Nature
-
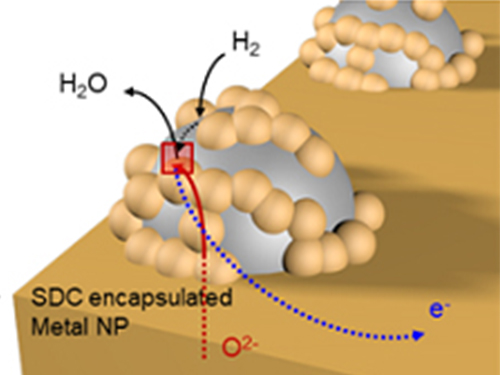 Unravelling Inherent Electrocatalysis to Improve the Performance of Hydrogen Fuel Cells
(Figure 1. Electrode structure for the precise evaluation of the metal nanoparticles’ electrochemical catalytic characteristics at a high temperature.)
A KAIST team presented an ideal electrode design to enhance the performance of high-temperature fuel cells. The new analytical platform with advanced nanoscale patterning method quantitatively revealed the electrochemical value of metal nanoparticles dispersed on the oxide electrode, thus leading to electrode design directions that can be used in a variety of eco-friendly energy technologies.
The team, working under Professor WooChul Jung and Professor Sang Ouk Kim at the Department of Materials Science and Engineering, described an accurate analysis of the reactivity of oxide electrodes boosted by metal nanoparticles, where all particles participate in the reaction. They identified how the metal catalysts activate hydrogen electro-oxidation on the ceria-based electrode surface and quantify how rapidly the reaction rate increases with the proper choice of metals.
Metal nanoparticles with diameters of 10 nanometers or less have become a key component in high-performance heterogeneous catalysts, primarily serving as a catalytic activator. Recent experimental and theoretical findings suggest that the optimization of the chemical nature at the metal and support interfaces is essential for performance improvement.
However, the high cost associated with cell fabrication and operation as well as poorer stability of metal nanoparticles at high temperatures have been a long-standing challenge. To solve this problem, the team utilized a globally recognized metal nano patterning technology that uses block copolymer self-assembled nano templates and succeeded in uniformly synthesizing metal particles 10 nanometers in size on the surface of oxide fuel cell electrodes. They also developed a technology to accurately analyze the catalyst characteristics of single particles at high temperatures and maximize the performance of a fuel cell with minimal catalyst use.
The research team confirmed that platinum, which is a commonly used metal catalyst, could boost fuel cell performance by as much as 21 times even at an amount of 300 nanograms, which only costs about 0.015 KRW.
The team quantitatively identified and compared the characteristics of widely used metal catalysts other than platinum, such as palladium, gold, and cobalt, and also elucidated the precise principle of catalyst performance through theoretical analysis.
(Figure 2. Comparison of the electrochemical catalytic characteristics for various 10nm metal nanoparticles (platinum, palladium, cobalt, gold) at a high temperature.)
Professor Jung said, "We have broken the conventional methods of increasing the amount of catalyst which have deemed inefficient and expensive. Our results suggest a clear idea for high performance fuel cells using very small amounts of nanoparticles. This technology can be applied to many different industrial fields, advancing the commercialization of eco-friendly energy technologies such as fuel cells that generate electricity and electrolytic cells that produce hydrogen from water.”
The research has been published as the cover article of Nature Nanotechnology in the March issue. This research was carried out with support from the Nano-Material Technology Development Program through the National Research Foundation of Korea.
2019.03.28 View 31757
Unravelling Inherent Electrocatalysis to Improve the Performance of Hydrogen Fuel Cells
(Figure 1. Electrode structure for the precise evaluation of the metal nanoparticles’ electrochemical catalytic characteristics at a high temperature.)
A KAIST team presented an ideal electrode design to enhance the performance of high-temperature fuel cells. The new analytical platform with advanced nanoscale patterning method quantitatively revealed the electrochemical value of metal nanoparticles dispersed on the oxide electrode, thus leading to electrode design directions that can be used in a variety of eco-friendly energy technologies.
The team, working under Professor WooChul Jung and Professor Sang Ouk Kim at the Department of Materials Science and Engineering, described an accurate analysis of the reactivity of oxide electrodes boosted by metal nanoparticles, where all particles participate in the reaction. They identified how the metal catalysts activate hydrogen electro-oxidation on the ceria-based electrode surface and quantify how rapidly the reaction rate increases with the proper choice of metals.
Metal nanoparticles with diameters of 10 nanometers or less have become a key component in high-performance heterogeneous catalysts, primarily serving as a catalytic activator. Recent experimental and theoretical findings suggest that the optimization of the chemical nature at the metal and support interfaces is essential for performance improvement.
However, the high cost associated with cell fabrication and operation as well as poorer stability of metal nanoparticles at high temperatures have been a long-standing challenge. To solve this problem, the team utilized a globally recognized metal nano patterning technology that uses block copolymer self-assembled nano templates and succeeded in uniformly synthesizing metal particles 10 nanometers in size on the surface of oxide fuel cell electrodes. They also developed a technology to accurately analyze the catalyst characteristics of single particles at high temperatures and maximize the performance of a fuel cell with minimal catalyst use.
The research team confirmed that platinum, which is a commonly used metal catalyst, could boost fuel cell performance by as much as 21 times even at an amount of 300 nanograms, which only costs about 0.015 KRW.
The team quantitatively identified and compared the characteristics of widely used metal catalysts other than platinum, such as palladium, gold, and cobalt, and also elucidated the precise principle of catalyst performance through theoretical analysis.
(Figure 2. Comparison of the electrochemical catalytic characteristics for various 10nm metal nanoparticles (platinum, palladium, cobalt, gold) at a high temperature.)
Professor Jung said, "We have broken the conventional methods of increasing the amount of catalyst which have deemed inefficient and expensive. Our results suggest a clear idea for high performance fuel cells using very small amounts of nanoparticles. This technology can be applied to many different industrial fields, advancing the commercialization of eco-friendly energy technologies such as fuel cells that generate electricity and electrolytic cells that produce hydrogen from water.”
The research has been published as the cover article of Nature Nanotechnology in the March issue. This research was carried out with support from the Nano-Material Technology Development Program through the National Research Foundation of Korea.
2019.03.28 View 31757 -
 Blue-enriched White Light to Wake You Up in the Morning
(from left: Professor Hyun Jung Chung, Professor Hyeon-Jeong Suk, Taesu Kim and Professor Kyungah Choi)
Here is a good news for those of who have difficulty with morning alertness. A KAIST research team proposed that a blue-enriched LED light can effectively help people overcome morning drowsiness. This study will provide the basis for major changes in future lighting strategies and thereby help create better indoor environments.
Considerable research has been devoted to unmasking circadian rhythms. The 2017 Nobel Prize in Physiology or Medicine went to Jeffrey C. Hall, Michael Rosbash, and Michael W. Young for unveiling the molecular mechanisms that control circadian rhythms. In particular, the relationship between light and its physiological effects has been investigated since the discovery of a novel, third type of photoreceptor in the human retina in the early 2000s. Rods and cones regulate visual effects, while the third type, photosensitive retinal ganglion cells, regulate a large variety of biological and behavioral processes including melatonin and cortisol secretion, alertness, and functional magnetic resonance imaging (fMRI).
Initial studies on light sources have shown that blue monochromatic, fully saturated lights are effective for stimulating physiological responses, but the relative effectiveness of commercially available white light sources is less well understood. Moreover, the research was more focused on the negative effects of blue light; for instance, when people are exposed to blue light at night, they have trouble achieving deep sleep because the light restrains melatonin secretion.
However, Professor Hyeon-Jeong Suk and Professor Kyungah Choi from the Department of Industrial Design and their team argue that the effects of blue-enriched morning light on physiological responses are time dependent, and that it has positive effects on melatonin levels and the subjective perception of alertness, mood, and visual comfort compared with warm white light.
The team conducted an experiment with 15 university students. They investigated whether an hour of morning light exposure with different chromaticity would affect their physiological and subjective responses differently. The decline of melatonin levels was significantly greater after the exposure to blue-enriched white light in comparison with warm white light.
Professor Suk said, “Light takes a huge part of our lives since we spend most of our time indoors. Light is one of the most powerful tools to affect changes in how we perceive and experience the environment around us.”
Professor Choi added, “When we investigate all of the psychological and physiological effects of light, we see there is much more to light than just efficient quantities. I believe that human-centric lighting strategies could be applied to a variety of environments, including residential areas, learning environments, and working spaces to improve our everyday lives.”
This research was collaborated with Professor Hyun Jung Chung from the Graduate School of Nanoscience and Technology and was published in Scientific Reports (10.1038/s41598-018-36791-5) on January 23, 2019.
Figure 1. Changes in melatonin secretion during day and night time
2019.03.06 View 13116
Blue-enriched White Light to Wake You Up in the Morning
(from left: Professor Hyun Jung Chung, Professor Hyeon-Jeong Suk, Taesu Kim and Professor Kyungah Choi)
Here is a good news for those of who have difficulty with morning alertness. A KAIST research team proposed that a blue-enriched LED light can effectively help people overcome morning drowsiness. This study will provide the basis for major changes in future lighting strategies and thereby help create better indoor environments.
Considerable research has been devoted to unmasking circadian rhythms. The 2017 Nobel Prize in Physiology or Medicine went to Jeffrey C. Hall, Michael Rosbash, and Michael W. Young for unveiling the molecular mechanisms that control circadian rhythms. In particular, the relationship between light and its physiological effects has been investigated since the discovery of a novel, third type of photoreceptor in the human retina in the early 2000s. Rods and cones regulate visual effects, while the third type, photosensitive retinal ganglion cells, regulate a large variety of biological and behavioral processes including melatonin and cortisol secretion, alertness, and functional magnetic resonance imaging (fMRI).
Initial studies on light sources have shown that blue monochromatic, fully saturated lights are effective for stimulating physiological responses, but the relative effectiveness of commercially available white light sources is less well understood. Moreover, the research was more focused on the negative effects of blue light; for instance, when people are exposed to blue light at night, they have trouble achieving deep sleep because the light restrains melatonin secretion.
However, Professor Hyeon-Jeong Suk and Professor Kyungah Choi from the Department of Industrial Design and their team argue that the effects of blue-enriched morning light on physiological responses are time dependent, and that it has positive effects on melatonin levels and the subjective perception of alertness, mood, and visual comfort compared with warm white light.
The team conducted an experiment with 15 university students. They investigated whether an hour of morning light exposure with different chromaticity would affect their physiological and subjective responses differently. The decline of melatonin levels was significantly greater after the exposure to blue-enriched white light in comparison with warm white light.
Professor Suk said, “Light takes a huge part of our lives since we spend most of our time indoors. Light is one of the most powerful tools to affect changes in how we perceive and experience the environment around us.”
Professor Choi added, “When we investigate all of the psychological and physiological effects of light, we see there is much more to light than just efficient quantities. I believe that human-centric lighting strategies could be applied to a variety of environments, including residential areas, learning environments, and working spaces to improve our everyday lives.”
This research was collaborated with Professor Hyun Jung Chung from the Graduate School of Nanoscience and Technology and was published in Scientific Reports (10.1038/s41598-018-36791-5) on January 23, 2019.
Figure 1. Changes in melatonin secretion during day and night time
2019.03.06 View 13116 -
 New Catalyst for Synthesizing Chiral Molecules Selectively
(from left: Dr. Yoonsu Park and Professor Sukbok Chang from the Department of Chemistry)
Molecules in nature often have “twin” molecules that look identical. In particular, the twin molecules that look like mirror images to each other are called enantiomers. However, even though they have the same type and number of elements, these twin molecules exhibit completely different properties.
Professor Sukbok Chang and Dr. Yoonsu Park from the Department of Chemistry developed a new catalyst capable of selectively synthesizing only one of the two enantiomers. Using this catalyst, the have succeeded in manufacturing the chiral lactam, an essential ingredient in pharmaceuticals, from a hydrocarbon compound.
Enantiomerism or chirality is considered very important for drug development. Biomaterials, such as DNAs and proteins also have chiral properties, but they exhibit different physiological activities depending on the types of drugs. One type of the enantiomer could be useful while the other is toxic. Hence, the technology for selective synthesizing (i.e. asymmetric synthesis) is required, but it is still regarded as a great challenge faced by modern chemistry to date.
The researchers solved this problem by developing a new catalyst. Earlier they presented their research on developing an iridium catalyst that converts hydrocarbons into high value γ-lactam compounds, and published it in Science in March 2018. However, the developed catalyst still had a limitation that both types of enantiomers are obtained without selectivity.
In this study, they found that among dozens of other catalyst candidates, iridium catalysts with chiral diamine scaffolds were able to select the correct enantiomer with a selectivity of 99% or more. This novel catalyst can be used to synthesize the various chiral γ-lactam as required. A left-handed γ-lactam and a right-handed γ-lactam can be produced using a left-handed iridium catalyst and a right-handed iridium catalyst, respectively.
They analyzed the reason for the high selectivity through computational chemistry simulations. They identified that temporal hydrogen bonding occurred between the chiral diamine catalysts and the hydrocarbon compound during the reaction. As a result of the hydrogen bonding, the formation of the left-handed lactam was boosted.
With their new catalyst, they also succeeded in synthesizing chiral lactam compounds with different structures. By using inexpensive and readily available feedstock hydrocarbons, the researchers produced a group of chiral lactams in different shapes. As their chirality and diverse structures enable lactams to function as an active compound in the body for antibiotic, anti-inflammatory, or anti-tumoral functions, this study may facilitate the development of potential drugs in a more efficient and cheaper way.
Professor Chang said, “We hope that our research on selectively producing core units of effective drugs will lead to developing new drugs that demonstrate fewer side-effects and higher efficacy. There are also economic advantages of this research because it uses hydrocarbon compounds, which can be abundantly found in nature, to produce high-value raw materials.
This research was published in Nature Catalysis(10.1038/s41929-019-0230-x) on February 19, 2019.
Figure 1. Asymmetric formation of chiral γ-lactam
Figure 2. Outline of research outcome
2019.03.05 View 8352
New Catalyst for Synthesizing Chiral Molecules Selectively
(from left: Dr. Yoonsu Park and Professor Sukbok Chang from the Department of Chemistry)
Molecules in nature often have “twin” molecules that look identical. In particular, the twin molecules that look like mirror images to each other are called enantiomers. However, even though they have the same type and number of elements, these twin molecules exhibit completely different properties.
Professor Sukbok Chang and Dr. Yoonsu Park from the Department of Chemistry developed a new catalyst capable of selectively synthesizing only one of the two enantiomers. Using this catalyst, the have succeeded in manufacturing the chiral lactam, an essential ingredient in pharmaceuticals, from a hydrocarbon compound.
Enantiomerism or chirality is considered very important for drug development. Biomaterials, such as DNAs and proteins also have chiral properties, but they exhibit different physiological activities depending on the types of drugs. One type of the enantiomer could be useful while the other is toxic. Hence, the technology for selective synthesizing (i.e. asymmetric synthesis) is required, but it is still regarded as a great challenge faced by modern chemistry to date.
The researchers solved this problem by developing a new catalyst. Earlier they presented their research on developing an iridium catalyst that converts hydrocarbons into high value γ-lactam compounds, and published it in Science in March 2018. However, the developed catalyst still had a limitation that both types of enantiomers are obtained without selectivity.
In this study, they found that among dozens of other catalyst candidates, iridium catalysts with chiral diamine scaffolds were able to select the correct enantiomer with a selectivity of 99% or more. This novel catalyst can be used to synthesize the various chiral γ-lactam as required. A left-handed γ-lactam and a right-handed γ-lactam can be produced using a left-handed iridium catalyst and a right-handed iridium catalyst, respectively.
They analyzed the reason for the high selectivity through computational chemistry simulations. They identified that temporal hydrogen bonding occurred between the chiral diamine catalysts and the hydrocarbon compound during the reaction. As a result of the hydrogen bonding, the formation of the left-handed lactam was boosted.
With their new catalyst, they also succeeded in synthesizing chiral lactam compounds with different structures. By using inexpensive and readily available feedstock hydrocarbons, the researchers produced a group of chiral lactams in different shapes. As their chirality and diverse structures enable lactams to function as an active compound in the body for antibiotic, anti-inflammatory, or anti-tumoral functions, this study may facilitate the development of potential drugs in a more efficient and cheaper way.
Professor Chang said, “We hope that our research on selectively producing core units of effective drugs will lead to developing new drugs that demonstrate fewer side-effects and higher efficacy. There are also economic advantages of this research because it uses hydrocarbon compounds, which can be abundantly found in nature, to produce high-value raw materials.
This research was published in Nature Catalysis(10.1038/s41929-019-0230-x) on February 19, 2019.
Figure 1. Asymmetric formation of chiral γ-lactam
Figure 2. Outline of research outcome
2019.03.05 View 8352 -
 New LSB with Theoretical Capacity over 90%
(Professor Hee-Tak Kim and Hyunwon Chu)
A KAIST research team has developed a lithium sulfur battery (LSB) that realizes 92% of the theoretical capacity and an areal capacity of 4mAh/cm2.
LSBs are gaining a great deal of attention as an alternative for lithium ion batteries (LIBs) because they have a theoretical energy density up to six to seven times higher than that of LIBs, and can be manufactured in a more cost-effective way.
However, LSBs face the obstacle of having a capacity reaching its theoretical maximum because they are prone to uncontrolled growth of lithium sulfide on the electrodes, which leads to blocking electron transfer.
To address the problem of electrode passivation, researchers introduced additional conductive agent into the electrode; however, it drastically lowered the energy density of LSBs, making it difficult to exceed 70% of the theoretical capacity.
Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering and his team replaced the lithium salt anions used in conventional LSB electrolytes with anions with a high donor number. The team successfully induced the three-dimensional growth of lithium sulfide on electrode surfaces and efficiently delayed the electrode passivation.
Based on this electrolyte design, the research team achieved 92% of the theoretical capacity with their high-capacity sulfur electrode (4mAh/cm2), which is equivalent to that of conventional LIB cathode. Furthermore, they were able to form a stable passivation film on the surface of the lithium anode and exhibited stable operation over 100 cycles.
This technology, which can be flexibly used with various types of sulfur electrodes, can mark a new milestone in the battery industry.
Professor Kim said, “We proposed a new physiochemical principle to overcome the limitations of conventional LSBs. I believe our achievement of obtaining 90% of the LBSs’ theoretical capacity without any capacity loss after 100 cycles will become a new milestone.”
This research, first-authored by Hyunwon Chu and Hyungjun Noh, was published in Nature Communications on January 14, 2019. It was also selected in the editor’s highlight for its outstanding achievements.
Figure 1. Lithium sulfur growth and its deposition mechanism for different sulfide growth behaviors
Figure 2. Capacity and cycle life characteristics of the LSBs
2019.02.11 View 9105
New LSB with Theoretical Capacity over 90%
(Professor Hee-Tak Kim and Hyunwon Chu)
A KAIST research team has developed a lithium sulfur battery (LSB) that realizes 92% of the theoretical capacity and an areal capacity of 4mAh/cm2.
LSBs are gaining a great deal of attention as an alternative for lithium ion batteries (LIBs) because they have a theoretical energy density up to six to seven times higher than that of LIBs, and can be manufactured in a more cost-effective way.
However, LSBs face the obstacle of having a capacity reaching its theoretical maximum because they are prone to uncontrolled growth of lithium sulfide on the electrodes, which leads to blocking electron transfer.
To address the problem of electrode passivation, researchers introduced additional conductive agent into the electrode; however, it drastically lowered the energy density of LSBs, making it difficult to exceed 70% of the theoretical capacity.
Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering and his team replaced the lithium salt anions used in conventional LSB electrolytes with anions with a high donor number. The team successfully induced the three-dimensional growth of lithium sulfide on electrode surfaces and efficiently delayed the electrode passivation.
Based on this electrolyte design, the research team achieved 92% of the theoretical capacity with their high-capacity sulfur electrode (4mAh/cm2), which is equivalent to that of conventional LIB cathode. Furthermore, they were able to form a stable passivation film on the surface of the lithium anode and exhibited stable operation over 100 cycles.
This technology, which can be flexibly used with various types of sulfur electrodes, can mark a new milestone in the battery industry.
Professor Kim said, “We proposed a new physiochemical principle to overcome the limitations of conventional LSBs. I believe our achievement of obtaining 90% of the LBSs’ theoretical capacity without any capacity loss after 100 cycles will become a new milestone.”
This research, first-authored by Hyunwon Chu and Hyungjun Noh, was published in Nature Communications on January 14, 2019. It was also selected in the editor’s highlight for its outstanding achievements.
Figure 1. Lithium sulfur growth and its deposition mechanism for different sulfide growth behaviors
Figure 2. Capacity and cycle life characteristics of the LSBs
2019.02.11 View 9105 -
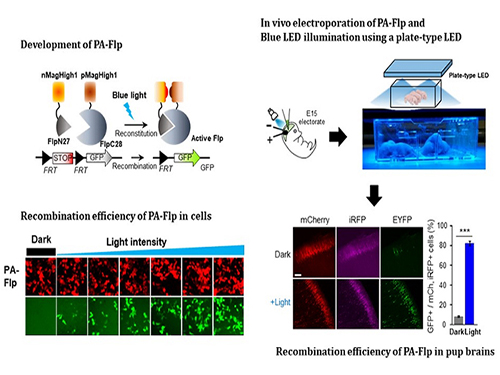 Noninvasive Light-Sensitive Recombinase for Deep Brain Genetic Manipulation
A KAIST team presented a noninvasive light-sensitive photoactivatable recombinase suitable for genetic manipulation in vivo. The highly light-sensitive property of photoactivatable Flp recombinase will be ideal for controlling genetic manipulation in deep mouse brain regions by illumination with a noninvasive light-emitting diode. This easy-to-use optogenetic module made by Professor Won Do Heo and his team will provide a side-effect free and expandable genetic manipulation tool for neuroscience research.
Spatiotemporal control of gene expression has been acclaimed as a valuable strategy for identifying functions of genes with complex neural circuits. Studies of complex brain functions require highly sophisticated and robust technologies that enable specific labeling and rapid genetic modification in live animals. A number of approaches for controlling the activity of proteins or expression of genes in a spatiotemporal manner using light, small molecules, hormones, and peptides have been developed for manipulating intact circuits or functions.
Among them, recombination-employing, chemically inducible systems are the most commonly used in vivo gene-modification systems. Other approaches include selective or conditional Cre-activation systems within subsets of green fluorescent protein-expressing cells or dual-promoter-driven intersectional populations of cells.
However, these methods are limited by the considerable time and effort required to establish knock-in mouse lines and by constraints on spatiotemporal control, which relies on a limited set of available genetic promoters and transgenic mouse resources.
Beyond these constraints, optogenetic approaches allow the activity of genetically defined neurons in the mouse brain to be controlled with high spatiotemporal resolution. However, an optogenetic module for gene-manipulation capable of revealing the spatiotemporal functions of specific target genes in the mouse brain has remained a challenge.
In the study published at Nature Communication on Jan. 18, the team featured photoactivatable Flp recombinase by searching out split sites of Flp recombinase that were not previously identified, being capable of reconstitution to be active. The team validated the highly light-sensitive, efficient performance of photoactivatable Flp recombinase through precise light targeting by showing transgene expression within anatomically confined mouse brain regions.
The concept of local genetic labeling presented here suggests a new approach for genetically identifying subpopulations of cells defined by the spatial and temporal characteristics of light delivery. To date, an optogenetic module for gene-manipulation capable of revealing spatiotemporal functions of specific target genes in the mouse brain has remained out of reach and no such light-inducible Flp system has been developed. Accordingly, the team sought to develop a photoactivatable Flp recombinase that takes full advantage of the high spatiotemporal control offered by light stimulation.
This activation through noninvasive light illumination deep inside the brain is advantageous in that it avoids chemical or optic fiber implantation-mediated side effects, such as off-target cytotoxicity or physical lesions that might influence animal physiology or behaviors. The technique provides expandable utilities for transgene expression systems upon Flp recombinase activity in vivo, by designing a viral vector for minimal leaky expression influenced by viral nascent promoters.
The team demonstrated the utility of PA-Flp as a noninvasive in vivo optogenetic manipulation tool for use in the mouse brain, even applicable for deep brain structures as it can reach the hippocampus or medial septum using external LED light illumination.
The study is the result of five years of research by Professor Heo, who has led the bio-imaging and optogenetics fields by developing his own bio-imaging and optogenetics technologies. “It will be a great advantage to control specific gene expression desired by LEDs with little physical and chemical stimulation that can affect the physiological phenomenon in living animals,” he explained.
2019.01.22 View 8548
Noninvasive Light-Sensitive Recombinase for Deep Brain Genetic Manipulation
A KAIST team presented a noninvasive light-sensitive photoactivatable recombinase suitable for genetic manipulation in vivo. The highly light-sensitive property of photoactivatable Flp recombinase will be ideal for controlling genetic manipulation in deep mouse brain regions by illumination with a noninvasive light-emitting diode. This easy-to-use optogenetic module made by Professor Won Do Heo and his team will provide a side-effect free and expandable genetic manipulation tool for neuroscience research.
Spatiotemporal control of gene expression has been acclaimed as a valuable strategy for identifying functions of genes with complex neural circuits. Studies of complex brain functions require highly sophisticated and robust technologies that enable specific labeling and rapid genetic modification in live animals. A number of approaches for controlling the activity of proteins or expression of genes in a spatiotemporal manner using light, small molecules, hormones, and peptides have been developed for manipulating intact circuits or functions.
Among them, recombination-employing, chemically inducible systems are the most commonly used in vivo gene-modification systems. Other approaches include selective or conditional Cre-activation systems within subsets of green fluorescent protein-expressing cells or dual-promoter-driven intersectional populations of cells.
However, these methods are limited by the considerable time and effort required to establish knock-in mouse lines and by constraints on spatiotemporal control, which relies on a limited set of available genetic promoters and transgenic mouse resources.
Beyond these constraints, optogenetic approaches allow the activity of genetically defined neurons in the mouse brain to be controlled with high spatiotemporal resolution. However, an optogenetic module for gene-manipulation capable of revealing the spatiotemporal functions of specific target genes in the mouse brain has remained a challenge.
In the study published at Nature Communication on Jan. 18, the team featured photoactivatable Flp recombinase by searching out split sites of Flp recombinase that were not previously identified, being capable of reconstitution to be active. The team validated the highly light-sensitive, efficient performance of photoactivatable Flp recombinase through precise light targeting by showing transgene expression within anatomically confined mouse brain regions.
The concept of local genetic labeling presented here suggests a new approach for genetically identifying subpopulations of cells defined by the spatial and temporal characteristics of light delivery. To date, an optogenetic module for gene-manipulation capable of revealing spatiotemporal functions of specific target genes in the mouse brain has remained out of reach and no such light-inducible Flp system has been developed. Accordingly, the team sought to develop a photoactivatable Flp recombinase that takes full advantage of the high spatiotemporal control offered by light stimulation.
This activation through noninvasive light illumination deep inside the brain is advantageous in that it avoids chemical or optic fiber implantation-mediated side effects, such as off-target cytotoxicity or physical lesions that might influence animal physiology or behaviors. The technique provides expandable utilities for transgene expression systems upon Flp recombinase activity in vivo, by designing a viral vector for minimal leaky expression influenced by viral nascent promoters.
The team demonstrated the utility of PA-Flp as a noninvasive in vivo optogenetic manipulation tool for use in the mouse brain, even applicable for deep brain structures as it can reach the hippocampus or medial septum using external LED light illumination.
The study is the result of five years of research by Professor Heo, who has led the bio-imaging and optogenetics fields by developing his own bio-imaging and optogenetics technologies. “It will be a great advantage to control specific gene expression desired by LEDs with little physical and chemical stimulation that can affect the physiological phenomenon in living animals,” he explained.
2019.01.22 View 8548 -
 Technology to Control Near-Field Thermal Radiation
(from left clockwise: Professor Seung Seob Lee, Professor Bong Jae Lee, PhD Mikyung Lim and PhD candidate Jaeman Song)
A KAIST research team succeeded in measuring and controlling the near-field thermal radiation between metallo-dielectric (MD) multilayer structures.
Their thermal radiation control technology can be applied to next-generation semiconductor packaging, thermophotovoltaic cells and thermal management systems. It also has the potential to be applied to a sustainable energy source for IoT sensors.
In the nanoscale gaps, thermal radiation between objects increases greatly with closer distances. The amount of heat transfer in this scale was found to be from 1,000 to 10,000 times greater than the blackbody radiation heat transfer, which was once considered the theoretical maximum for the rate of thermal radiation. This phenomenon is called near-field thermal radiation. With recent developments in nanotechnology, research into near-field thermal radiation between various materials has been actively carried out.
Surface polariton coupling generated from nanostructures has been of particular interest because it enhances the amount of near-field thermal radiation between two objects, and allows the spectral control of near-field thermal radiation. This advantage has motivated much of the recent theoretical research on the application of near-field thermal radiation using nanostructures, such as thin films, multilayer nanostructures, and nanowires. Nevertheless, thus far, most of the studies have focused on measuring near-field thermal radiation between isotropic materials.
A joint team led by Professor Bong Jae Lee and Professor Seung Seob Lee from the Department of Mechanical Engineering succeeded in measuring near-field thermal radiation according to the vacuum distance between MD multilayer nanostructures by using a custom MEMS (Micro-Electro-Mechanical Systems)-device-integrated platform with three-axis nanopositioner.
MD multilayer nanostructures refer to structures in which metal and dielectric layers with regular thickness alternate. The MD single-layer pair is referred to as a unit cell, and the ratio of the thickness occupied by the metal layer in the unit cell is called the fill factor.
By measuring the near-field thermal radiation with a varying number of unit cells and the fill factor of the multilayer nanostructures, the team demonstrated that the surface plasmon polariton coupling enhances near-field thermal radiation greatly, and allows spectral control over the heat transfer.
Professor B. J. Lee said, “The isotropic materials that have so far been studied experimentally had limited spectral control over the near-field thermal radiation. Our near-field thermal radiation control technology using multilayer nanostructures is expected to become the first step toward developing various near-field thermal radiation applications.”
This research, led by PhD Mikyung Lim and PhD candidate Jaeman Song, was published in Nature Communications on October 16.
Figure 1. Experimental setup for measuring near-field thermal radiation between MD multilayers
Figure 2. Investigation of manipulated near-field heat flux by modifying the surface conditions with MD multilayers
2019.01.04 View 7303
Technology to Control Near-Field Thermal Radiation
(from left clockwise: Professor Seung Seob Lee, Professor Bong Jae Lee, PhD Mikyung Lim and PhD candidate Jaeman Song)
A KAIST research team succeeded in measuring and controlling the near-field thermal radiation between metallo-dielectric (MD) multilayer structures.
Their thermal radiation control technology can be applied to next-generation semiconductor packaging, thermophotovoltaic cells and thermal management systems. It also has the potential to be applied to a sustainable energy source for IoT sensors.
In the nanoscale gaps, thermal radiation between objects increases greatly with closer distances. The amount of heat transfer in this scale was found to be from 1,000 to 10,000 times greater than the blackbody radiation heat transfer, which was once considered the theoretical maximum for the rate of thermal radiation. This phenomenon is called near-field thermal radiation. With recent developments in nanotechnology, research into near-field thermal radiation between various materials has been actively carried out.
Surface polariton coupling generated from nanostructures has been of particular interest because it enhances the amount of near-field thermal radiation between two objects, and allows the spectral control of near-field thermal radiation. This advantage has motivated much of the recent theoretical research on the application of near-field thermal radiation using nanostructures, such as thin films, multilayer nanostructures, and nanowires. Nevertheless, thus far, most of the studies have focused on measuring near-field thermal radiation between isotropic materials.
A joint team led by Professor Bong Jae Lee and Professor Seung Seob Lee from the Department of Mechanical Engineering succeeded in measuring near-field thermal radiation according to the vacuum distance between MD multilayer nanostructures by using a custom MEMS (Micro-Electro-Mechanical Systems)-device-integrated platform with three-axis nanopositioner.
MD multilayer nanostructures refer to structures in which metal and dielectric layers with regular thickness alternate. The MD single-layer pair is referred to as a unit cell, and the ratio of the thickness occupied by the metal layer in the unit cell is called the fill factor.
By measuring the near-field thermal radiation with a varying number of unit cells and the fill factor of the multilayer nanostructures, the team demonstrated that the surface plasmon polariton coupling enhances near-field thermal radiation greatly, and allows spectral control over the heat transfer.
Professor B. J. Lee said, “The isotropic materials that have so far been studied experimentally had limited spectral control over the near-field thermal radiation. Our near-field thermal radiation control technology using multilayer nanostructures is expected to become the first step toward developing various near-field thermal radiation applications.”
This research, led by PhD Mikyung Lim and PhD candidate Jaeman Song, was published in Nature Communications on October 16.
Figure 1. Experimental setup for measuring near-field thermal radiation between MD multilayers
Figure 2. Investigation of manipulated near-field heat flux by modifying the surface conditions with MD multilayers
2019.01.04 View 7303 -
 Ultrathin Digital Camera Inspired by Xenos Peckii Eyes
(Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering)
The visual system of Xenos peckii, an endoparasite of paper wasps, demonstrates distinct benefits for high sensitivity and high resolution, differing from the compound eyes of most insects. Taking their unique features, a KAIST team developed an ultrathin digital camera that emulates the unique eyes of Xenos peckii.
The ultrathin digital camera offers a wide field of view and high resolution in a slimmer body compared to existing imaging systems. It is expected to support various applications, such as monitoring equipment, medical imaging devices, and mobile imaging systems.
Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering and his team are known for mimicking biological visual organs. The team’s past research includes an LED lens based on the abdominal segments of fireflies and biologically inspired anti-reflective structures.
Recently, the demand for ultrathin digital cameras has increased, due to the miniaturization of electronic and optical devices. However, most camera modules use multiple lenses along the optical axis to compensate for optical aberrations, resulting in a larger volume as well as a thicker total track length of digital cameras. Resolution and sensitivity would be compromised if these modules were to be simply reduced in size and thickness.
To address this issue, the team have developed micro-optical components, inspired from the visual system of Xenos peckii, and combined them with a CMOS (complementary metal oxide semiconductor) image sensor to achieve an ultrathin digital camera.
This new camera, measuring less than 2mm in thickness, emulates the eyes of Xenos peckii by using dozens of microprism arrays and microlens arrays. A microprism and microlens pair form a channel and the light-absorbing medium between the channels reduces optical crosstalk. Each channel captures the partial image at slightly different orientation, and the retrieved partial images are combined into a single image, thereby ensuring a wide field of view and high resolution.
Professor Jeong said, “We have proposed a novel method of fabricating an ultrathin camera. As the first insect-inspired, ultrathin camera that integrates a microcamera on a conventional CMOS image sensor array, our study will have a significant impact in optics and related fields.”
This research, led by PhD candidates Dongmin Keum and Kyung-Won Jang, was published in Light: Science & Applications on October 24, 2018.
Figure 1. Natural Xenos peckii eye and the biological inspiration for the ultrathin digital camera (Light: Science & Applications 2018)
Figure 2. Optical images captured by the bioinspired ultrathin digital camera (Light: Science & Applications 2018)
2018.12.31 View 10003
Ultrathin Digital Camera Inspired by Xenos Peckii Eyes
(Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering)
The visual system of Xenos peckii, an endoparasite of paper wasps, demonstrates distinct benefits for high sensitivity and high resolution, differing from the compound eyes of most insects. Taking their unique features, a KAIST team developed an ultrathin digital camera that emulates the unique eyes of Xenos peckii.
The ultrathin digital camera offers a wide field of view and high resolution in a slimmer body compared to existing imaging systems. It is expected to support various applications, such as monitoring equipment, medical imaging devices, and mobile imaging systems.
Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering and his team are known for mimicking biological visual organs. The team’s past research includes an LED lens based on the abdominal segments of fireflies and biologically inspired anti-reflective structures.
Recently, the demand for ultrathin digital cameras has increased, due to the miniaturization of electronic and optical devices. However, most camera modules use multiple lenses along the optical axis to compensate for optical aberrations, resulting in a larger volume as well as a thicker total track length of digital cameras. Resolution and sensitivity would be compromised if these modules were to be simply reduced in size and thickness.
To address this issue, the team have developed micro-optical components, inspired from the visual system of Xenos peckii, and combined them with a CMOS (complementary metal oxide semiconductor) image sensor to achieve an ultrathin digital camera.
This new camera, measuring less than 2mm in thickness, emulates the eyes of Xenos peckii by using dozens of microprism arrays and microlens arrays. A microprism and microlens pair form a channel and the light-absorbing medium between the channels reduces optical crosstalk. Each channel captures the partial image at slightly different orientation, and the retrieved partial images are combined into a single image, thereby ensuring a wide field of view and high resolution.
Professor Jeong said, “We have proposed a novel method of fabricating an ultrathin camera. As the first insect-inspired, ultrathin camera that integrates a microcamera on a conventional CMOS image sensor array, our study will have a significant impact in optics and related fields.”
This research, led by PhD candidates Dongmin Keum and Kyung-Won Jang, was published in Light: Science & Applications on October 24, 2018.
Figure 1. Natural Xenos peckii eye and the biological inspiration for the ultrathin digital camera (Light: Science & Applications 2018)
Figure 2. Optical images captured by the bioinspired ultrathin digital camera (Light: Science & Applications 2018)
2018.12.31 View 10003 -
 From Concept to Reality: Changing Color of Light Using a Spatiotemporal Boundary
(from left: Professor Bumki Min, PhD candidate Jaehyeon Son and PhD Kanghee Lee)
A KAIST team developed an optical technique to change the color (frequency) of light using a spatiotemporal boundary. The research focuses on realizing a spatiotemporal boundary with a much higher degree of freedom than the results of previous studies by fabricating a thin metal structure on a semiconductor surface. Such a spatiotemporal boundary is expected to be applicable to an ultra-thin film type optical device capable of changing the color of light.
The optical frequency conversion device plays a key role in precision measurement and communication technology, and the device has been developed mainly based on optical nonlinearity.
If the intensity of light is very strong, the optical medium responds nonlinearly so the nonlinear optical phenomena, such as frequency doubling or frequency mixing, can be observed. Such optical nonlinear phenomena are realized usually by the interaction between a high-intensity laser and a nonlinear medium.
As an alternative method frequency conversion is observed by temporally modifying the optical properties of the medium through which light travels using an external stimulus. Since frequency conversion in this way can be observed even in weak light, such a technique could be particularly useful in communication technology.
However, rapid optical property modification of the medium by an external stimulus and subsequent light frequency conversion techniques have been researched only in the pertubative regime, and it has been difficult to realize these theoretical results in practical applications.
To realize such a conceptual idea, Professor Bumki Min from the Department of Mechanical Engineering and his team collaborated with Professor Wonju Jeon from the Department of Mechanical Engineering and Professor Fabian Rotermund from the Department of Physics. They developed an artificial optical material (metamaterial) by arranging a metal microstructure that mimics an atomic structure and succeeded in creating a spatiotemporal boundary by changing the optical property of the artificial material abruptly.
While previous studies only slightly modified the refractive index of the medium, this study provided a spatiotemporal boundary as a platform for freely designing and changing the spectral properties of the medium. Using this, the research team developed a device that can control the frequency of light to a large degree.
The research team said a spatiotemporal boundary, which was only conceptually considered in previous research and realized in the pertubative regime, was developed as a step that can be realized and applied.
Professor Min said, “The frequency conversion of light becomes designable and predictable, so our research could be applied in many optical applications. This research will present a new direction for time-variant media research projects in the field of optics.”
This research, led by PhD Kanghee Lee and PhD candidate Jaehyeon Son, was published online in Nature Photonics on October 8, 2018.
This work was supported by the National Research Foundation of Korea (NRF) through the government of Korea. The work was also supported by the Center for Advanced Meta-Materials (CAMM) funded by the Korea Government (MSIP) as the Global Frontier Project (NRF-2014M3A6B3063709).
Figure 1. The frequency conversion process of light using a spatiotemporal boundary.
Figure 2. The complex amplitude of light at the converted frequency with the variation of a spatiotemporal boundary.
2018.11.29 View 9450
From Concept to Reality: Changing Color of Light Using a Spatiotemporal Boundary
(from left: Professor Bumki Min, PhD candidate Jaehyeon Son and PhD Kanghee Lee)
A KAIST team developed an optical technique to change the color (frequency) of light using a spatiotemporal boundary. The research focuses on realizing a spatiotemporal boundary with a much higher degree of freedom than the results of previous studies by fabricating a thin metal structure on a semiconductor surface. Such a spatiotemporal boundary is expected to be applicable to an ultra-thin film type optical device capable of changing the color of light.
The optical frequency conversion device plays a key role in precision measurement and communication technology, and the device has been developed mainly based on optical nonlinearity.
If the intensity of light is very strong, the optical medium responds nonlinearly so the nonlinear optical phenomena, such as frequency doubling or frequency mixing, can be observed. Such optical nonlinear phenomena are realized usually by the interaction between a high-intensity laser and a nonlinear medium.
As an alternative method frequency conversion is observed by temporally modifying the optical properties of the medium through which light travels using an external stimulus. Since frequency conversion in this way can be observed even in weak light, such a technique could be particularly useful in communication technology.
However, rapid optical property modification of the medium by an external stimulus and subsequent light frequency conversion techniques have been researched only in the pertubative regime, and it has been difficult to realize these theoretical results in practical applications.
To realize such a conceptual idea, Professor Bumki Min from the Department of Mechanical Engineering and his team collaborated with Professor Wonju Jeon from the Department of Mechanical Engineering and Professor Fabian Rotermund from the Department of Physics. They developed an artificial optical material (metamaterial) by arranging a metal microstructure that mimics an atomic structure and succeeded in creating a spatiotemporal boundary by changing the optical property of the artificial material abruptly.
While previous studies only slightly modified the refractive index of the medium, this study provided a spatiotemporal boundary as a platform for freely designing and changing the spectral properties of the medium. Using this, the research team developed a device that can control the frequency of light to a large degree.
The research team said a spatiotemporal boundary, which was only conceptually considered in previous research and realized in the pertubative regime, was developed as a step that can be realized and applied.
Professor Min said, “The frequency conversion of light becomes designable and predictable, so our research could be applied in many optical applications. This research will present a new direction for time-variant media research projects in the field of optics.”
This research, led by PhD Kanghee Lee and PhD candidate Jaehyeon Son, was published online in Nature Photonics on October 8, 2018.
This work was supported by the National Research Foundation of Korea (NRF) through the government of Korea. The work was also supported by the Center for Advanced Meta-Materials (CAMM) funded by the Korea Government (MSIP) as the Global Frontier Project (NRF-2014M3A6B3063709).
Figure 1. The frequency conversion process of light using a spatiotemporal boundary.
Figure 2. The complex amplitude of light at the converted frequency with the variation of a spatiotemporal boundary.
2018.11.29 View 9450 -
 Lens-free OLEDs with Efficiency comparable to that of Inorganic LEDs
(from left: Professor Seunghyup Yoo and PhD candidate Jinouk Song)
The use of organic light-emitting diodes (OLEDs) has extended to various applications, but their efficiency is still lagging behind inorganic light-emitting diodes. In this research, a KAIST team provided a systematic way to yield OLEDs with an external quantum efficiency (EQE) greater than 50% with an external scattering medium.
Having properties suitable for thin and flexible devices, OLEDs are popular light sources for displays, such as mobile devices and high quality TVs. In recent years, numerous efforts have been made to apply OLEDs in lighting as well as light sources for vehicles.
For such applications, high efficiency is of the upmost importance for the successful deployment of light sources. Thanks to continuous research and the development of OLEDs, their efficiency is steadily on the rise, and a level equivalent to inorganic LEDs has been demonstrated in some reports.
However, these highly efficient OLEDs were often achieved with a macroscopic lens or complex internal nanostructures, which undermines the key advantages of OLEDs as an affordable planar light sources and tends to hinder their stable operation, thus putting a limitation to their commercialization.
Among various methods proven effective for OLED light extraction, a team led by Professor Seunghyup Yoo at the School of Electrical Engineering focused on the external scattering-based approach, as it can maintain planar geometry and compatibility with flexibility. It is also able to be fabricated on a large scale at a low cost and causes no interference with electrical properties of OLEDs.
Conventionally, research on enhancing OLED light extraction using light scattering has been conducted empirically in many cases. This time, the team developed comprehensive and analytical methodology to theoretically predict structures that maximize efficiency.
Considering OLEDs with the external scattering layers as a whole rather than two separate entities, the researchers combined the mathematical description of the scattering phenomena with the optical model for light emission within an OLED to rapidly predict the characteristics of many devices with various structures. Based on this approach, the team theoretically predicted the optimal combination of scattering layers and OLED architectures that can lead to the maximum efficiency.
Following this theoretical prediction, the team experimentally produced the optimal light scattering film and incorporated it to OLEDs with orange emitters having a high degree of horizontal dipole orientation. As a result, the team successfully realized OLEDs exhibiting EQE of 56% and power efficiency of 221 lm/W. This is one of the highest efficiencies ever realized for an OLED unit device without the help of a macroscopic lens or internal light extraction structures.
Professor Yoo said, “There are various technologies developed for improving OLED light extraction efficiency; nevertheless, most of them have not reached a level of practical use. This research mainly provides a systematic way to attain an EQE of 50% or higher in OLEDs while keeping in mind the constraints for commercialization. The approach shown here can readily be applied to lighting devices or sensors of wearable devices.”.
This research, co-led by Professor Jang-Joo Kim from Seoul National University and Professor Yun-Hi Kim from Gyeongsang National University, was published in Nature Communications on August 10, 2018. (J. Song et al. Nature Communications, 9, 3207. DOI: 10.1038/s41467-018-05671-x)
Figure 1.Photographs of OLEDs with SiO₂ -embedded scattering layers according to scatterance
2018.10.26 View 10280
Lens-free OLEDs with Efficiency comparable to that of Inorganic LEDs
(from left: Professor Seunghyup Yoo and PhD candidate Jinouk Song)
The use of organic light-emitting diodes (OLEDs) has extended to various applications, but their efficiency is still lagging behind inorganic light-emitting diodes. In this research, a KAIST team provided a systematic way to yield OLEDs with an external quantum efficiency (EQE) greater than 50% with an external scattering medium.
Having properties suitable for thin and flexible devices, OLEDs are popular light sources for displays, such as mobile devices and high quality TVs. In recent years, numerous efforts have been made to apply OLEDs in lighting as well as light sources for vehicles.
For such applications, high efficiency is of the upmost importance for the successful deployment of light sources. Thanks to continuous research and the development of OLEDs, their efficiency is steadily on the rise, and a level equivalent to inorganic LEDs has been demonstrated in some reports.
However, these highly efficient OLEDs were often achieved with a macroscopic lens or complex internal nanostructures, which undermines the key advantages of OLEDs as an affordable planar light sources and tends to hinder their stable operation, thus putting a limitation to their commercialization.
Among various methods proven effective for OLED light extraction, a team led by Professor Seunghyup Yoo at the School of Electrical Engineering focused on the external scattering-based approach, as it can maintain planar geometry and compatibility with flexibility. It is also able to be fabricated on a large scale at a low cost and causes no interference with electrical properties of OLEDs.
Conventionally, research on enhancing OLED light extraction using light scattering has been conducted empirically in many cases. This time, the team developed comprehensive and analytical methodology to theoretically predict structures that maximize efficiency.
Considering OLEDs with the external scattering layers as a whole rather than two separate entities, the researchers combined the mathematical description of the scattering phenomena with the optical model for light emission within an OLED to rapidly predict the characteristics of many devices with various structures. Based on this approach, the team theoretically predicted the optimal combination of scattering layers and OLED architectures that can lead to the maximum efficiency.
Following this theoretical prediction, the team experimentally produced the optimal light scattering film and incorporated it to OLEDs with orange emitters having a high degree of horizontal dipole orientation. As a result, the team successfully realized OLEDs exhibiting EQE of 56% and power efficiency of 221 lm/W. This is one of the highest efficiencies ever realized for an OLED unit device without the help of a macroscopic lens or internal light extraction structures.
Professor Yoo said, “There are various technologies developed for improving OLED light extraction efficiency; nevertheless, most of them have not reached a level of practical use. This research mainly provides a systematic way to attain an EQE of 50% or higher in OLEDs while keeping in mind the constraints for commercialization. The approach shown here can readily be applied to lighting devices or sensors of wearable devices.”.
This research, co-led by Professor Jang-Joo Kim from Seoul National University and Professor Yun-Hi Kim from Gyeongsang National University, was published in Nature Communications on August 10, 2018. (J. Song et al. Nature Communications, 9, 3207. DOI: 10.1038/s41467-018-05671-x)
Figure 1.Photographs of OLEDs with SiO₂ -embedded scattering layers according to scatterance
2018.10.26 View 10280 -
 Skin Hardness to Estimate Better Human Thermal Status
(Professor Young-Ho Cho and Researcher Sunghyun Yoon)
Under the same temperature and humidity, human thermal status may vary due to individual body constitution and climatic environment. A KAIST research team previously developed a wearable sweat rate sensor for human thermal comfort monitoring. Furthering the development, this time they proposed skin hardness as an additional, independent physiological sign to estimate human thermal status more accurately. This novel approach can be applied to developing systems incorporating human-machine interaction, which requires accurate information about human thermal status.
Professor Young-Ho Cho and his team from the Department of Bio and Brain Engineering had previously studied skin temperature and sweat rate to determine human thermal comfort, and developed a watch-type sweat rate sensor that accurately and steadily detects thermal comfort last February (title: Wearable Sweat Rate Sensors for Human Thermal Comfort Monitoring ).
However, skin temperature and sweat rate are still not enough to estimate exact human thermal comfort. Hence, an additional indicator is required for enhancing the accuracy and reliability of the estimation and the team selected skin hardness. When people feel hot or cold, arrector pili muscles connected to hair follicles contract and expand, and skin hardness comes from this contraction and relaxation of the muscles. Based on the phenomenon of changing skin hardness, the team proposed skin hardness as a new indicator for measuring human thermal sensation.
With this new estimation model using three physiological signs for estimating human thermal status, the team conducted human experiments and verified that skin hardness is effective and independent from the two conventional physiological signs. Adding skin hardness to the conventional model can reduce errors by 23.5%, which makes its estimation more reliable.
The team will develop a sensor that detects skin hardness and applies it to cognitive air-conditioning and heating systems that better interact with humans than existing systems.
Professor Cho said, “Introducing this new indicator, skin hardness, elevates the reliability of measuring human thermal comfort regardless of individual body constitution and climatic environment. Based on this method, we can develop a personalized air conditioning and heating system that will allow affective interaction between humans and machines by sharing both physical and mental health conditions and emotions.”
This research, led by researchers Sunghyun Yoon and Jai Kyoung Sim, was published in Scientific Reports, Vol.8, Article No.12027 on August 13, 2018. (pp.1-6)
Figure 1. Measuring human thermal status through skin hardness
Figure 2. The instrument used for measuring human thermal status through skin hardness
2018.10.17 View 7551
Skin Hardness to Estimate Better Human Thermal Status
(Professor Young-Ho Cho and Researcher Sunghyun Yoon)
Under the same temperature and humidity, human thermal status may vary due to individual body constitution and climatic environment. A KAIST research team previously developed a wearable sweat rate sensor for human thermal comfort monitoring. Furthering the development, this time they proposed skin hardness as an additional, independent physiological sign to estimate human thermal status more accurately. This novel approach can be applied to developing systems incorporating human-machine interaction, which requires accurate information about human thermal status.
Professor Young-Ho Cho and his team from the Department of Bio and Brain Engineering had previously studied skin temperature and sweat rate to determine human thermal comfort, and developed a watch-type sweat rate sensor that accurately and steadily detects thermal comfort last February (title: Wearable Sweat Rate Sensors for Human Thermal Comfort Monitoring ).
However, skin temperature and sweat rate are still not enough to estimate exact human thermal comfort. Hence, an additional indicator is required for enhancing the accuracy and reliability of the estimation and the team selected skin hardness. When people feel hot or cold, arrector pili muscles connected to hair follicles contract and expand, and skin hardness comes from this contraction and relaxation of the muscles. Based on the phenomenon of changing skin hardness, the team proposed skin hardness as a new indicator for measuring human thermal sensation.
With this new estimation model using three physiological signs for estimating human thermal status, the team conducted human experiments and verified that skin hardness is effective and independent from the two conventional physiological signs. Adding skin hardness to the conventional model can reduce errors by 23.5%, which makes its estimation more reliable.
The team will develop a sensor that detects skin hardness and applies it to cognitive air-conditioning and heating systems that better interact with humans than existing systems.
Professor Cho said, “Introducing this new indicator, skin hardness, elevates the reliability of measuring human thermal comfort regardless of individual body constitution and climatic environment. Based on this method, we can develop a personalized air conditioning and heating system that will allow affective interaction between humans and machines by sharing both physical and mental health conditions and emotions.”
This research, led by researchers Sunghyun Yoon and Jai Kyoung Sim, was published in Scientific Reports, Vol.8, Article No.12027 on August 13, 2018. (pp.1-6)
Figure 1. Measuring human thermal status through skin hardness
Figure 2. The instrument used for measuring human thermal status through skin hardness
2018.10.17 View 7551 -
 Trigger of the Hyperactivation of Fibrosis Identified
(Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering)
Scientists have been investigating the negative effects that the hyperactivation of fibrosis has on fibrotic diseases and cancer. A KAIST research team unveiled a positive feedback loop that bi-stably activates fibroblasts in collaboration with Samsung Medical Center. This finding will contribute to developing therapeutic targets for both fibrosis and cancer.
Human fibroblasts are dormant in normal tissue, but show radical activation during wound healing. However, the principle that induces their explosive activation has not yet been identified.
Here, Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering, in collaboration with Professor Seok-Hyung Kim from Samsung Medical Center, discovered the principle of a circuit that continuously activates fibroblasts.
They constructed a positive feedback loops (PFLs) where Twist1, Prrx1, and Tenascin-C (TNC) molecules consecutively activate fibroblasts. They confirmed that these are the main inducers of fibroblast activation by conducting various experiments, including molecular biological tests, mathematical modeling, animal testing, and computer simulations to conclude that they are the main inducers of fibroblast activation.
According to their research, Twist 1 is a key regulator of cancer-associated fibroblasts, which directly upregulates Prrx1 and then triggers TNC, which also increases Twist1 expression. This circuit consequently forms a Twist-Prrx1-TNC positive feedback loop.
Activated fibroblasts need to be deactivated after wounds are healed. However, if the PFLs continue, the fibroblasts become the major cause of worsening fibrotic diseases and cancers.
Therefore, the team expects that Twist1-Prrx1-TNC positive PFLs will be applied for novel and effective therapeutic targeting of fibrotic diseases and cancers.
This research was published in Nature Communications on August 1, 2018.
Figure 1. Twist1 increases tenascin-c expression in cancer-associated fibroblasts. Twist1 is a potent but indirect inducer of tenascin-c (TNC), which is essential for maintaining Twist1 expression in cancer-associated fibroblasts (CAFs).
Figure 2. Summary of the study. The Twist1-Prrx1-TNC positive feedback regulation provides clues for understanding the activation of fibroblasts during wound healing under normal conditions, as well as abnormally activated fibroblasts in pathological conditions such as cancerous and fibrotic diseases. Under normal conditions, the PFL acts as a reversible bistable switch by which the activation of fibroblasts is “ON" above a sufficient level of stimulation and “OFF" for the withdrawal of the stimulus. However, this switch can be permanently turned on under pathologic conditions by continued activation of the PFL, resulting in sustained proliferation of fibroblasts.
2018.10.11 View 7287
Trigger of the Hyperactivation of Fibrosis Identified
(Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering)
Scientists have been investigating the negative effects that the hyperactivation of fibrosis has on fibrotic diseases and cancer. A KAIST research team unveiled a positive feedback loop that bi-stably activates fibroblasts in collaboration with Samsung Medical Center. This finding will contribute to developing therapeutic targets for both fibrosis and cancer.
Human fibroblasts are dormant in normal tissue, but show radical activation during wound healing. However, the principle that induces their explosive activation has not yet been identified.
Here, Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering, in collaboration with Professor Seok-Hyung Kim from Samsung Medical Center, discovered the principle of a circuit that continuously activates fibroblasts.
They constructed a positive feedback loops (PFLs) where Twist1, Prrx1, and Tenascin-C (TNC) molecules consecutively activate fibroblasts. They confirmed that these are the main inducers of fibroblast activation by conducting various experiments, including molecular biological tests, mathematical modeling, animal testing, and computer simulations to conclude that they are the main inducers of fibroblast activation.
According to their research, Twist 1 is a key regulator of cancer-associated fibroblasts, which directly upregulates Prrx1 and then triggers TNC, which also increases Twist1 expression. This circuit consequently forms a Twist-Prrx1-TNC positive feedback loop.
Activated fibroblasts need to be deactivated after wounds are healed. However, if the PFLs continue, the fibroblasts become the major cause of worsening fibrotic diseases and cancers.
Therefore, the team expects that Twist1-Prrx1-TNC positive PFLs will be applied for novel and effective therapeutic targeting of fibrotic diseases and cancers.
This research was published in Nature Communications on August 1, 2018.
Figure 1. Twist1 increases tenascin-c expression in cancer-associated fibroblasts. Twist1 is a potent but indirect inducer of tenascin-c (TNC), which is essential for maintaining Twist1 expression in cancer-associated fibroblasts (CAFs).
Figure 2. Summary of the study. The Twist1-Prrx1-TNC positive feedback regulation provides clues for understanding the activation of fibroblasts during wound healing under normal conditions, as well as abnormally activated fibroblasts in pathological conditions such as cancerous and fibrotic diseases. Under normal conditions, the PFL acts as a reversible bistable switch by which the activation of fibroblasts is “ON" above a sufficient level of stimulation and “OFF" for the withdrawal of the stimulus. However, this switch can be permanently turned on under pathologic conditions by continued activation of the PFL, resulting in sustained proliferation of fibroblasts.
2018.10.11 View 7287 -
 Effective Drug Delivery to Heart with Tannic Acid
(Professor Haeshin Lee from the Department of Chemistry) Typical methods of drug delivery to the heart require surgical procedures involving incisions in the chest wall and bones. To efficiently treat cardiovascular and related vascular diseases without surgery, a KAIST research team developed a heart-targeting drug delivery technology using tannin acid via intravenous systemic injection. This method can be applied to the development of a variety of new protein-based drugs.
Cardiovascular-circulatory disease is currently the second leading cause of death in Korea. A typical example of this disease is myocardial infarction caused by poor oxygen and nutrient supply due to narrowed coronary arteries and poor blood flow to the heart.
Although there have been numerous research projects to develop chemotherapeutic drugs and therapeutic proteins, clinics still rely on surgical procedures. Drug delivery can be an alternative, but it is quite challenging because ceaseless dynamic cycles of the heart and massive exchanges of blood mean administered therapeutics do not stay inside the heart very long.
Professor Haeshin Lee from the Department of Chemistry and his team employed tannic acid (TA), which is known for giving bitter taste to wines. It is one of the most abundant polyphenols and can be easily found in plants, such as fruits, vegetables, cacao, and others. TA has also been used as a multifunctional coating molecule.
Using these properties of TA, the team complexed protein and peptide therapeutics with tannic acid and succeeded in targeting protein and peptide therapeutics to the heart. TA, coated on the surface of a granulated protein complex, helps maintain cardiac function because it adheres to extracellular matrices, elastin, and collagens in heart tissues allowing the protein to stay attached to the heart tissue for a longer period.
The team confirmed that these Tannic-acid-modified proteins stay in blood vessels five days longer than with protein-only injections. Additionally they found that TA-protein complexes do not show any cardiac toxicity and do not cause noticeable pathology.
The team has been continuously developing biomaterials for medical applications by testing various polyphenolic materials that feature adhesive and coating properties, including tannic acid. They have injected a mixture of TA and fibroblast growth factors (FGF) into animal models with myocardial infarctions. After four weeks, they confirmed that the infarction was reduced and the left ventricular pressure and cardiac output were almost normalized.
Professor Lee said, “Although there have been numerous drugs related to heart disease, so far there has not been efficient drug delivery to the heart so this technology will be able to reformulate existing drugs into new and more efficient drugs.”
This research, jointly led by Dr. Ki-Suk Kim from the Predictive Model Research Center, was published in Nature Biomedical Engineering on April 30 ( http://www.nature.com/articles/s41551-018-0227-9 ).
Figure 1. Schematic for the heart-targeting mechanism of TANNylated protein nanocomplexes: (1) size-dependent permeation, (2) phenolic (that is, TA), and (3) internalization by internalization by myoblasts
Figure 2. Effect of TA based protein complexes on cardiac cell transport efficiency and viral gene expression efficiency and therapeutic function in animal models with myocardial infarction
2018.09.18 View 6185
Effective Drug Delivery to Heart with Tannic Acid
(Professor Haeshin Lee from the Department of Chemistry) Typical methods of drug delivery to the heart require surgical procedures involving incisions in the chest wall and bones. To efficiently treat cardiovascular and related vascular diseases without surgery, a KAIST research team developed a heart-targeting drug delivery technology using tannin acid via intravenous systemic injection. This method can be applied to the development of a variety of new protein-based drugs.
Cardiovascular-circulatory disease is currently the second leading cause of death in Korea. A typical example of this disease is myocardial infarction caused by poor oxygen and nutrient supply due to narrowed coronary arteries and poor blood flow to the heart.
Although there have been numerous research projects to develop chemotherapeutic drugs and therapeutic proteins, clinics still rely on surgical procedures. Drug delivery can be an alternative, but it is quite challenging because ceaseless dynamic cycles of the heart and massive exchanges of blood mean administered therapeutics do not stay inside the heart very long.
Professor Haeshin Lee from the Department of Chemistry and his team employed tannic acid (TA), which is known for giving bitter taste to wines. It is one of the most abundant polyphenols and can be easily found in plants, such as fruits, vegetables, cacao, and others. TA has also been used as a multifunctional coating molecule.
Using these properties of TA, the team complexed protein and peptide therapeutics with tannic acid and succeeded in targeting protein and peptide therapeutics to the heart. TA, coated on the surface of a granulated protein complex, helps maintain cardiac function because it adheres to extracellular matrices, elastin, and collagens in heart tissues allowing the protein to stay attached to the heart tissue for a longer period.
The team confirmed that these Tannic-acid-modified proteins stay in blood vessels five days longer than with protein-only injections. Additionally they found that TA-protein complexes do not show any cardiac toxicity and do not cause noticeable pathology.
The team has been continuously developing biomaterials for medical applications by testing various polyphenolic materials that feature adhesive and coating properties, including tannic acid. They have injected a mixture of TA and fibroblast growth factors (FGF) into animal models with myocardial infarctions. After four weeks, they confirmed that the infarction was reduced and the left ventricular pressure and cardiac output were almost normalized.
Professor Lee said, “Although there have been numerous drugs related to heart disease, so far there has not been efficient drug delivery to the heart so this technology will be able to reformulate existing drugs into new and more efficient drugs.”
This research, jointly led by Dr. Ki-Suk Kim from the Predictive Model Research Center, was published in Nature Biomedical Engineering on April 30 ( http://www.nature.com/articles/s41551-018-0227-9 ).
Figure 1. Schematic for the heart-targeting mechanism of TANNylated protein nanocomplexes: (1) size-dependent permeation, (2) phenolic (that is, TA), and (3) internalization by internalization by myoblasts
Figure 2. Effect of TA based protein complexes on cardiac cell transport efficiency and viral gene expression efficiency and therapeutic function in animal models with myocardial infarction
2018.09.18 View 6185