen in existing wearable robots. In particular, the fabric cover that wraps around the entire thigh from the robot's hip joint is a stylish element that respects the wearer's self-esteem and individuality, like fashionable athletic wear. It also acts as a device for the wearer to psychologically feel safe in interacting with the robot and blending in with the general public. This presents a new aesthetic for wearable robots where function and form are harmonized.
<Figure 3. WSF1 Vision Concept's Operating Principle. It walks autonomously and is worn from the front while the user is seated.>
KAIST Professor Hyunjoon Park said of the award, "We are focusing on using technology, aesthetics, and human-centered innovation to present advanced technical solutions as easy, enjoyable, and cool experiences for users. Based on Angel Robotics Inc.'s vision of 'recreating human ability with technology,' the WSF1 VISION Concept aimed to break away from the traditional framework of wearable robots and deliver a design experience that adds dignity, independence, and new style to the user's life."
<Figure 4. WSF1 Vision Concept Detail Image>
A physical model of the WSF1 VISION Concept is scheduled to be unveiled in the Future Hall of the 2025 Gwangju Design Biennale from August 30 to November 2. The theme is 'Po-yong-ji-deok' (the virtue of inclusion), and it will showcase the role of design language in creating an inclusive future society.
<Figure 5. WSF1 Vision Concept: Image of a Person Wearing and Walking>
2025.08.09 View 136 -
 Key Figures in the Establishment of KAIST, Specially Invited to the Presidential Office’s National Appointment Ceremony
KAIST announced on August 6 that Professor Emeritus Jung-Woong Ra from the Department of Electrical Engineering and Won-ki Kwon, former Vice Minister of the Ministry of Science and Technology, who played pivotal roles in the establishment of KAIST, were selected as special guests for the 'National Appointment Ceremony' hosted by the Presidential Office on August 15th.
The Presidential Office selected special invitees across eight categories for the ceremony. These include individuals born in 1945 (referred to as 'Liberation Babies'), those involved in the founding of KAIST in 1971, independence activists and national patriots, overseas workers in Germany and the Middle East, AI industry professionals, residents from regions facing depopulation, leading figures in K-culture, military personnel, firefighters, police officers, families of fallen public servants and victims of social disasters, as well as promising talents in economics, science, culture, and the arts.
Considering the historical significance of its establishment and its symbolic meaning for the development of national science and technology, KAIST Professor Emeritus Jung-Woong Ra, who was a key figure in the establishment of the Department of Electrical Engineering after being appointed as a professor in 1971, and former Vice Minister Kwon Won-ki, who was the first practical leader of the establishment project. Both were officially included on the special invitation list.
Briefing from the Presidential Office regarding the 'National Appointment Ceremony' (2025.07.28) https://www.president.go.kr/newsroom/briefing/grehGMuP
2025.08.06 View 242
Key Figures in the Establishment of KAIST, Specially Invited to the Presidential Office’s National Appointment Ceremony
KAIST announced on August 6 that Professor Emeritus Jung-Woong Ra from the Department of Electrical Engineering and Won-ki Kwon, former Vice Minister of the Ministry of Science and Technology, who played pivotal roles in the establishment of KAIST, were selected as special guests for the 'National Appointment Ceremony' hosted by the Presidential Office on August 15th.
The Presidential Office selected special invitees across eight categories for the ceremony. These include individuals born in 1945 (referred to as 'Liberation Babies'), those involved in the founding of KAIST in 1971, independence activists and national patriots, overseas workers in Germany and the Middle East, AI industry professionals, residents from regions facing depopulation, leading figures in K-culture, military personnel, firefighters, police officers, families of fallen public servants and victims of social disasters, as well as promising talents in economics, science, culture, and the arts.
Considering the historical significance of its establishment and its symbolic meaning for the development of national science and technology, KAIST Professor Emeritus Jung-Woong Ra, who was a key figure in the establishment of the Department of Electrical Engineering after being appointed as a professor in 1971, and former Vice Minister Kwon Won-ki, who was the first practical leader of the establishment project. Both were officially included on the special invitation list.
Briefing from the Presidential Office regarding the 'National Appointment Ceremony' (2025.07.28) https://www.president.go.kr/newsroom/briefing/grehGMuP
2025.08.06 View 242 -
 KAIST Enables On-Site Disease Diagnosis in Just 3 Minutes... Nanozyme Reaction Selectivity Improved 38-Fold
<(From Left) Professor Jinwoo Lee, Ph.D candidate Seonhye Park and Ph.D candidate Daeeun Choi from Chemical & Biomolecular Engineering>
To enable early diagnosis of acute illnesses and effective management of chronic conditions, point-of-care testing (POCT) technology—diagnostics conducted near the patient—is drawing global attention. The key to POCT lies in enzymes that recognize and react precisely with specific substances. However, traditional natural enzymes are expensive and unstable, and nanozymes (enzyme-mimicking catalysts) have suffered from low reaction selectivity. Now, a Korean research team has developed a high-sensitivity sensor platform that achieves 38 times higher selectivity than existing nanozymes and allows disease diagnostics visible to the naked eye within just 3 minutes.
On the 28th, KAIST (President Kwang Hyung Lee) announced that Professor Jinwoo Lee’s research team from the Department of Chemical & Biomolecular Engineering, in collaboration with teams led by Professor Jeong Woo Han at Seoul National University and Professor Moon Il Kim at Gachon University, has developed a new single-atom catalyst that selectively performs only peroxidase-like reactions while maintaining high reaction efficiency.
Using bodily fluids such as blood, urine, or saliva, this diagnostic platform enables test results to be read within minutes even outside hospital settings—greatly improving medical accessibility and ensuring timely treatment. The key lies in the visual detection of biomarkers (disease indicators) through color changes triggered by enzyme reactions. However, natural enzymes are expensive and easily degraded in diagnostic environments, limiting their storage and distribution.
To address this, inorganic nanozyme materials have been developed as substitutes. Yet, they typically lack selectivity—when hydrogen peroxide is used as a substrate, the same catalyst triggers both peroxidase-like reactions (which cause color change) and catalase-like reactions (which remove the substrate), reducing diagnostic signal accuracy.
To control catalyst selectivity at the atomic level, the researchers used an innovative structural design: attaching chlorine (Cl) ligands in a three-dimensional configuration to the central ruthenium (Ru) atom to fine-tune its chemical properties. This enabled them to isolate only the desired diagnostic signal.
<Figure1. The catalyst in this study (ruthenium single-atom catalyst) exhibits peroxidase-like activity with selectivity akin to natural enzymes through three-dimensional directional ligand coordination. Due to the absence of competing catalase activity, selective peroxidase-like reactions proceed under biomimetic conditions. In contrast, conventional single-atom catalysts with active sites arranged on planar surfaces exhibit dual functionality depending on pH. Under neutral conditions, their catalase activity leads to hydrogen peroxide depletion, hindering accurate detection. The catalyst in this study eliminates such interference, enabling direct detection of biomarkers through coupled reactions with oxidases without the need for cumbersome steps like buffer replacement. The ability to simultaneously detect multiple target substances under biomimetic conditions demonstrates the practicality of ruthenium single-atom catalysts for on-site diagnostics>
Experimental results showed that the new catalyst achieved over 38-fold improvement in selectivity compared to existing nanozymes, with significantly increased sensitivity and speed in detecting hydrogen peroxide. Even in near-physiological conditions (pH 6.0), the catalyst maintained its performance, proving its applicability in real-world diagnostics.
By incorporating the catalyst and oxidase into a paper-based sensor, the team created a system that could simultaneously detect four key biomarkers related to health: glucose, lactate, cholesterol, and choline—all with a simple color change.
This platform is broadly applicable across various disease diagnostics and can deliver results within 3 minutes without complex instruments or pH adjustments. The findings show that diagnostic performance can be dramatically improved without changing the platform itself, but rather by engineering the catalyst structure.
<Figure 2.(a) Schematic diagram of the paper sensor (Zone 1: glucose oxidase immobilized; Zone 2: lactate oxidase immobilized; Zone 3: choline oxidase immobilized; Zone 4: cholesterol oxidase immobilized; Zone 5: no oxidase enzyme). (b) Single biomarker (single disease indicator) detection using the ruthenium single‑atom catalyst–based paper sensor.(c) Multiple biomarker (multiple disease indicator) detection using the ruthenium single‑atom catalyst–based paper sensor>
Professor Jinwoo Lee of KAIST commented, “This study is significant in that it simultaneously achieves enzyme-level selectivity and reactivity by structurally designing single-atom catalysts.” He added that “the structure–function-based catalyst design strategy can be extended to the development of various metal-based catalysts and other reaction domains where selectivity is critical.”
Seonhye Park and Daeeun Choi, both Ph.D. candidates at KAIST, are co-first authors. The research was published on July 6, 2025, in the prestigious journal Advanced Materials
-Title: Breaking the Selectivity Barrier of Single-Atom Nanozymes Through Out-of-Plane Ligand Coordinatio
- Authors: Seonhye Park (KAIST, co–first author), Daeeun Choi (KAIST, co–first author), Kyu In Shim (SNU, co–first author), Phuong Thy Nguyen (Gachon Univ., co–first author), Seongbeen Kim (KAIST), Seung Yeop Yi (KAIST), Moon Il Kim (Gachon Univ., corresponding author), Jeong Woo Han (SNU, corresponding author), Jinwoo Lee (KAIST, corresponding author
-DOI: https://doi.org/10.1002/adma.202506480
This research was supported by the Ministry of Science and ICT and the National Research Foundation of Korea (NRF).
2025.07.29 View 501
KAIST Enables On-Site Disease Diagnosis in Just 3 Minutes... Nanozyme Reaction Selectivity Improved 38-Fold
<(From Left) Professor Jinwoo Lee, Ph.D candidate Seonhye Park and Ph.D candidate Daeeun Choi from Chemical & Biomolecular Engineering>
To enable early diagnosis of acute illnesses and effective management of chronic conditions, point-of-care testing (POCT) technology—diagnostics conducted near the patient—is drawing global attention. The key to POCT lies in enzymes that recognize and react precisely with specific substances. However, traditional natural enzymes are expensive and unstable, and nanozymes (enzyme-mimicking catalysts) have suffered from low reaction selectivity. Now, a Korean research team has developed a high-sensitivity sensor platform that achieves 38 times higher selectivity than existing nanozymes and allows disease diagnostics visible to the naked eye within just 3 minutes.
On the 28th, KAIST (President Kwang Hyung Lee) announced that Professor Jinwoo Lee’s research team from the Department of Chemical & Biomolecular Engineering, in collaboration with teams led by Professor Jeong Woo Han at Seoul National University and Professor Moon Il Kim at Gachon University, has developed a new single-atom catalyst that selectively performs only peroxidase-like reactions while maintaining high reaction efficiency.
Using bodily fluids such as blood, urine, or saliva, this diagnostic platform enables test results to be read within minutes even outside hospital settings—greatly improving medical accessibility and ensuring timely treatment. The key lies in the visual detection of biomarkers (disease indicators) through color changes triggered by enzyme reactions. However, natural enzymes are expensive and easily degraded in diagnostic environments, limiting their storage and distribution.
To address this, inorganic nanozyme materials have been developed as substitutes. Yet, they typically lack selectivity—when hydrogen peroxide is used as a substrate, the same catalyst triggers both peroxidase-like reactions (which cause color change) and catalase-like reactions (which remove the substrate), reducing diagnostic signal accuracy.
To control catalyst selectivity at the atomic level, the researchers used an innovative structural design: attaching chlorine (Cl) ligands in a three-dimensional configuration to the central ruthenium (Ru) atom to fine-tune its chemical properties. This enabled them to isolate only the desired diagnostic signal.
<Figure1. The catalyst in this study (ruthenium single-atom catalyst) exhibits peroxidase-like activity with selectivity akin to natural enzymes through three-dimensional directional ligand coordination. Due to the absence of competing catalase activity, selective peroxidase-like reactions proceed under biomimetic conditions. In contrast, conventional single-atom catalysts with active sites arranged on planar surfaces exhibit dual functionality depending on pH. Under neutral conditions, their catalase activity leads to hydrogen peroxide depletion, hindering accurate detection. The catalyst in this study eliminates such interference, enabling direct detection of biomarkers through coupled reactions with oxidases without the need for cumbersome steps like buffer replacement. The ability to simultaneously detect multiple target substances under biomimetic conditions demonstrates the practicality of ruthenium single-atom catalysts for on-site diagnostics>
Experimental results showed that the new catalyst achieved over 38-fold improvement in selectivity compared to existing nanozymes, with significantly increased sensitivity and speed in detecting hydrogen peroxide. Even in near-physiological conditions (pH 6.0), the catalyst maintained its performance, proving its applicability in real-world diagnostics.
By incorporating the catalyst and oxidase into a paper-based sensor, the team created a system that could simultaneously detect four key biomarkers related to health: glucose, lactate, cholesterol, and choline—all with a simple color change.
This platform is broadly applicable across various disease diagnostics and can deliver results within 3 minutes without complex instruments or pH adjustments. The findings show that diagnostic performance can be dramatically improved without changing the platform itself, but rather by engineering the catalyst structure.
<Figure 2.(a) Schematic diagram of the paper sensor (Zone 1: glucose oxidase immobilized; Zone 2: lactate oxidase immobilized; Zone 3: choline oxidase immobilized; Zone 4: cholesterol oxidase immobilized; Zone 5: no oxidase enzyme). (b) Single biomarker (single disease indicator) detection using the ruthenium single‑atom catalyst–based paper sensor.(c) Multiple biomarker (multiple disease indicator) detection using the ruthenium single‑atom catalyst–based paper sensor>
Professor Jinwoo Lee of KAIST commented, “This study is significant in that it simultaneously achieves enzyme-level selectivity and reactivity by structurally designing single-atom catalysts.” He added that “the structure–function-based catalyst design strategy can be extended to the development of various metal-based catalysts and other reaction domains where selectivity is critical.”
Seonhye Park and Daeeun Choi, both Ph.D. candidates at KAIST, are co-first authors. The research was published on July 6, 2025, in the prestigious journal Advanced Materials
-Title: Breaking the Selectivity Barrier of Single-Atom Nanozymes Through Out-of-Plane Ligand Coordinatio
- Authors: Seonhye Park (KAIST, co–first author), Daeeun Choi (KAIST, co–first author), Kyu In Shim (SNU, co–first author), Phuong Thy Nguyen (Gachon Univ., co–first author), Seongbeen Kim (KAIST), Seung Yeop Yi (KAIST), Moon Il Kim (Gachon Univ., corresponding author), Jeong Woo Han (SNU, corresponding author), Jinwoo Lee (KAIST, corresponding author
-DOI: https://doi.org/10.1002/adma.202506480
This research was supported by the Ministry of Science and ICT and the National Research Foundation of Korea (NRF).
2025.07.29 View 501 -
 Vulnerability Found: One Packet Can Paralyze Smartphones
<(From left) Professor Yongdae Kim, PhD candidate Tuan Dinh Hoang, PhD candidate Taekkyung Oh from KAIST, Professor CheolJun Park from Kyung Hee University; and Professor Insu Yun from KAIST>
Smartphones must stay connected to mobile networks at all times to function properly. The core component that enables this constant connectivity is the communication modem (Baseband) inside the device. KAIST researchers, using their self-developed testing framework called 'LLFuzz (Lower Layer Fuzz),' have discovered security vulnerabilities in the lower layers of smartphone communication modems and demonstrated the necessity of standardizing 'mobile communication modem security testing.'
*Standardization: In mobile communication, conformance testing, which verifies normal operation in normal situations, has been standardized. However, standards for handling abnormal packets have not yet been established, hence the need for standardized security testing.
Professor Yongdae Kim's team from the School of Electrical Engineering at KAIST, in a joint research effort with Professor CheolJun Park's team from Kyung Hee University, announced on the 25th of July that they have discovered critical security vulnerabilities in the lower layers of smartphone communication modems. These vulnerabilities can incapacitate smartphone communication with just a single manipulated wireless packet (a data transmission unit in a network). In particular, these vulnerabilities are extremely severe as they can potentially lead to remote code execution (RCE)
The research team utilized their self-developed 'LLFuzz' analysis framework to analyze the lower layer state transitions and error handling logic of the modem to detect security vulnerabilities. LLFuzz was able to precisely extract vulnerabilities caused by implementation errors by comparing and analyzing 3GPP* standard-based state machines with actual device responses.
*3GPP: An international collaborative organization that creates global mobile communication standards.
The research team conducted experiments on 15 commercial smartphones from global manufacturers, including Apple, Samsung Electronics, Google, and Xiaomi, and discovered a total of 11 vulnerabilities. Among these, seven were assigned official CVE (Common Vulnerabilities and Exposures) numbers, and manufacturers applied security patches for these vulnerabilities. However, the remaining four have not yet been publicly disclosed.
While previous security research primarily focused on higher layers of mobile communication, such as NAS (Network Access Stratum) and RRC (Radio Resource Control), the research team concentrated on analyzing the error handling logic of mobile communication's lower layers, which manufacturers have often neglected.
These vulnerabilities occurred in the lower layers of the communication modem (RLC, MAC, PDCP, PHY*), and due to their structural characteristics where encryption or authentication is not applied, operational errors could be induced simply by injecting external signals.
*RLC, MAC, PDCP, PHY: Lower layers of LTE/5G communication, responsible for wireless resource allocation, error control, encryption, and physical layer transmission.
The research team released a demo video showing that when they injected a manipulated wireless packet (malformed MAC packet) into commercial smartphones via a Software-Defined Radio (SDR) device using packets generated on an experimental laptop, the smartphone's communication modem (Baseband) immediately crashed
※ Experiment video: https://drive.google.com/file/d/1NOwZdu_Hf4ScG7LkwgEkHLa_nSV4FPb_/view?usp=drive_link
The video shows data being normally transmitted at 23MB per second on the fast.com page, but immediately after the manipulated packet is injected, the transmission stops and the mobile communication signal disappears. This intuitively demonstrates that a single wireless packet can cripple a commercial device's communication modem.
The vulnerabilities were found in the 'modem chip,' a core component of smartphones responsible for calls, texts, and data communication, making it a very important component.
Qualcomm: Affects over 90 chipsets, including CVE-2025-21477, CVE-2024-23385.
MediaTek: Affects over 80 chipsets, including CVE-2024-20076, CVE-2024-20077, CVE-2025-20659.
Samsung: CVE-2025-26780 (targets the latest chipsets like Exynos 2400, 5400).
Apple: CVE-2024-27870 (shares the same vulnerability as Qualcomm CVE).
The problematic modem chips (communication components) are not only in premium smartphones but also in low-end smartphones, tablets, smartwatches, and IoT devices, leading to the widespread potential for user harm due to their broad diffusion.
Furthermore, the research team experimentally tested 5G vulnerabilities in the lower layers and found two vulnerabilities in just two weeks. Considering that 5G vulnerability checks have not been generally conducted, it is possible that many more vulnerabilities exist in the mobile communication lower layers of baseband chips.
Professor Yongdae Kim explained, "The lower layers of smartphone communication modems are not subject to encryption or authentication, creating a structural risk where devices can accept arbitrary signals from external sources." He added, "This research demonstrates the necessity of standardizing mobile communication modem security testing for smartphones and other IoT devices."
The research team is continuing additional analysis of the 5G lower layers using LLFuzz and is also developing tools for testing LTE and 5G upper layers. They are also pursuing collaborations for future tool disclosure. The team's stance is that "as technological complexity increases, systemic security inspection systems must evolve in parallel."
First author Tuan Dinh Hoang, a Ph.D. student in the School of Electrical Engineering, will present the research results in August at USENIX Security 2025, one of the world's most prestigious conferences in cybersecurity.
※ Paper Title: LLFuzz: An Over-the-Air Dynamic Testing Framework for Cellular Baseband Lower Layers (Tuan Dinh Hoang and Taekkyung Oh, KAIST; CheolJun Park, Kyung Hee Univ.; Insu Yun and Yongdae Kim, KAIST)
※ Usenix paper site: https://www.usenix.org/conference/usenixsecurity25/presentation/hoang (Not yet public), Lab homepage paper: https://syssec.kaist.ac.kr/pub/2025/LLFuzz_Tuan.pdf
※ Open-source repository: https://github.com/SysSec-KAIST/LLFuzz (To be released)
This research was conducted with support from the Institute of Information & Communications Technology Planning & Evaluation (IITP) funded by the Ministry of Science and ICT.
2025.07.25 View 698
Vulnerability Found: One Packet Can Paralyze Smartphones
<(From left) Professor Yongdae Kim, PhD candidate Tuan Dinh Hoang, PhD candidate Taekkyung Oh from KAIST, Professor CheolJun Park from Kyung Hee University; and Professor Insu Yun from KAIST>
Smartphones must stay connected to mobile networks at all times to function properly. The core component that enables this constant connectivity is the communication modem (Baseband) inside the device. KAIST researchers, using their self-developed testing framework called 'LLFuzz (Lower Layer Fuzz),' have discovered security vulnerabilities in the lower layers of smartphone communication modems and demonstrated the necessity of standardizing 'mobile communication modem security testing.'
*Standardization: In mobile communication, conformance testing, which verifies normal operation in normal situations, has been standardized. However, standards for handling abnormal packets have not yet been established, hence the need for standardized security testing.
Professor Yongdae Kim's team from the School of Electrical Engineering at KAIST, in a joint research effort with Professor CheolJun Park's team from Kyung Hee University, announced on the 25th of July that they have discovered critical security vulnerabilities in the lower layers of smartphone communication modems. These vulnerabilities can incapacitate smartphone communication with just a single manipulated wireless packet (a data transmission unit in a network). In particular, these vulnerabilities are extremely severe as they can potentially lead to remote code execution (RCE)
The research team utilized their self-developed 'LLFuzz' analysis framework to analyze the lower layer state transitions and error handling logic of the modem to detect security vulnerabilities. LLFuzz was able to precisely extract vulnerabilities caused by implementation errors by comparing and analyzing 3GPP* standard-based state machines with actual device responses.
*3GPP: An international collaborative organization that creates global mobile communication standards.
The research team conducted experiments on 15 commercial smartphones from global manufacturers, including Apple, Samsung Electronics, Google, and Xiaomi, and discovered a total of 11 vulnerabilities. Among these, seven were assigned official CVE (Common Vulnerabilities and Exposures) numbers, and manufacturers applied security patches for these vulnerabilities. However, the remaining four have not yet been publicly disclosed.
While previous security research primarily focused on higher layers of mobile communication, such as NAS (Network Access Stratum) and RRC (Radio Resource Control), the research team concentrated on analyzing the error handling logic of mobile communication's lower layers, which manufacturers have often neglected.
These vulnerabilities occurred in the lower layers of the communication modem (RLC, MAC, PDCP, PHY*), and due to their structural characteristics where encryption or authentication is not applied, operational errors could be induced simply by injecting external signals.
*RLC, MAC, PDCP, PHY: Lower layers of LTE/5G communication, responsible for wireless resource allocation, error control, encryption, and physical layer transmission.
The research team released a demo video showing that when they injected a manipulated wireless packet (malformed MAC packet) into commercial smartphones via a Software-Defined Radio (SDR) device using packets generated on an experimental laptop, the smartphone's communication modem (Baseband) immediately crashed
※ Experiment video: https://drive.google.com/file/d/1NOwZdu_Hf4ScG7LkwgEkHLa_nSV4FPb_/view?usp=drive_link
The video shows data being normally transmitted at 23MB per second on the fast.com page, but immediately after the manipulated packet is injected, the transmission stops and the mobile communication signal disappears. This intuitively demonstrates that a single wireless packet can cripple a commercial device's communication modem.
The vulnerabilities were found in the 'modem chip,' a core component of smartphones responsible for calls, texts, and data communication, making it a very important component.
Qualcomm: Affects over 90 chipsets, including CVE-2025-21477, CVE-2024-23385.
MediaTek: Affects over 80 chipsets, including CVE-2024-20076, CVE-2024-20077, CVE-2025-20659.
Samsung: CVE-2025-26780 (targets the latest chipsets like Exynos 2400, 5400).
Apple: CVE-2024-27870 (shares the same vulnerability as Qualcomm CVE).
The problematic modem chips (communication components) are not only in premium smartphones but also in low-end smartphones, tablets, smartwatches, and IoT devices, leading to the widespread potential for user harm due to their broad diffusion.
Furthermore, the research team experimentally tested 5G vulnerabilities in the lower layers and found two vulnerabilities in just two weeks. Considering that 5G vulnerability checks have not been generally conducted, it is possible that many more vulnerabilities exist in the mobile communication lower layers of baseband chips.
Professor Yongdae Kim explained, "The lower layers of smartphone communication modems are not subject to encryption or authentication, creating a structural risk where devices can accept arbitrary signals from external sources." He added, "This research demonstrates the necessity of standardizing mobile communication modem security testing for smartphones and other IoT devices."
The research team is continuing additional analysis of the 5G lower layers using LLFuzz and is also developing tools for testing LTE and 5G upper layers. They are also pursuing collaborations for future tool disclosure. The team's stance is that "as technological complexity increases, systemic security inspection systems must evolve in parallel."
First author Tuan Dinh Hoang, a Ph.D. student in the School of Electrical Engineering, will present the research results in August at USENIX Security 2025, one of the world's most prestigious conferences in cybersecurity.
※ Paper Title: LLFuzz: An Over-the-Air Dynamic Testing Framework for Cellular Baseband Lower Layers (Tuan Dinh Hoang and Taekkyung Oh, KAIST; CheolJun Park, Kyung Hee Univ.; Insu Yun and Yongdae Kim, KAIST)
※ Usenix paper site: https://www.usenix.org/conference/usenixsecurity25/presentation/hoang (Not yet public), Lab homepage paper: https://syssec.kaist.ac.kr/pub/2025/LLFuzz_Tuan.pdf
※ Open-source repository: https://github.com/SysSec-KAIST/LLFuzz (To be released)
This research was conducted with support from the Institute of Information & Communications Technology Planning & Evaluation (IITP) funded by the Ministry of Science and ICT.
2025.07.25 View 698 -
 Immune Signals Directly Modulate Brain's Emotional Circuits: Unraveling the Mechanism Behind Anxiety-Inducing Behaviors
KAIST's Department of Brain and Cognitive Sciences, led by Professor Jeong-Tae Kwon, has collaborated with MIT and Harvard Medical School to make a groundbreaking discovery. For the first time globally, their joint research has revealed that cytokines, released during immune responses, directly influence the brain's emotional circuits to regulate anxiety behavior.
The study provided experimental evidence for a bidirectional regulatory mechanism: inflammatory cytokines IL-17A and IL-17C act on specific neurons in the amygdala, a region known for emotional regulation, increasing their excitability and consequently inducing anxiety. Conversely, the anti-inflammatory cytokine IL-10 was found to suppress excitability in these very same neurons, thereby contributing to anxiety alleviation.
In a mouse model, the research team observed that while skin inflammation was mitigated by immunotherapy (IL-17RA antibody), anxiety levels paradoxically rose. This was attributed to elevated circulating IL-17 family cytokines leading to the overactivation of amygdala neurons.
Key finding: Inflammatory cytokines IL-17A/17C promote anxiety by acting on excitable amygdala neurons (via IL-17RA/RE receptors), whereas anti-inflammatory cytokine IL-10 alleviates anxiety by suppressing excitability through IL-10RA receptors on the same neurons.
The researchers further elucidated that the anti-inflammatory cytokine IL-10 works to reduce the excitability of these amygdala neurons, thereby mitigating anxiety responses.
This research marks the first instance of demonstrating that immune responses, such as infections or inflammation, directly impact emotional regulation at the level of brain circuits, extending beyond simple physical reactions. This is a profoundly significant achievement, as it proposes a crucial biological mechanism that interlinks immunity, emotion, and behavior through identical neurons within the brain.
The findings of this research were published in the esteemed international journal Cell on April 17th of this year.
Paper Information:
Title: Inflammatory and anti-inflammatory cytokines bidirectionally modulate amygdala circuits regulating anxiety
Journal: Cell (Vol. 188, 2190–2220), April 17, 2025
DOI: https://doi.org/10.1016/j.cell.2025.03.005
Corresponding Authors: Professor Gloria Choi (MIT), Professor Jun R. Huh (Harvard Medical School)
2025.07.24 View 513
Immune Signals Directly Modulate Brain's Emotional Circuits: Unraveling the Mechanism Behind Anxiety-Inducing Behaviors
KAIST's Department of Brain and Cognitive Sciences, led by Professor Jeong-Tae Kwon, has collaborated with MIT and Harvard Medical School to make a groundbreaking discovery. For the first time globally, their joint research has revealed that cytokines, released during immune responses, directly influence the brain's emotional circuits to regulate anxiety behavior.
The study provided experimental evidence for a bidirectional regulatory mechanism: inflammatory cytokines IL-17A and IL-17C act on specific neurons in the amygdala, a region known for emotional regulation, increasing their excitability and consequently inducing anxiety. Conversely, the anti-inflammatory cytokine IL-10 was found to suppress excitability in these very same neurons, thereby contributing to anxiety alleviation.
In a mouse model, the research team observed that while skin inflammation was mitigated by immunotherapy (IL-17RA antibody), anxiety levels paradoxically rose. This was attributed to elevated circulating IL-17 family cytokines leading to the overactivation of amygdala neurons.
Key finding: Inflammatory cytokines IL-17A/17C promote anxiety by acting on excitable amygdala neurons (via IL-17RA/RE receptors), whereas anti-inflammatory cytokine IL-10 alleviates anxiety by suppressing excitability through IL-10RA receptors on the same neurons.
The researchers further elucidated that the anti-inflammatory cytokine IL-10 works to reduce the excitability of these amygdala neurons, thereby mitigating anxiety responses.
This research marks the first instance of demonstrating that immune responses, such as infections or inflammation, directly impact emotional regulation at the level of brain circuits, extending beyond simple physical reactions. This is a profoundly significant achievement, as it proposes a crucial biological mechanism that interlinks immunity, emotion, and behavior through identical neurons within the brain.
The findings of this research were published in the esteemed international journal Cell on April 17th of this year.
Paper Information:
Title: Inflammatory and anti-inflammatory cytokines bidirectionally modulate amygdala circuits regulating anxiety
Journal: Cell (Vol. 188, 2190–2220), April 17, 2025
DOI: https://doi.org/10.1016/j.cell.2025.03.005
Corresponding Authors: Professor Gloria Choi (MIT), Professor Jun R. Huh (Harvard Medical School)
2025.07.24 View 513 -
 Approaches to Human-Robot Interaction Using Biosignals
<(From left) Dr. Hwa-young Jeong, Professor Kyung-seo Park, Dr. Yoon-tae Jeong, Dr. Ji-hoon Seo, Professor Min-kyu Je, Professor Jung Kim >
A joint research team led by Professor Jung Kim of KAIST Department of Mechanical Engineering and Professor Min-kyu Je of the Department of Electrical and Electronic Engineering recently published a review paper on the latest trends and advancements in intuitive Human-Robot Interaction (HRI) using bio-potential and bio-impedance in the internationally renowned academic journal 'Nature Reviews Electrical Engineering'.
This review paper is the result of a collaborative effort by Dr. Kyung-seo Park (DGIST, co-first author), Dr. Hwa-young Jeong (EPFL, co-first author), Dr. Yoon-tae Jeong (IMEC), and Dr. Ji-hoon Seo (UCSD), all doctoral graduates from the two laboratories. Nature Reviews Electrical Engineering is a review specialized journal in the field of electrical, electronic, and artificial intelligence technology, newly launched by Nature Publishing Group last year. It is known to invite world-renowned scholars in the field through strict selection criteria. Professor Jung Kim's research team's paper, titled "Using bio-potential and bio-impedance for intuitive human-robot interaction," was published on July 18, 2025. (DOI: https://doi.org/10.1038/s44287-025-00191-5)
This review paper explains how biosignals can be used to quickly and accurately detect movement intentions and introduces advancements in movement prediction technology based on neural signals and muscle activity. It also focuses on the crucial role of integrated circuits (ICs) in maximizing low-noise performance and energy efficiency in biosignal sensing, covering thelatest development trends in low-noise, low-power designs for accurately measuring bio-potential and impedance signals.
The review emphasizes the importance of hybrid and multi-modal sensing approaches, presenting the possibility of building robust, intuitive, and scalable HRI systems. The research team stressed that collaboration between sensor and IC design fields is essential for the practical application of biosignal-based HRI systems and stated that interdisciplinary collaboration will play a significant role in the development of next-generation HRI technology. Dr. Hwa-young Jeong, a co-first author of the paper, presented the potential of bio-potential and impedance signals to make human-robot interaction more intuitive and efficient, predicting that it will make significant contributions to the development of HRI technologies such as rehabilitation robots and robotic prostheses using biosignals in the future. This research was supported by several research projects, including the Human Plus Project of the National Research Foundation of Korea.
2025.07.24 View 577
Approaches to Human-Robot Interaction Using Biosignals
<(From left) Dr. Hwa-young Jeong, Professor Kyung-seo Park, Dr. Yoon-tae Jeong, Dr. Ji-hoon Seo, Professor Min-kyu Je, Professor Jung Kim >
A joint research team led by Professor Jung Kim of KAIST Department of Mechanical Engineering and Professor Min-kyu Je of the Department of Electrical and Electronic Engineering recently published a review paper on the latest trends and advancements in intuitive Human-Robot Interaction (HRI) using bio-potential and bio-impedance in the internationally renowned academic journal 'Nature Reviews Electrical Engineering'.
This review paper is the result of a collaborative effort by Dr. Kyung-seo Park (DGIST, co-first author), Dr. Hwa-young Jeong (EPFL, co-first author), Dr. Yoon-tae Jeong (IMEC), and Dr. Ji-hoon Seo (UCSD), all doctoral graduates from the two laboratories. Nature Reviews Electrical Engineering is a review specialized journal in the field of electrical, electronic, and artificial intelligence technology, newly launched by Nature Publishing Group last year. It is known to invite world-renowned scholars in the field through strict selection criteria. Professor Jung Kim's research team's paper, titled "Using bio-potential and bio-impedance for intuitive human-robot interaction," was published on July 18, 2025. (DOI: https://doi.org/10.1038/s44287-025-00191-5)
This review paper explains how biosignals can be used to quickly and accurately detect movement intentions and introduces advancements in movement prediction technology based on neural signals and muscle activity. It also focuses on the crucial role of integrated circuits (ICs) in maximizing low-noise performance and energy efficiency in biosignal sensing, covering thelatest development trends in low-noise, low-power designs for accurately measuring bio-potential and impedance signals.
The review emphasizes the importance of hybrid and multi-modal sensing approaches, presenting the possibility of building robust, intuitive, and scalable HRI systems. The research team stressed that collaboration between sensor and IC design fields is essential for the practical application of biosignal-based HRI systems and stated that interdisciplinary collaboration will play a significant role in the development of next-generation HRI technology. Dr. Hwa-young Jeong, a co-first author of the paper, presented the potential of bio-potential and impedance signals to make human-robot interaction more intuitive and efficient, predicting that it will make significant contributions to the development of HRI technologies such as rehabilitation robots and robotic prostheses using biosignals in the future. This research was supported by several research projects, including the Human Plus Project of the National Research Foundation of Korea.
2025.07.24 View 577