research
Scientists at KAIST have developed a new way of making fuel cell membranes using nanoscale fasteners, paving the way for lower-cost, higher-efficiency and more easily manufactured fuel cells.
The internal workings of fuel cells vary, but basically all types mix hydrogen and oxygen to produce a chemical reaction that delivers usable electricity and exhausts ordinary water as a by-product. One of the most efficient types is the proton exchange membrane (PEM) fuel cell, which operates at low enough temperatures to be used in homes and vehicles.
To generate electricity, PEM fuel cells rely on two chemical compartments separated by a permeable catalyst membrane. This membrane acts as an electrolyte; a negative electrode is bonded to one side of the membrane and a positive electrode is bonded to the other. The electrolyte membrane is often based on a polymer of perfluorosulfonic acid. Due to its high cost, however, a less expensive hydrocarbon-based electrolyte membrane has attracted interest in this technology sector.
Until now, the challenge in adopting such a hydrocarbon membrane has been that the interface between the electrode and hydrocarbon membrane is weak. This causes the membrane to delaminate relatively easily, falling apart and losing efficiency with use.
Professor Hee-Tak Kim of the Department of Chemical and Biomolecular Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and his research team have developed a new fastening system that bonds the two materials mechanically rather than chemically. This opens the way to the development of fuel cell membranes that are less expensive, easier to manufacture, stronger and more efficient.
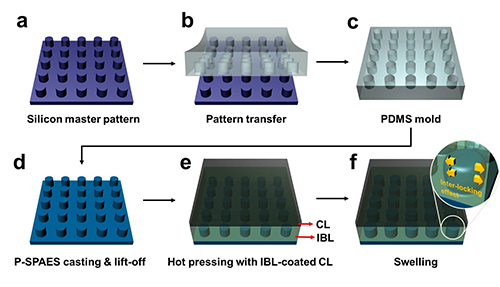
The researchers achieved this by moulding a pattern of tiny cylindrical pillars on the face of the hydrocarbon membrane. The pillars protrude into a softened skin of the electrode with heat. The mechanical bond sets and strengthens as the material cools and absorbs water. The pillar-patterned hydrocarbon membrane is cast using silicone moulds.
Professor Kim said, “This physically fastened bond is almost five times stronger and harder to separate than current bonds between the same layers.”
The new interlocking method also appears to offer a way to bond many types of hydrocarbon membranes that, until now, have been rejected because they couldn’t be fastened robustly. This would make hydrocarbon membranes practical for a number of applications beyond fuel cells such as rechargeable “redox flow” batteries.
The research team is now developing a stronger and more scalable interlocking interface for their nanoscale fasteners.
Picture: Schematic Diagram of the Fabrication of the Pillar P-SPAES Membrane and Its Working Principle of Interlocking Effects
-
research KAIST reveals for the first time the mechanism by which alcohol triggers liver inflammation
<(From left)Dr. Keungmo Yang, Professor Won-Il Jeong, Ph.D candidate Kyurae Kim> Excessive alcohol consumption causes alcoholic liver disease, and about 20% of these cases progress to alcohol-associated steatohepatitis (ASH), which can lead to liver cirrhosis and liver failure. Early diagnosis and treatment are therefore extremely important. A KAIST research team has identified a new molecular mechanism in which alcohol-damaged liver cells increase reactive oxygen species (ROS), leading
2025-07-17 -
research KAIST Successfully Implements 3D Brain-Mimicking Platform with 6x Higher Precision
<(From left) Dr. Dongjo Yoon, Professor Je-Kyun Park from the Department of Bio and Brain Engineering, (upper right) Professor Yoonkey Nam, Dr. Soo Jee Kim> Existing three-dimensional (3D) neuronal culture technology has limitations in brain research due to the difficulty of precisely replicating the brain's complex multilayered structure and the lack of a platform that can simultaneously analyze both structure and function. A KAIST research team has successfully developed an integrated
2025-07-16 -
research KAIST Develops Robots That React to Danger Like Humans
<(From left) Ph.D candidate See-On Park, Professor Jongwon Lee, and Professor Shinhyun Choi> In the midst of the co-development of artificial intelligence and robotic advancements, developing technologies that enable robots to efficiently perceive and respond to their surroundings like humans has become a crucial task. In this context, Korean researchers are gaining attention for newly implementing an artificial sensory nervous system that mimics the sensory nervous system of living org
2025-07-16 -
research A KAIST Team Engineers a Microbial Platform for Efficient Lutein Production
<(From Left) Ph.D. Candidate Hyunmin Eun, Distinguished Professor Sang Yup Lee, , Dr. Cindy Pricilia Surya Prabowo> The application of systems metabolic engineering strategies, along with the construction of an electron channeling system, has enabled the first gram-per-liter scale production of lutein from Corynebacterium glutamicum, providing a viable alternative to plant-derived lutein production. A research group at KAIST has successfully engineered a microbial strain capable o
2025-07-14 -
research KAIST Ushers in Era of Predicting ‘Optimal Alloys’ Using AI, Without High-Temperature Experiments
<Picture1.(From Left) Prof. Seungbum Hong, Ph.D candidate Youngwoo Choi> Steel alloys used in automobiles and machinery parts are typically manufactured through a melting process at high temperatures. The phenomenon where the components remain unchanged during melting is called “congruent melting.” KAIST researchers have now addressed this process—traditionally only possible through high-temperature experiments—using artificial intelligence (AI). This study dra
2025-07-14