KU
-
 KAIST-KU Joint Research Center Opens
The Joint Research Center partnering KAIST and Khalifa University has been completed and the opening of the KAIST center was held on July 5, 2019, following the opening at Khalifa in April.
The joint research center will explore the most impactful technologies that will change people’s lives in the face of the new industrial environment brought about by the Fourth Industrial Revolution. The breakthroughs include smart transportation and smart healthcare such as wireless electric vehicles, unmanned vehicles, and wearable healthcare devices. The two institutions signed an MOU on the Joint Research Agreement on the Technology Development for the Fourth Industrial Revolution in 2018.
This is the second phase of collaboration following the partnership agreement that was signed in 2010 between the two institutions, which aimed to provide the best science and technology education as well as develop nuclear energy in the UAE.
The Khalifa University delegation, headed by Executive Vice President Arif Sultan Al Hammadi and Senior Vice President of Research and Development Steven Griffiths, flew in to attend the ceremony at KAIST. President Sung-Chul Shin, Vice President for Research Hyun Wook Park, Vice President for Planning and Budget Su-chan Chae, Associate Vice President of the International Office Man-Sung Yim joined and Co-Directors of the Joint Research Center Daniel Choi from Khalifa and Jong-Hyun Kim from KAIST also participated in the opening ceremony.
2019.07.06 View 8192
KAIST-KU Joint Research Center Opens
The Joint Research Center partnering KAIST and Khalifa University has been completed and the opening of the KAIST center was held on July 5, 2019, following the opening at Khalifa in April.
The joint research center will explore the most impactful technologies that will change people’s lives in the face of the new industrial environment brought about by the Fourth Industrial Revolution. The breakthroughs include smart transportation and smart healthcare such as wireless electric vehicles, unmanned vehicles, and wearable healthcare devices. The two institutions signed an MOU on the Joint Research Agreement on the Technology Development for the Fourth Industrial Revolution in 2018.
This is the second phase of collaboration following the partnership agreement that was signed in 2010 between the two institutions, which aimed to provide the best science and technology education as well as develop nuclear energy in the UAE.
The Khalifa University delegation, headed by Executive Vice President Arif Sultan Al Hammadi and Senior Vice President of Research and Development Steven Griffiths, flew in to attend the ceremony at KAIST. President Sung-Chul Shin, Vice President for Research Hyun Wook Park, Vice President for Planning and Budget Su-chan Chae, Associate Vice President of the International Office Man-Sung Yim joined and Co-Directors of the Joint Research Center Daniel Choi from Khalifa and Jong-Hyun Kim from KAIST also participated in the opening ceremony.
2019.07.06 View 8192 -
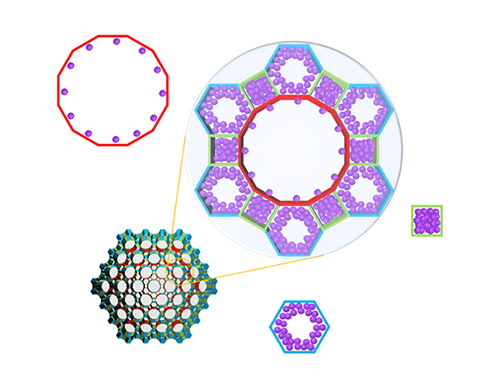 Real-Time Analysis of MOF Adsorption Behavior
Researchers have developed a technology to analyze the adsorption behavior of molecules in each individual pore of a metal organic framework (MOF). This system has large specific surface areas, allowing for the real-time observation of the adsorption process of an MOF, a new material effective for sorting carbon dioxide, hydrogen, and methane.
Accurate measurements and assessments of gas adsorption isotherms are important for characterizing porous materials and developing their applications. The existing technology is only able to measure the amount of gas molecules adsorbed to the material, without directly observing the adsorption behavior.
The research team led by Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability (EEWS) prescribed a real time gas adsorption crystallography system by integrating an existing X-ray diffraction (XRD) measurement device that can provide structural information and a gas adsorption measurement device.
Specifically, the system allowed the observation of a mesoporous MOF that has multiple pores rather than a single pore structure. The research team categorized the adsorption behaviors of MOF molecules by pore type, followed by observations and measurements, resulting in the identification of a stepwise adsorption process that was previously not possible to analyze.
Further, the team systematically and quantitatively analyzed how the pore structure and the type of adsorption molecule affect the adsorption behavior to suggest what type of MOF structure is appropriate as a storage material for each type of adsorption behavior.
Professor Kang said, “We quantitatively analyzed each pore molecule in real time to identify the effects of chemical and structural properties of pores on adsorption behavior.” He continued, “By understanding the real-time adsorption behavior of molecules at the level of the pores that form the material, rather than the whole material, we will be able to apply this technology to develop a new high-capacity storage material.”
This research was published in Nature Chemistry online on May 13, 2019 under the title ‘Isotherms of Individual Pores by Gas Adsorption Crystallography’.
(Figure. Schematic illustration of molecules adsorbed on metal organic frameworks with different pores of various structures, where the In-situ X-ray crystallography has been developed to classify each pore structure and analyze the position of the molecule to determine the amount of molecules adsorbed to each pore.)
2019.06.18 View 39959
Real-Time Analysis of MOF Adsorption Behavior
Researchers have developed a technology to analyze the adsorption behavior of molecules in each individual pore of a metal organic framework (MOF). This system has large specific surface areas, allowing for the real-time observation of the adsorption process of an MOF, a new material effective for sorting carbon dioxide, hydrogen, and methane.
Accurate measurements and assessments of gas adsorption isotherms are important for characterizing porous materials and developing their applications. The existing technology is only able to measure the amount of gas molecules adsorbed to the material, without directly observing the adsorption behavior.
The research team led by Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability (EEWS) prescribed a real time gas adsorption crystallography system by integrating an existing X-ray diffraction (XRD) measurement device that can provide structural information and a gas adsorption measurement device.
Specifically, the system allowed the observation of a mesoporous MOF that has multiple pores rather than a single pore structure. The research team categorized the adsorption behaviors of MOF molecules by pore type, followed by observations and measurements, resulting in the identification of a stepwise adsorption process that was previously not possible to analyze.
Further, the team systematically and quantitatively analyzed how the pore structure and the type of adsorption molecule affect the adsorption behavior to suggest what type of MOF structure is appropriate as a storage material for each type of adsorption behavior.
Professor Kang said, “We quantitatively analyzed each pore molecule in real time to identify the effects of chemical and structural properties of pores on adsorption behavior.” He continued, “By understanding the real-time adsorption behavior of molecules at the level of the pores that form the material, rather than the whole material, we will be able to apply this technology to develop a new high-capacity storage material.”
This research was published in Nature Chemistry online on May 13, 2019 under the title ‘Isotherms of Individual Pores by Gas Adsorption Crystallography’.
(Figure. Schematic illustration of molecules adsorbed on metal organic frameworks with different pores of various structures, where the In-situ X-ray crystallography has been developed to classify each pore structure and analyze the position of the molecule to determine the amount of molecules adsorbed to each pore.)
2019.06.18 View 39959 -
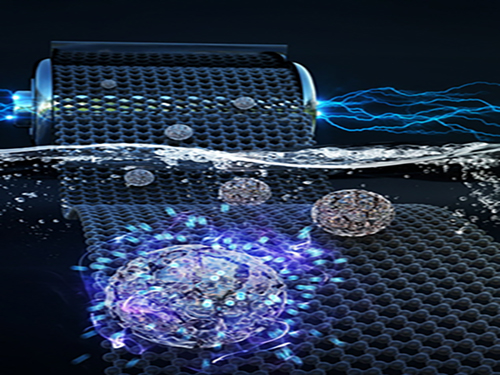 Faster and More Powerful Aqueous Hybrid Capacitor
(Professor Jeung Ku Kang from the Graduate School of EEWS)
A KAIST research team made it one step closer to realizing safe energy storage with high energy density, high power density, and a longer cycle life. This hybrid storage alternative shows power density 100 times faster than conventional batteries, allowing it to be charged within a few seconds. Hence, it is suitable for small portable electronic devices.
Conventional electrochemical energy storage systems, including lithium-ion batteries (LIBs), have a high voltage range and energy density, but are subject to safety issues raised by flammable organic electrolytes, which are used to ensure the beneficial properties. Additionally, they suffer from slow electrochemical reaction rates, which lead to a poor charging rate and low power density with a capacity that fades quickly, resulting in a short cycle life.
On the other hand, capacitors based on aqueous electrolytes are receiving a great deal of attention because they are considered to be safe and environmentally friendly alternatives. However, aqueous electrolytes lag behind energy storage systems based on organic electrolytes in terms of energy density due to their limited voltage range and low capacitance.
Hence, developing aqueous energy storage with high energy density and a long cycle life in addition to the high power density that enables fast charging is the most challenging task for advancing next-generation electrochemical energy storage devices.
Here, Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability and his team developed an aqueous hybrid capacitor (AHC) that boasts high energy density, high power, and excellent cycle stability by synthesizing two types of porous metal oxide nanoclusters on graphene to create positive and negative electrodes for AHCs.
The porous metal oxide nanoparticles are composed of nanoclusters as small as two to three nanometers and have mesopores that are smaller than five nanometers. In these porous structures, ions can be rapidly transferred to the material surfaces and a large number of ions can be stored inside the metal oxide particles very quickly due to their small particle size and large surface area.
The team applied porous manganese oxide on graphene for positive electrodes and porous iron oxide on graphene for negative electrodes to design an aqueous hybrid capacitor that can operate at an extended voltage range of 2V.
Professor Kang said, “This newly developed AHC with high capacity and power density driven from porous metal oxide electrodes will contribute to commercializing a new type of energy storage system. This technology allows ultra-fast charging within several seconds, making it suitable as a power source for mobile devices or electric vehicles where solar energy is directly stored as electricity.”
This research, co-led by Professor Hyung Mo Jeong from Kangwon National University, was published in Advanced Functional Materials on August 15, 2018.
Figure 1. Image that shows properties of porous metal oxide nanoparticles formed on graphene in the aqueous hybrid capacitor
2018.11.09 View 8242
Faster and More Powerful Aqueous Hybrid Capacitor
(Professor Jeung Ku Kang from the Graduate School of EEWS)
A KAIST research team made it one step closer to realizing safe energy storage with high energy density, high power density, and a longer cycle life. This hybrid storage alternative shows power density 100 times faster than conventional batteries, allowing it to be charged within a few seconds. Hence, it is suitable for small portable electronic devices.
Conventional electrochemical energy storage systems, including lithium-ion batteries (LIBs), have a high voltage range and energy density, but are subject to safety issues raised by flammable organic electrolytes, which are used to ensure the beneficial properties. Additionally, they suffer from slow electrochemical reaction rates, which lead to a poor charging rate and low power density with a capacity that fades quickly, resulting in a short cycle life.
On the other hand, capacitors based on aqueous electrolytes are receiving a great deal of attention because they are considered to be safe and environmentally friendly alternatives. However, aqueous electrolytes lag behind energy storage systems based on organic electrolytes in terms of energy density due to their limited voltage range and low capacitance.
Hence, developing aqueous energy storage with high energy density and a long cycle life in addition to the high power density that enables fast charging is the most challenging task for advancing next-generation electrochemical energy storage devices.
Here, Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability and his team developed an aqueous hybrid capacitor (AHC) that boasts high energy density, high power, and excellent cycle stability by synthesizing two types of porous metal oxide nanoclusters on graphene to create positive and negative electrodes for AHCs.
The porous metal oxide nanoparticles are composed of nanoclusters as small as two to three nanometers and have mesopores that are smaller than five nanometers. In these porous structures, ions can be rapidly transferred to the material surfaces and a large number of ions can be stored inside the metal oxide particles very quickly due to their small particle size and large surface area.
The team applied porous manganese oxide on graphene for positive electrodes and porous iron oxide on graphene for negative electrodes to design an aqueous hybrid capacitor that can operate at an extended voltage range of 2V.
Professor Kang said, “This newly developed AHC with high capacity and power density driven from porous metal oxide electrodes will contribute to commercializing a new type of energy storage system. This technology allows ultra-fast charging within several seconds, making it suitable as a power source for mobile devices or electric vehicles where solar energy is directly stored as electricity.”
This research, co-led by Professor Hyung Mo Jeong from Kangwon National University, was published in Advanced Functional Materials on August 15, 2018.
Figure 1. Image that shows properties of porous metal oxide nanoparticles formed on graphene in the aqueous hybrid capacitor
2018.11.09 View 8242 -
 Aqueous Storage Device Needs Only 20 Seconds to Go
(from left: PhD candidate Il Woo Ock and Professor Jeung Ku Kang)
A KAIST research team developed a new hybrid energy storage device that can be charged in less than half a minute. It employs aqueous electrolytes instead of flammable organic solvents, so it is both environmentally friendly and safe. It also facilitates a boosting charge with high energy density, which makes it suitable for portable electronic devices.
Professor Jeung Ku Kang and his team from the Graduate School of Energy, Environment, Water, and Sustainability developed this hybrid energy storage with high energy and power densities along over a long cycle life by assembling fibre-like polymer chain anodes and sub-nanoscale metal oxide cathodes on graphene.
Conventional aqueous electrolyte-based energy storage devices have a limitation for boosting charges and high energy density due to low driving voltage and a shortage of anode materials.
Energy storage device capacity is determined by the two electrodes, and the balance between cathode and anode leads to high stability. In general, two electrodes show differences in electrical properties and differ in ion storage mechanism processes, resulting in poor storage and stability from the imbalance.
The research team came up with new structures and materials to facilitate rapid speed in energy exchange on the surfaces of the electrodes and minimize the energy loss between the two electrodes.
The team made anodes with graphene-based polymer chain materials. The web-like structure of graphene leads to a high surface area, thereby allowing higher capacitance.
For cathode materials, the team used metal oxide in sub-nanoscale structures to elevate atom-by-ion redox reactions. This method realized higher energy density and faster energy exchange while minimizing energy loss.
The developed device can be charged within 20 to 30 seconds using a low-power charging system, such as a USB switching charger or a flexible photovoltaic cell. The developed aqueous hybrid energy device shows more than 100-fold higher power density compared to conventional aqueous batteries and can be rapidly recharged. Further, the device showed high stability with its capacity maintained at 100% at a high charge/discharge current.
Professor Kang said, “This eco-friendly technology can be easily manufactured and is highly applicable. In particular, its high capacity and high stability, compared to existing technologies, could contribute to the commercialization of aqueous capacitors. The device can be rapidly charged using a low-power charging system, and thus can be applied to portable electronic device.”
This research, led by a PhD candidate Il Woo Ock, was published in Advanced Energy Materials on January 15.
Figure 1. Switching wearable LED kit with two AHCs in series charged by a flexible photovoltaic cell
Figure 2. Schematic diagram for aqueous hybrid capacitors
Figure 3. TEM images of anode and cathode
2018.02.28 View 13208
Aqueous Storage Device Needs Only 20 Seconds to Go
(from left: PhD candidate Il Woo Ock and Professor Jeung Ku Kang)
A KAIST research team developed a new hybrid energy storage device that can be charged in less than half a minute. It employs aqueous electrolytes instead of flammable organic solvents, so it is both environmentally friendly and safe. It also facilitates a boosting charge with high energy density, which makes it suitable for portable electronic devices.
Professor Jeung Ku Kang and his team from the Graduate School of Energy, Environment, Water, and Sustainability developed this hybrid energy storage with high energy and power densities along over a long cycle life by assembling fibre-like polymer chain anodes and sub-nanoscale metal oxide cathodes on graphene.
Conventional aqueous electrolyte-based energy storage devices have a limitation for boosting charges and high energy density due to low driving voltage and a shortage of anode materials.
Energy storage device capacity is determined by the two electrodes, and the balance between cathode and anode leads to high stability. In general, two electrodes show differences in electrical properties and differ in ion storage mechanism processes, resulting in poor storage and stability from the imbalance.
The research team came up with new structures and materials to facilitate rapid speed in energy exchange on the surfaces of the electrodes and minimize the energy loss between the two electrodes.
The team made anodes with graphene-based polymer chain materials. The web-like structure of graphene leads to a high surface area, thereby allowing higher capacitance.
For cathode materials, the team used metal oxide in sub-nanoscale structures to elevate atom-by-ion redox reactions. This method realized higher energy density and faster energy exchange while minimizing energy loss.
The developed device can be charged within 20 to 30 seconds using a low-power charging system, such as a USB switching charger or a flexible photovoltaic cell. The developed aqueous hybrid energy device shows more than 100-fold higher power density compared to conventional aqueous batteries and can be rapidly recharged. Further, the device showed high stability with its capacity maintained at 100% at a high charge/discharge current.
Professor Kang said, “This eco-friendly technology can be easily manufactured and is highly applicable. In particular, its high capacity and high stability, compared to existing technologies, could contribute to the commercialization of aqueous capacitors. The device can be rapidly charged using a low-power charging system, and thus can be applied to portable electronic device.”
This research, led by a PhD candidate Il Woo Ock, was published in Advanced Energy Materials on January 15.
Figure 1. Switching wearable LED kit with two AHCs in series charged by a flexible photovoltaic cell
Figure 2. Schematic diagram for aqueous hybrid capacitors
Figure 3. TEM images of anode and cathode
2018.02.28 View 13208 -
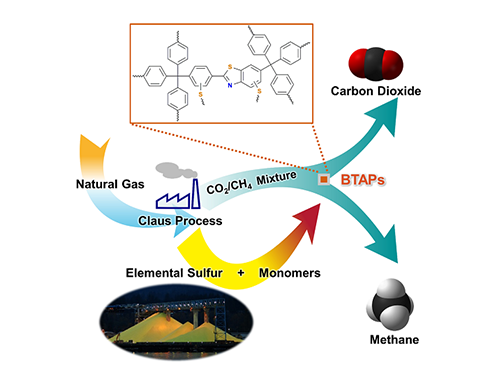 Direct Utilization of Elemental Sulfur for Microporous Polymer Synthesis
Using elemental sulfur as an alternative chemical feedstock, KAIST researchers have produced novel microporous polymers to sift CO2 from methane in natural-gas processing.
Methane, a primary component of natural gas, has emerged recently as an important energy source, largely owing to its abundance and relatively clean nature compared with other fossil fuels. In order to use natural gas as a fuel, however, it must undergo a procedure called “hydrodesulfurization” or “natural gas sweetening” to reduce sulfur-dioxide emissions from combustion of fossil fuels. This process leads to excessive and involuntary production of elemental sulfur. Although sulfur is one of the world’s most versatile and common elements, it has relatively few large-scale applications, mostly for gunpowder and sulfuric acid production.
Thus, the development of synthetic and processing methods to convert sulfur into useful chemicals remains a challenge. A research team led by Professor Ali Coskun from the Graduate School of EEWS (Energy, Environment, Water and Sustainability) at Korea Advanced Institute of Science and Technology (KAIST) has recently introduced a new approach to resolving this problem by employing elemental sulfur directly in the synthesis of microporous polymers for the process of natural-gas sweetening.
Natural gas, containing varying amounts of carbon dioxide (CO2) and hydrogen sulfide (H2S), is generally treated with amine solutions, followed by the regeneration of these solutions at increased temperatures to release captured CO2 and H2S. A two-step separation is involved in removing these gases. The amine solutions first remove H2S, and then CO2 is separated from methane (CH4) with either amine solutions or porous sorbents such as microporous polymers.
Using elemental sulfur and organic linkers, the research team developed a solvent and catalyst-free strategy for the synthesis of ultramicroporous benzothiazole polymers (BTAPs) in quantitative yields. BTAPs were found to be highly porous and showed exceptional physiochemical stability. In-situ chemical impregnation of sulfur within the micropores increased CO2 affinity of the sorbent, while limiting diffusion of CH4. BTAPs, as low-cost, scalable solid-sorbents, showed outstanding CO2 separation ability for flue gas, as well as for natural and landfill gas conditions.
The team noted that: “Each year, millions of tons of elemental sulfur are generated as a by-product of petroleum refining and natural-gas processing, but industries and businesses lacked good ideas for using it. Our research provides a solution: the direct utilization of elemental sulfur into the synthesis of ultramicroporous polymers that can be recycled back into an efficient and sustainable process for CO2 separation. Our novel polymeric materials offer new possibilities for the application of a little-used natural resource, sulfur, to provide a sustainable solution to challenging environmental issues.”
This work was published online in Chem on September 8, 2016 and also highlighted in C&EN (Chemical & Engineering News) by the American Chemical Society (ACS) on September 19, 2016. The research paper was entitled “Direct Utilization of Elemental Sulfur in the Synthesis of Microporous Polymers for Natural Gas Sweetening.” (DOI: 10.1016/j.chempr.2016.08.003)
Figure 1. A Schematic Image of Direct Utilization of Elemental Sulfur
This image shows direct utilization of elemental sulfur in the synthesis of microporous polymers and its gas separation performance.
Figure 2. BTAP’s Breakthrough Experiment under Pre-mixed Gas Conditions
This data presents the breakthrough measurements for CO2-containing binary gas-mixture streams with different feed-gas compositions to investigate the CO2 capture capacity of ultramicroporous benzothiazole polymers (BTAPs) for large-scale applications under simulated conditions of natural and landfill gases.
2016.10.05 View 9661
Direct Utilization of Elemental Sulfur for Microporous Polymer Synthesis
Using elemental sulfur as an alternative chemical feedstock, KAIST researchers have produced novel microporous polymers to sift CO2 from methane in natural-gas processing.
Methane, a primary component of natural gas, has emerged recently as an important energy source, largely owing to its abundance and relatively clean nature compared with other fossil fuels. In order to use natural gas as a fuel, however, it must undergo a procedure called “hydrodesulfurization” or “natural gas sweetening” to reduce sulfur-dioxide emissions from combustion of fossil fuels. This process leads to excessive and involuntary production of elemental sulfur. Although sulfur is one of the world’s most versatile and common elements, it has relatively few large-scale applications, mostly for gunpowder and sulfuric acid production.
Thus, the development of synthetic and processing methods to convert sulfur into useful chemicals remains a challenge. A research team led by Professor Ali Coskun from the Graduate School of EEWS (Energy, Environment, Water and Sustainability) at Korea Advanced Institute of Science and Technology (KAIST) has recently introduced a new approach to resolving this problem by employing elemental sulfur directly in the synthesis of microporous polymers for the process of natural-gas sweetening.
Natural gas, containing varying amounts of carbon dioxide (CO2) and hydrogen sulfide (H2S), is generally treated with amine solutions, followed by the regeneration of these solutions at increased temperatures to release captured CO2 and H2S. A two-step separation is involved in removing these gases. The amine solutions first remove H2S, and then CO2 is separated from methane (CH4) with either amine solutions or porous sorbents such as microporous polymers.
Using elemental sulfur and organic linkers, the research team developed a solvent and catalyst-free strategy for the synthesis of ultramicroporous benzothiazole polymers (BTAPs) in quantitative yields. BTAPs were found to be highly porous and showed exceptional physiochemical stability. In-situ chemical impregnation of sulfur within the micropores increased CO2 affinity of the sorbent, while limiting diffusion of CH4. BTAPs, as low-cost, scalable solid-sorbents, showed outstanding CO2 separation ability for flue gas, as well as for natural and landfill gas conditions.
The team noted that: “Each year, millions of tons of elemental sulfur are generated as a by-product of petroleum refining and natural-gas processing, but industries and businesses lacked good ideas for using it. Our research provides a solution: the direct utilization of elemental sulfur into the synthesis of ultramicroporous polymers that can be recycled back into an efficient and sustainable process for CO2 separation. Our novel polymeric materials offer new possibilities for the application of a little-used natural resource, sulfur, to provide a sustainable solution to challenging environmental issues.”
This work was published online in Chem on September 8, 2016 and also highlighted in C&EN (Chemical & Engineering News) by the American Chemical Society (ACS) on September 19, 2016. The research paper was entitled “Direct Utilization of Elemental Sulfur in the Synthesis of Microporous Polymers for Natural Gas Sweetening.” (DOI: 10.1016/j.chempr.2016.08.003)
Figure 1. A Schematic Image of Direct Utilization of Elemental Sulfur
This image shows direct utilization of elemental sulfur in the synthesis of microporous polymers and its gas separation performance.
Figure 2. BTAP’s Breakthrough Experiment under Pre-mixed Gas Conditions
This data presents the breakthrough measurements for CO2-containing binary gas-mixture streams with different feed-gas compositions to investigate the CO2 capture capacity of ultramicroporous benzothiazole polymers (BTAPs) for large-scale applications under simulated conditions of natural and landfill gases.
2016.10.05 View 9661