battery
-
 Rechargeable Lithium Sulfur Battery for Greater Battery Capacity
Professor Do Kyung Kim from the Department of Material Science and Engineering and Professor Jang Wook Choi from the Graduate School of EEWS have been featured in the lead story of the renowned nanoscience journal Advanced Materials for their research on the lithium sulfur battery. This new type of battery developed by Professor Kim is expected to have a longer life battery life and [higher] energy density than currently commercial batteries.
With ample energy density up to 2100Wh/kg—almost 5.4 times that of lithium ion batteries—lithium sulfur batteries can withstand the sharp decrease in energy capacity resulting from charging and discharging—which has been considered the inherent limitation of the conventional batteries.
Professor Kim and his research team used one-dimensional, vertical alignment of 75nm tick, 15μm long sulfur nanowires to maximize electric conductivity. Then, to prevent loss of battery life, they carbon-coated each nanowire and prohibited direct contact between the sulfur and electrolyte.
The result was one of the most powerful batteries in terms of both energy performance and density. Compared to conventional batteries which suffer from continuous decrease in energy capacity after being discharged, the lithium sulfur battery maintained 99.2% of its initial capacity after being charged and discharged 300 times and up to 70% even after 1000 times.
Professor Kim claims that his new battery is an important step forward towards a high-performance rechargeable battery which is a vital technology for unmanned vehicles, electric automobiles and energy storage. He hopes that his research can solve the problems of battery-capacity loss and contribute to South Korea’s leading position in battery technology. Professor Kim’s research team has filed applications for one domestic and international patent for their research.
2013.12.11 View 15410
Rechargeable Lithium Sulfur Battery for Greater Battery Capacity
Professor Do Kyung Kim from the Department of Material Science and Engineering and Professor Jang Wook Choi from the Graduate School of EEWS have been featured in the lead story of the renowned nanoscience journal Advanced Materials for their research on the lithium sulfur battery. This new type of battery developed by Professor Kim is expected to have a longer life battery life and [higher] energy density than currently commercial batteries.
With ample energy density up to 2100Wh/kg—almost 5.4 times that of lithium ion batteries—lithium sulfur batteries can withstand the sharp decrease in energy capacity resulting from charging and discharging—which has been considered the inherent limitation of the conventional batteries.
Professor Kim and his research team used one-dimensional, vertical alignment of 75nm tick, 15μm long sulfur nanowires to maximize electric conductivity. Then, to prevent loss of battery life, they carbon-coated each nanowire and prohibited direct contact between the sulfur and electrolyte.
The result was one of the most powerful batteries in terms of both energy performance and density. Compared to conventional batteries which suffer from continuous decrease in energy capacity after being discharged, the lithium sulfur battery maintained 99.2% of its initial capacity after being charged and discharged 300 times and up to 70% even after 1000 times.
Professor Kim claims that his new battery is an important step forward towards a high-performance rechargeable battery which is a vital technology for unmanned vehicles, electric automobiles and energy storage. He hopes that his research can solve the problems of battery-capacity loss and contribute to South Korea’s leading position in battery technology. Professor Kim’s research team has filed applications for one domestic and international patent for their research.
2013.12.11 View 15410 -
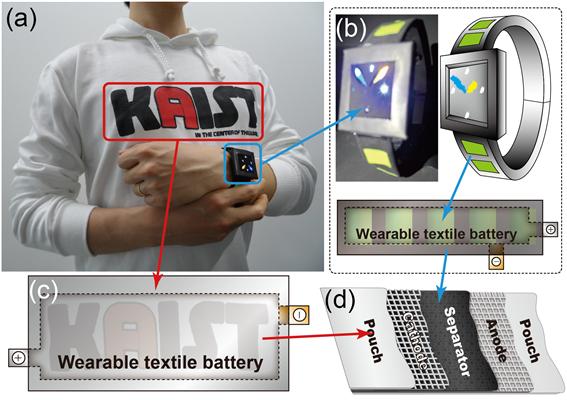 Technology Developed for Flexible, Foldable & Rechargeable Battery
Flexible, Foldable & Rechargeable Battery
The research group of professors Jang-Wook Choi & Jung-Yong Lee from the Graduate School of EEWS and Taek-Soo Kim from the Department of Mechanical Engineering at KAIST has developed technology for flexible and foldable batteries which are rechargeable using solar energy. The research result was published in the online issue of Nano Letters on November 5.
Trial versions of flexible and wearable electronics are being developed and introduced in the market such as Galaxy Gear, Apple’s i-Watch, and Google Glass. Research is being conducted to make the batteries softer and more wearable and to compete in the fast-growing market for flexible electronics.
This new technology is expected to be applied to the development of wearable computers as well as winter outdoor clothing since it is flexible and light. The research group expects that the new technology can be applied to current battery production lines without additional investment.
Professor Choi said, “It can be used as a core-source technology in the rechargeable battery industry in the future. Various wearable mobile electronic products can be developed through cooperation and collaboration within the industry.”
2013.11.21 View 13872
Technology Developed for Flexible, Foldable & Rechargeable Battery
Flexible, Foldable & Rechargeable Battery
The research group of professors Jang-Wook Choi & Jung-Yong Lee from the Graduate School of EEWS and Taek-Soo Kim from the Department of Mechanical Engineering at KAIST has developed technology for flexible and foldable batteries which are rechargeable using solar energy. The research result was published in the online issue of Nano Letters on November 5.
Trial versions of flexible and wearable electronics are being developed and introduced in the market such as Galaxy Gear, Apple’s i-Watch, and Google Glass. Research is being conducted to make the batteries softer and more wearable and to compete in the fast-growing market for flexible electronics.
This new technology is expected to be applied to the development of wearable computers as well as winter outdoor clothing since it is flexible and light. The research group expects that the new technology can be applied to current battery production lines without additional investment.
Professor Choi said, “It can be used as a core-source technology in the rechargeable battery industry in the future. Various wearable mobile electronic products can be developed through cooperation and collaboration within the industry.”
2013.11.21 View 13872 -
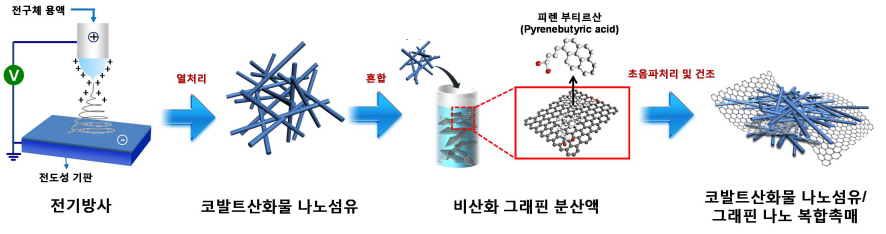 Core Technology for Lithium Air Secondary Battery Developed
KAIST-Kyonggi University joint research team developed composite catalyst out of nano fiber and graphene
Five times improvement in capacity compared to lithium-ion secondary battery, driving 800 km at maximum
The core technology for lithium air secondary battery, the next generation high capacity battery, has been developed.
A research team formed by KAIST Department of Materials Science’s Professors Il-Doo Kim and Seokwoo Jeon, and Kyonggi University Department of Materials Science’s Professor Yong-Joon Park has created a lithium air secondary battery, with five times greater storage than the lithium-ion secondary battery, by developing a nano fiber-graphene composite catalyst. The research results are published in the August 8th online edition of Nano Letters.
A cathode of a lithium-ion battery consists of graphite and an anode of the battery consists of a lithium transition metal oxide. Lithium-ion batteries are widely used in mobile phones and laptops. However, lithium-ion batteries cannot support electric vehicles, providing energy for only 160 kilometers on one full charge. The lithium air secondary battery just developed by the research team uses lithium on the cathode and oxygen on the anode. It is earning a popular acknowledgement among the next generation secondary battery research community for having lightweight mass and high energy density.
However, lithium-ion batteries remain difficult to commercialize because of their short lifespan. Lithium and oxygen meet up to form lithium oxide (Li2O2) at discharge, and decompose again at charge. In a traditional lithium air battery, this cycle does not occur smoothly and results in high resistance, thereby reducing the lifespan of the battery. It is thus essential to develop high efficiency catalyst that facilitates the formation and decomposition of lithium oxides.
The research team used electric radiation to develop a nano composite catalyst by mixing cobalt oxide nano fiber and graphene. The performance of the battery has been maximized by settling nonoxidative graphene, which has high specific surface area and electrical conductivity, on catalyst active cobalt oxide nano fiber. Applying the nano composite catalyst on both poles of the lithium air battery resulted in an improved lifespan of over 80 recharge cycles with capacity greater than 100mAh/g, five times greater than a lithium ion battery. The newly discovered charge-discharge property is the highest among the reported performances of the lithium air battery so far.
The lithium air battery is cheap to make, as the main materials are metal oxide and graphene. “There are yet more issues to resolve such as stability, but we will collaborate with other organizations to open up the era of electronic vehicles,” said Professor Il-Doo Kim. “We hope to contribute to vitalizing the fields of next generation lithium air battery by leading nanocatalyst synthesis technology, one of the core materials in the fields of secondary battery,” Professor Kim spoke of his aspiration.
The graduate students participated in the research are Won-Hee Ryu, a postdoctorate at KAIST Department of Materials Science, Sungho Song, a PhD candidate at KAIST Department of Materials Science, and Taek-Han Yoon, a graduate student at Kyonggi University.
Picture I: Schematic Diagram of Lithium Air Battery Made of Nano Composite Catalysts
Picture II: Images of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts
Picture III: Images of Manufacturing Process of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts for Lithium Air Battery
2013.10.18 View 13752
Core Technology for Lithium Air Secondary Battery Developed
KAIST-Kyonggi University joint research team developed composite catalyst out of nano fiber and graphene
Five times improvement in capacity compared to lithium-ion secondary battery, driving 800 km at maximum
The core technology for lithium air secondary battery, the next generation high capacity battery, has been developed.
A research team formed by KAIST Department of Materials Science’s Professors Il-Doo Kim and Seokwoo Jeon, and Kyonggi University Department of Materials Science’s Professor Yong-Joon Park has created a lithium air secondary battery, with five times greater storage than the lithium-ion secondary battery, by developing a nano fiber-graphene composite catalyst. The research results are published in the August 8th online edition of Nano Letters.
A cathode of a lithium-ion battery consists of graphite and an anode of the battery consists of a lithium transition metal oxide. Lithium-ion batteries are widely used in mobile phones and laptops. However, lithium-ion batteries cannot support electric vehicles, providing energy for only 160 kilometers on one full charge. The lithium air secondary battery just developed by the research team uses lithium on the cathode and oxygen on the anode. It is earning a popular acknowledgement among the next generation secondary battery research community for having lightweight mass and high energy density.
However, lithium-ion batteries remain difficult to commercialize because of their short lifespan. Lithium and oxygen meet up to form lithium oxide (Li2O2) at discharge, and decompose again at charge. In a traditional lithium air battery, this cycle does not occur smoothly and results in high resistance, thereby reducing the lifespan of the battery. It is thus essential to develop high efficiency catalyst that facilitates the formation and decomposition of lithium oxides.
The research team used electric radiation to develop a nano composite catalyst by mixing cobalt oxide nano fiber and graphene. The performance of the battery has been maximized by settling nonoxidative graphene, which has high specific surface area and electrical conductivity, on catalyst active cobalt oxide nano fiber. Applying the nano composite catalyst on both poles of the lithium air battery resulted in an improved lifespan of over 80 recharge cycles with capacity greater than 100mAh/g, five times greater than a lithium ion battery. The newly discovered charge-discharge property is the highest among the reported performances of the lithium air battery so far.
The lithium air battery is cheap to make, as the main materials are metal oxide and graphene. “There are yet more issues to resolve such as stability, but we will collaborate with other organizations to open up the era of electronic vehicles,” said Professor Il-Doo Kim. “We hope to contribute to vitalizing the fields of next generation lithium air battery by leading nanocatalyst synthesis technology, one of the core materials in the fields of secondary battery,” Professor Kim spoke of his aspiration.
The graduate students participated in the research are Won-Hee Ryu, a postdoctorate at KAIST Department of Materials Science, Sungho Song, a PhD candidate at KAIST Department of Materials Science, and Taek-Han Yoon, a graduate student at Kyonggi University.
Picture I: Schematic Diagram of Lithium Air Battery Made of Nano Composite Catalysts
Picture II: Images of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts
Picture III: Images of Manufacturing Process of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts for Lithium Air Battery
2013.10.18 View 13752 -
 International Workshop on EEWS 2010 was held.
On October 7 and 8th at Fusion Hall of KI Building, KAIST, the 2010 International Workshop on EEWS (Energy, Environment, Water, and Sustainability) was held.
The third to be held, forty national and international academic professionals including Mark Shannon, professor at University of Illinois at Urbana-Champaign, Domen Kazunari, Tokyo University professor, Dong Sub Kim, CTO of SK Energy and Doyoung Seung, Senior Vice President of GS Caltex, participated at this year’s workshop.
In twelve sessions, themes including Artificial Photosynthesis, Wireless Power Transfer, Green Aviation, Safe Nuclear Fuel Reuse, Fuel Cells in Action, LED 2.0, Foundation of Energy-Water Nexus, and Flexible Battery & Solar Cell were presented and discussed.
“Through this workshop, current EEWS policy and research progress from different countries and the future of related technologies will be foreseen,” said Jae Kyu Lee, Dean of KAIST EEWS Initiative. “I hope it became an opportunity to create cooperative relationships with leading researchers.”
EEWS is a research project conducted by KAIST to solve global issues that mankind faces today such as depletion of energy, environmental pollution, water shortage, and sustainability.
2010.10.15 View 20325
International Workshop on EEWS 2010 was held.
On October 7 and 8th at Fusion Hall of KI Building, KAIST, the 2010 International Workshop on EEWS (Energy, Environment, Water, and Sustainability) was held.
The third to be held, forty national and international academic professionals including Mark Shannon, professor at University of Illinois at Urbana-Champaign, Domen Kazunari, Tokyo University professor, Dong Sub Kim, CTO of SK Energy and Doyoung Seung, Senior Vice President of GS Caltex, participated at this year’s workshop.
In twelve sessions, themes including Artificial Photosynthesis, Wireless Power Transfer, Green Aviation, Safe Nuclear Fuel Reuse, Fuel Cells in Action, LED 2.0, Foundation of Energy-Water Nexus, and Flexible Battery & Solar Cell were presented and discussed.
“Through this workshop, current EEWS policy and research progress from different countries and the future of related technologies will be foreseen,” said Jae Kyu Lee, Dean of KAIST EEWS Initiative. “I hope it became an opportunity to create cooperative relationships with leading researchers.”
EEWS is a research project conducted by KAIST to solve global issues that mankind faces today such as depletion of energy, environmental pollution, water shortage, and sustainability.
2010.10.15 View 20325 -
 Prof. Song Develops Nano-Structure to Enhance Power of Rechargeable Lithium-ion Battery
A team of scientists led by Prof. Hyun-Joon Song of the Department of Chemistry, KAIST, developed a nano-structure that could increase the power of rechargeable lithium-ion batteries, university sources said on Monday (Feb. 16).
The research team found that a nano-structured material using copper oxide (CuO) could produce lithium-ion batteries with some 50 percent more capacity than conventional products. The study was published in the online edition of peer-review journal Advanced Materials.
In rechargeable lithium-ion batteries, lithium ions move between the battery"s anode and cathode. The high-energy density of the batteries led to their common use in consumer electronics products, expecially portable devices. Their demand in automotive and aerospace applications is growing, and nano-structured, or nano-enabled batteries are emerging as the new generation of lithium-ion batteries for their edge in recharging time, capacity and battery life.
Graphite has been a popular material for cathodes in lithium-ion batteries. However, graphite cathodes are also blamed for lost capacity due to their consumption of lithium ions, which are linked to shorter battery life.
As such, scientists have been looking for materials that could replace graphite in cathodes, and silicon and metal oxide have been studied as possible alternatives.
2009.02.17 View 14134
Prof. Song Develops Nano-Structure to Enhance Power of Rechargeable Lithium-ion Battery
A team of scientists led by Prof. Hyun-Joon Song of the Department of Chemistry, KAIST, developed a nano-structure that could increase the power of rechargeable lithium-ion batteries, university sources said on Monday (Feb. 16).
The research team found that a nano-structured material using copper oxide (CuO) could produce lithium-ion batteries with some 50 percent more capacity than conventional products. The study was published in the online edition of peer-review journal Advanced Materials.
In rechargeable lithium-ion batteries, lithium ions move between the battery"s anode and cathode. The high-energy density of the batteries led to their common use in consumer electronics products, expecially portable devices. Their demand in automotive and aerospace applications is growing, and nano-structured, or nano-enabled batteries are emerging as the new generation of lithium-ion batteries for their edge in recharging time, capacity and battery life.
Graphite has been a popular material for cathodes in lithium-ion batteries. However, graphite cathodes are also blamed for lost capacity due to their consumption of lithium ions, which are linked to shorter battery life.
As such, scientists have been looking for materials that could replace graphite in cathodes, and silicon and metal oxide have been studied as possible alternatives.
2009.02.17 View 14134 -
 Method to Synthesize New Lithium Ion Battery Cathode Material Identified
A KAIST research team headed by Prof. Do-Kyung Kim at the Department of Materials Science and Engineering developed a technology to synthesize a new lithium ion battery spinel cathode which is regarded as a core part of hybrid and lithium battery cars.
The research was conducted in collaboration with a research team of Prof. Yi Cui at Stanford University"s Department of Chemistry. Their findings were introduced in the November issue of Nano Letters, one of the leading academic journals in nano-science.
The newly synthesized lithium ion battery spinel cathode known as spinel LiMn2O4 nanorods is attracting interests as an alternative cathode material since it is a low-cost, environmentally friendly substance for Li-ion battery cathodes. Its raw material is also highly available.
Lithium ion batteries with high energy and power density are important for consumer electronic devices, portable power tools, and vehicle electrification. LixCoO2 is a commonly used cathode material in commercial lithium iron batteries. However, the high cost, toxicity, and limited abundance of cobalt have been recognized to be disadvantageous.
2008.11.20 View 15197
Method to Synthesize New Lithium Ion Battery Cathode Material Identified
A KAIST research team headed by Prof. Do-Kyung Kim at the Department of Materials Science and Engineering developed a technology to synthesize a new lithium ion battery spinel cathode which is regarded as a core part of hybrid and lithium battery cars.
The research was conducted in collaboration with a research team of Prof. Yi Cui at Stanford University"s Department of Chemistry. Their findings were introduced in the November issue of Nano Letters, one of the leading academic journals in nano-science.
The newly synthesized lithium ion battery spinel cathode known as spinel LiMn2O4 nanorods is attracting interests as an alternative cathode material since it is a low-cost, environmentally friendly substance for Li-ion battery cathodes. Its raw material is also highly available.
Lithium ion batteries with high energy and power density are important for consumer electronic devices, portable power tools, and vehicle electrification. LixCoO2 is a commonly used cathode material in commercial lithium iron batteries. However, the high cost, toxicity, and limited abundance of cobalt have been recognized to be disadvantageous.
2008.11.20 View 15197