-
 Artist in residence program at KAIST
A new and innovative program to support artists’ creative activities was launched by KAIST for the first time in Korean college history.
The artist in residence program is an unusual collaboration between engineers and artists. Out of hundreds of applicants for the program, three writers were selected: a novelist, web-based cartoonist, and screen writer. In late October, they became the residents of KAIST.
Housing and an office are provided for the writers in addition to a monthly cash stipend of 800,000 won for up to six months. A variety of programs will be available as well including a tour of laboratories to help the writers get ideas and inspiration.
KAIST’s Vice President Jun-Ho Oh, the founder of the program, said, “Artists can freshen their imagination, and KAIST members can benefit from them to promote creative and innovative ideas through exchange and collaboration.”
2013.11.04 View 6868
Artist in residence program at KAIST
A new and innovative program to support artists’ creative activities was launched by KAIST for the first time in Korean college history.
The artist in residence program is an unusual collaboration between engineers and artists. Out of hundreds of applicants for the program, three writers were selected: a novelist, web-based cartoonist, and screen writer. In late October, they became the residents of KAIST.
Housing and an office are provided for the writers in addition to a monthly cash stipend of 800,000 won for up to six months. A variety of programs will be available as well including a tour of laboratories to help the writers get ideas and inspiration.
KAIST’s Vice President Jun-Ho Oh, the founder of the program, said, “Artists can freshen their imagination, and KAIST members can benefit from them to promote creative and innovative ideas through exchange and collaboration.”
2013.11.04 View 6868 -
 KAIST ranked 60th in the 2013 QS World University Rankings
KAST achieved the highest ranking in its history in the QS World University Rankings by Quacquarelli Symonds.
The British company, which specializes in education, has ranked KAIST 60th in the world in its 2013 QS World University Rankings, as announced on its homepage on September 10th. KAIST was ranked 198th in 2006 and was promoted to 63rd in 2012.
This year, MIT was first, followed by Harvard in second, Cambridge in third, University College of London in fourth, and Imperial College in fifth.
Seoul National University, in 35th place, was ranked the highest among Korean universities. Six other Korean universities were included in the top 200, including KAIST, POSTECH which ranked 107th, Yonsei University in 114th place, Korea University in 145th, and Sungkyunkwan University in 162nd.
QS World University Rankings are assessed based on six different criteria: academic reputation (40%), citations per faculty (20%), student-to-faculty ratio (20%), employer reputation (10%), international student ratio (5%), and foreign faculty ratio (5%).
KAIST improved over 10 points from last year in the areas of employer reputation and citations per faculty.
“This has been accomplished through the challenging spirit and passion of the KAIST family including the students and the faculty. All possible support will be provided for them to excel in research and education”, said the president, Steve Kang.
KAIST also ranked sixth in the 2013 Chosun Daily Newspaper Asian University Rankings and first in the Chungang Daily Newspaper University Rankings for five years from 2008 to 2012.
2013.11.04 View 6493
KAIST ranked 60th in the 2013 QS World University Rankings
KAST achieved the highest ranking in its history in the QS World University Rankings by Quacquarelli Symonds.
The British company, which specializes in education, has ranked KAIST 60th in the world in its 2013 QS World University Rankings, as announced on its homepage on September 10th. KAIST was ranked 198th in 2006 and was promoted to 63rd in 2012.
This year, MIT was first, followed by Harvard in second, Cambridge in third, University College of London in fourth, and Imperial College in fifth.
Seoul National University, in 35th place, was ranked the highest among Korean universities. Six other Korean universities were included in the top 200, including KAIST, POSTECH which ranked 107th, Yonsei University in 114th place, Korea University in 145th, and Sungkyunkwan University in 162nd.
QS World University Rankings are assessed based on six different criteria: academic reputation (40%), citations per faculty (20%), student-to-faculty ratio (20%), employer reputation (10%), international student ratio (5%), and foreign faculty ratio (5%).
KAIST improved over 10 points from last year in the areas of employer reputation and citations per faculty.
“This has been accomplished through the challenging spirit and passion of the KAIST family including the students and the faculty. All possible support will be provided for them to excel in research and education”, said the president, Steve Kang.
KAIST also ranked sixth in the 2013 Chosun Daily Newspaper Asian University Rankings and first in the Chungang Daily Newspaper University Rankings for five years from 2008 to 2012.
2013.11.04 View 6493 -
 A beginner's book for engineering co-published by KAIST professors
A group of KAIST professors published a book (Korean) to draw public attention to engineering.
The book, titled ‘What is Engineering?’, was co-published by 19 KAIST engineering professors for future engineering students and readers interested in exploring what engineering is about.
The co-authors wish to show talented students how important, fun, and helpful engineering is in improving our everyday life even though it is still not a very popular major in Korea.The book contains general knowledge in a wide array of engieering fields such as mechanics, civil, electrical & electronics, materials, chemistry, aerospace, marine systems, nuclear, industrial design, knowledge service, and bio and brain.One of the co-authors who planned for the publication of this book, Pung-Hyun Sung, a professor of nuclear and quantum engineering, said, “Various information, including the history, roles and future prospects of engineering, is contained in this book. It will be a precious guide for future engineers and members of the general public who are interested in understanding why engineering is so important.”
2013.11.04 View 5956
A beginner's book for engineering co-published by KAIST professors
A group of KAIST professors published a book (Korean) to draw public attention to engineering.
The book, titled ‘What is Engineering?’, was co-published by 19 KAIST engineering professors for future engineering students and readers interested in exploring what engineering is about.
The co-authors wish to show talented students how important, fun, and helpful engineering is in improving our everyday life even though it is still not a very popular major in Korea.The book contains general knowledge in a wide array of engieering fields such as mechanics, civil, electrical & electronics, materials, chemistry, aerospace, marine systems, nuclear, industrial design, knowledge service, and bio and brain.One of the co-authors who planned for the publication of this book, Pung-Hyun Sung, a professor of nuclear and quantum engineering, said, “Various information, including the history, roles and future prospects of engineering, is contained in this book. It will be a precious guide for future engineers and members of the general public who are interested in understanding why engineering is so important.”
2013.11.04 View 5956 -
 Collaboration with Korea Institute of Energy Research
KAIST and the Korea Institute of Energy Research (KIER) agreed on September 4th to further collaboration on energy research such as the development of nano-based hybrid solar cells, bio-fuels, artificial photosynthesis, and carbon dioxide reduction.
The two institutions will select 11 research projects to focus on their cooperation. President Steve Kang (in the right) stood with Jooho Whang, the president of KIER (in the left), holding the signed memorandum of understanding.
2013.11.04 View 8431
Collaboration with Korea Institute of Energy Research
KAIST and the Korea Institute of Energy Research (KIER) agreed on September 4th to further collaboration on energy research such as the development of nano-based hybrid solar cells, bio-fuels, artificial photosynthesis, and carbon dioxide reduction.
The two institutions will select 11 research projects to focus on their cooperation. President Steve Kang (in the right) stood with Jooho Whang, the president of KIER (in the left), holding the signed memorandum of understanding.
2013.11.04 View 8431 -
 KAIST's classes now available to take from all around the world
Signed a partnership agreement with Coursera to provide millions of people with online courses in science and technology.
The Korea Advanced Institute of Science and Technology (KAIST), a world-leading research university focusing on science, engineering and technology, joined a new, online platform for open access that serves the needs of Korean and global learners.
KAIST and Coursera, the world"s largest provider of massive open online courses (MOOCs), agreed on October 14th, 2013 to partner for the provision of internet-based open learning, through which the university expects to reinforce its current education initiative, Education 3.0.Steve Kang, president of KAIST, was upbeat about the partnership."We know the benefits and importance of online education that will significantly impact the landscape of today"s higher education. Hopefully, our partnership with Coursera will expand our initiative to continuously provide quality education globally."
With its network of 107 prestigious partner universities and public institutions worldwide, Coursera offers 482 free online courses across a wide field of humanities, science, engineering, and business to 5 million students around the globe.
KAIST will be able to utilize top-notch online courses and lecture contents available on the company"s website. The university can also supply its online courses to the global community, allowing the faculty"s top quality lectures to reach hundreds and thousands of students and adult learners throughout the world.Incorporating advanced information and communications technology, KAIST has implemented a new, smart education program, Education 3.0, since 2012 to effectively meet the growing demands of creating a better and more interactive learning and teaching environment for students and faculty. Under Education 3.0, students study online and meet in groups with a professor for discussions and problem solving.
Tae-Eog Lee, Director of the Center for Excellence in Learning & Teaching at KAIST, said:"We received a phenomenal response from students and professors to the courses made available under Education 3.0. For this year alone, we are offering 60 courses, such classes as calculus, general biology, basic programming, design and communication, bioengineering fundamentals, and logic and artificial intelligence."
Professor Lee added:"It has turned out that our education initiative is not only useful to our students but also quite popular among learners outside the university and Korea. It"s a great thing that KAIST can contribute to the world"s concerted efforts to provide equal opportunities for learning. At the same time, we look forward to seeing the benefits of MOOC-based content being used in our classrooms."
Founded in 2012 by two eminent Stanford University professors, Coursera has held a strong lead in MOOCs. Unlike the traditional online education model, open courseware (OCW), designed for simply sharing lecture materials including videos, slides, and data through the internet, MOOCs develop and evaluate courses, lecture contents, and delivery quality to meet high academic standards—In order to earn credits, subscribers (universities and students) are required to submit course registration, specification, and description; student attendance roster; homework and assignments; and assessment.
Daphne Koller, co-founder of Coursera, commented on the partnership agreement with KAIST:"We are honored to have so many brilliant minds working together to expand educational opportunities globally. To be able to offer courses from professors at the forefront of their fields to millions of people is truly remarkable, and our students remind us daily of the value of spreading this knowledge globally."
Among the partner universities and institutions are Stanford University, California Institute of Technology, Columbia University, École Polytechnique Fédérale de Lausanne, Technion-Israel Institute of Technology, the National University of Singapore, the University of Tokyo, the World Bank, and Shanghai Jiao Tong University.
President Steve Kang (in the left) singed a partnership agreement with Dr. Daphne Koller (in the right), president and CEO of Coursera.
2013.11.04 View 10282
KAIST's classes now available to take from all around the world
Signed a partnership agreement with Coursera to provide millions of people with online courses in science and technology.
The Korea Advanced Institute of Science and Technology (KAIST), a world-leading research university focusing on science, engineering and technology, joined a new, online platform for open access that serves the needs of Korean and global learners.
KAIST and Coursera, the world"s largest provider of massive open online courses (MOOCs), agreed on October 14th, 2013 to partner for the provision of internet-based open learning, through which the university expects to reinforce its current education initiative, Education 3.0.Steve Kang, president of KAIST, was upbeat about the partnership."We know the benefits and importance of online education that will significantly impact the landscape of today"s higher education. Hopefully, our partnership with Coursera will expand our initiative to continuously provide quality education globally."
With its network of 107 prestigious partner universities and public institutions worldwide, Coursera offers 482 free online courses across a wide field of humanities, science, engineering, and business to 5 million students around the globe.
KAIST will be able to utilize top-notch online courses and lecture contents available on the company"s website. The university can also supply its online courses to the global community, allowing the faculty"s top quality lectures to reach hundreds and thousands of students and adult learners throughout the world.Incorporating advanced information and communications technology, KAIST has implemented a new, smart education program, Education 3.0, since 2012 to effectively meet the growing demands of creating a better and more interactive learning and teaching environment for students and faculty. Under Education 3.0, students study online and meet in groups with a professor for discussions and problem solving.
Tae-Eog Lee, Director of the Center for Excellence in Learning & Teaching at KAIST, said:"We received a phenomenal response from students and professors to the courses made available under Education 3.0. For this year alone, we are offering 60 courses, such classes as calculus, general biology, basic programming, design and communication, bioengineering fundamentals, and logic and artificial intelligence."
Professor Lee added:"It has turned out that our education initiative is not only useful to our students but also quite popular among learners outside the university and Korea. It"s a great thing that KAIST can contribute to the world"s concerted efforts to provide equal opportunities for learning. At the same time, we look forward to seeing the benefits of MOOC-based content being used in our classrooms."
Founded in 2012 by two eminent Stanford University professors, Coursera has held a strong lead in MOOCs. Unlike the traditional online education model, open courseware (OCW), designed for simply sharing lecture materials including videos, slides, and data through the internet, MOOCs develop and evaluate courses, lecture contents, and delivery quality to meet high academic standards—In order to earn credits, subscribers (universities and students) are required to submit course registration, specification, and description; student attendance roster; homework and assignments; and assessment.
Daphne Koller, co-founder of Coursera, commented on the partnership agreement with KAIST:"We are honored to have so many brilliant minds working together to expand educational opportunities globally. To be able to offer courses from professors at the forefront of their fields to millions of people is truly remarkable, and our students remind us daily of the value of spreading this knowledge globally."
Among the partner universities and institutions are Stanford University, California Institute of Technology, Columbia University, École Polytechnique Fédérale de Lausanne, Technion-Israel Institute of Technology, the National University of Singapore, the University of Tokyo, the World Bank, and Shanghai Jiao Tong University.
President Steve Kang (in the left) singed a partnership agreement with Dr. Daphne Koller (in the right), president and CEO of Coursera.
2013.11.04 View 10282 -
 KAIST Hosted the 6th International Presidential Forum on Global Research Universities
More than 120 global leaders from higher education, private and public sectors, to discuss the promotion of economic growth through knowledge creation and entrepreneurship
The Korea Advanced Institute of Science and Technology (KAIST) held the 6th International Presidential Forum on Global Research Universities (IPFGRU) on October 15th at the Westin Chosun Hotel in Seoul, Republic of Korea.
About 64 presidents and vice presidents from 57 research universities in 28 nations attended for a presentation and panel discussion on the topic of “The Role and Responsibility of Research Universities: Knowledge Creation, Technology Transfer, and Entrepreneurship.”Annually held, the forum is organized to promote excellence and innovation in higher education and provide a place for discussion among prominent research university leaders and key policy-makers in the private and public sectors from across the world.Among the notable universities attending the 2013 forum were the University of California, Irvine, the École Polytechnique Fédérale de Lausanne, Technische Universität Berlin, Technion-Israel Institute of Technology, Tokyo Institute of Technology, Rice University, the University of Waterloo, and Massachusetts Institute of Technology (MIT). Government officials as well as representatives from business and industry such as Samsung Electronics, Korea Telecom, and Elsevier also joined the event.
The forum was proceeded with three separate sessions: Enabling Knowledge Creation, Entrepreneurship & University-Based Technology Transfer, and Higher Education & Strategic Knowledge Creation: Specialization & Performance, through which speakers and panelists examined how universities have played a role in knowledge creation and technology transfer, and ultimately how they have contributed to the development of national economies.
Keynote speakers were Michael Drake, chancellor of UC Irvine, and Jörg Steinbach, president of Technische Universität Berlin. Forum participants shared their experiences and insights in starting up knowledge- and technolgy-based new businesses.
Steve Kang, president of KAIST, talked about the purpose of the 2013 IPFGRU:
“In the face of an ever-changing economic climate driven by shifts in technological advancement, demographic trends, and global integration, the role of research universities is becoming ever more significant in achieving sustainable economic growth. This forum will help participants from around the world to define the choices ahead as universities seek the most productive and beneficial models for cooperation with industry, venture startups, and government.”For the 2013 IPFGRU, Ministry of Science, ICT, and Future Planning, ROK, Saudi Aramco, Samsung Heavy Industries, S-Oil, Elsevier, Thomson Reuters, and the Korea Economic Daily were forum sponsors.
2013.11.04 View 11057
KAIST Hosted the 6th International Presidential Forum on Global Research Universities
More than 120 global leaders from higher education, private and public sectors, to discuss the promotion of economic growth through knowledge creation and entrepreneurship
The Korea Advanced Institute of Science and Technology (KAIST) held the 6th International Presidential Forum on Global Research Universities (IPFGRU) on October 15th at the Westin Chosun Hotel in Seoul, Republic of Korea.
About 64 presidents and vice presidents from 57 research universities in 28 nations attended for a presentation and panel discussion on the topic of “The Role and Responsibility of Research Universities: Knowledge Creation, Technology Transfer, and Entrepreneurship.”Annually held, the forum is organized to promote excellence and innovation in higher education and provide a place for discussion among prominent research university leaders and key policy-makers in the private and public sectors from across the world.Among the notable universities attending the 2013 forum were the University of California, Irvine, the École Polytechnique Fédérale de Lausanne, Technische Universität Berlin, Technion-Israel Institute of Technology, Tokyo Institute of Technology, Rice University, the University of Waterloo, and Massachusetts Institute of Technology (MIT). Government officials as well as representatives from business and industry such as Samsung Electronics, Korea Telecom, and Elsevier also joined the event.
The forum was proceeded with three separate sessions: Enabling Knowledge Creation, Entrepreneurship & University-Based Technology Transfer, and Higher Education & Strategic Knowledge Creation: Specialization & Performance, through which speakers and panelists examined how universities have played a role in knowledge creation and technology transfer, and ultimately how they have contributed to the development of national economies.
Keynote speakers were Michael Drake, chancellor of UC Irvine, and Jörg Steinbach, president of Technische Universität Berlin. Forum participants shared their experiences and insights in starting up knowledge- and technolgy-based new businesses.
Steve Kang, president of KAIST, talked about the purpose of the 2013 IPFGRU:
“In the face of an ever-changing economic climate driven by shifts in technological advancement, demographic trends, and global integration, the role of research universities is becoming ever more significant in achieving sustainable economic growth. This forum will help participants from around the world to define the choices ahead as universities seek the most productive and beneficial models for cooperation with industry, venture startups, and government.”For the 2013 IPFGRU, Ministry of Science, ICT, and Future Planning, ROK, Saudi Aramco, Samsung Heavy Industries, S-Oil, Elsevier, Thomson Reuters, and the Korea Economic Daily were forum sponsors.
2013.11.04 View 11057 -
 A powerful strategy for developing microbial cell factories by employing synthetic small RNAs
The current systems for the production of chemicals, fuels and materials heavily rely on the use of fossil resources. Due to the increasing concerns on climate change and other environmental problems, however, there has been much interest in developing biorefineries for the production of such chemicals, fuels and materials from renewable resources. For the biorefineries to be competitive with the traditional fossil resource-based refineries, development of high performance microorganisms is the most important as it will affect the overall economics of the process most significantly. Metabolic engineering, which can be defined as purposeful modification of cellular metabolic and regulatory networks with an aim to improve the production of a desired product, has been successfully employed to improve the performance of the cell. However, it is not trivial to engineer the cellular metabolism and regulatory circuits in the cell due to their high complexity.
In metabolic engineering, it is important to find the genes that need to be amplified and attenuated in order to increase the product formation rate while minimizing the production of undesirable byproducts. Gene knock-out experiments are often performed to delete those metabolic fluxes that will consequently result in the increase of the desired product formation. However, gene knock-out experiments require much effort and time to perform, and are difficult to do for a large number of genes. Furthermore, the gene knock-out experiments performed in one strain cannot be transferred to another organism and thus the whole experimental process has to be repeated. This is a big problem in developing a high performance microbial cell factory because it is required to find the best platform strain among many different strains. Therefore, researchers have been eager to develop a strategy that allows rapid identification of multiple genes to be attenuated in multiple strains at the same time.
A Korean research team led by Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering from the Korea Advanced Institute of Science and Technology (KAIST) reported the development of a strategy for efficiently developing microbial cell factories by employing synthetic small RNAs (sRNAs). They first reported the development of such system in Nature Biotechnology last February. This strategy of employing synthetic sRNAs in metabolic engineering has been receiving great interest worldwide as it allows easy, rapid, high-throughput, tunable, and un-doable knock-down of multiple genes in multiple strains at the same time. The research team published a paper online on August 8 as a cover page (September issue) in Nature Protocols, describing the detailed strategy and protocol to employ synthetic sRNAs for metabolic engineering.
In this paper, researchers described the detailed step-by-step protocol for synthetic sRNA-based gene expression control, including the sRNA design principles. Tailor-made synthetic sRNAs can be easily manipulated by using conventional gene cloning method. The use of synthetic sRNAs for gene expression regulation provides several advantages such as portability, conditionality, and tunability in high-throughput experiments. Plasmid-based synthetic sRNA expression system does not leave any scar on the chromosome, and can be easily transferred to many other host strains to be examined. Thus, the construction of libraries and examination of different host strains are much easier than the conventional hard-coded gene manipulation systems. Also, the expression of genes can be conditionally repressed by controlling the production of synthetic sRNAs. Synthetic sRNAs possessing different repression efficiencies make it possible to finely tune the gene expression levels as well. Furthermore, synthetic sRNAs allow knock-down of the expression of essential genes, which was not possible by conventional gene knock-out experiments.
Synthetic sRNAs can be utilized for diverse experiments where gene expression regulation is needed. One of promising applications is high-throughput screening of the target genes to be manipulated and multiple strains simultaneously to enhance the production of chemicals and materials of interest. Such simultaneous optimization of gene targets and strains has been one of the big challenges in metabolic engineering. Another application is to fine tune the expression of the screened genes for flux optimization, which would enhance chemical production further by balancing the flux between biomass formation and target chemical production. Synthetic sRNAs can also be applied to finely regulating genetic interactions in a circuit or network, which is essential in synthetic biology. Once a sRNA scaffold-harboring plasmid is constructed, tailor-made, synthetic sRNAs can be made within 3-4 days, followed by the desired application experiments.
Dr. Eytan Zlotorynski, an editor at Nature Protocols, said "This paper describes the detailed protocol for the design and applications of synthetic sRNA. The method, which has many advantages, is likely to become common practice, and prove useful for metabolic engineering and synthetic biology studies."
This paper published in Nature Protocols will be useful for all researchers in academia and industry who are interested in the use of synthetic sRNAs for fundamental and applied biological and biotechnological studies.
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012-C1AAA001-2012M1A2A2026556) and the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) of the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea.
2013.10.31 View 10190
A powerful strategy for developing microbial cell factories by employing synthetic small RNAs
The current systems for the production of chemicals, fuels and materials heavily rely on the use of fossil resources. Due to the increasing concerns on climate change and other environmental problems, however, there has been much interest in developing biorefineries for the production of such chemicals, fuels and materials from renewable resources. For the biorefineries to be competitive with the traditional fossil resource-based refineries, development of high performance microorganisms is the most important as it will affect the overall economics of the process most significantly. Metabolic engineering, which can be defined as purposeful modification of cellular metabolic and regulatory networks with an aim to improve the production of a desired product, has been successfully employed to improve the performance of the cell. However, it is not trivial to engineer the cellular metabolism and regulatory circuits in the cell due to their high complexity.
In metabolic engineering, it is important to find the genes that need to be amplified and attenuated in order to increase the product formation rate while minimizing the production of undesirable byproducts. Gene knock-out experiments are often performed to delete those metabolic fluxes that will consequently result in the increase of the desired product formation. However, gene knock-out experiments require much effort and time to perform, and are difficult to do for a large number of genes. Furthermore, the gene knock-out experiments performed in one strain cannot be transferred to another organism and thus the whole experimental process has to be repeated. This is a big problem in developing a high performance microbial cell factory because it is required to find the best platform strain among many different strains. Therefore, researchers have been eager to develop a strategy that allows rapid identification of multiple genes to be attenuated in multiple strains at the same time.
A Korean research team led by Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering from the Korea Advanced Institute of Science and Technology (KAIST) reported the development of a strategy for efficiently developing microbial cell factories by employing synthetic small RNAs (sRNAs). They first reported the development of such system in Nature Biotechnology last February. This strategy of employing synthetic sRNAs in metabolic engineering has been receiving great interest worldwide as it allows easy, rapid, high-throughput, tunable, and un-doable knock-down of multiple genes in multiple strains at the same time. The research team published a paper online on August 8 as a cover page (September issue) in Nature Protocols, describing the detailed strategy and protocol to employ synthetic sRNAs for metabolic engineering.
In this paper, researchers described the detailed step-by-step protocol for synthetic sRNA-based gene expression control, including the sRNA design principles. Tailor-made synthetic sRNAs can be easily manipulated by using conventional gene cloning method. The use of synthetic sRNAs for gene expression regulation provides several advantages such as portability, conditionality, and tunability in high-throughput experiments. Plasmid-based synthetic sRNA expression system does not leave any scar on the chromosome, and can be easily transferred to many other host strains to be examined. Thus, the construction of libraries and examination of different host strains are much easier than the conventional hard-coded gene manipulation systems. Also, the expression of genes can be conditionally repressed by controlling the production of synthetic sRNAs. Synthetic sRNAs possessing different repression efficiencies make it possible to finely tune the gene expression levels as well. Furthermore, synthetic sRNAs allow knock-down of the expression of essential genes, which was not possible by conventional gene knock-out experiments.
Synthetic sRNAs can be utilized for diverse experiments where gene expression regulation is needed. One of promising applications is high-throughput screening of the target genes to be manipulated and multiple strains simultaneously to enhance the production of chemicals and materials of interest. Such simultaneous optimization of gene targets and strains has been one of the big challenges in metabolic engineering. Another application is to fine tune the expression of the screened genes for flux optimization, which would enhance chemical production further by balancing the flux between biomass formation and target chemical production. Synthetic sRNAs can also be applied to finely regulating genetic interactions in a circuit or network, which is essential in synthetic biology. Once a sRNA scaffold-harboring plasmid is constructed, tailor-made, synthetic sRNAs can be made within 3-4 days, followed by the desired application experiments.
Dr. Eytan Zlotorynski, an editor at Nature Protocols, said "This paper describes the detailed protocol for the design and applications of synthetic sRNA. The method, which has many advantages, is likely to become common practice, and prove useful for metabolic engineering and synthetic biology studies."
This paper published in Nature Protocols will be useful for all researchers in academia and industry who are interested in the use of synthetic sRNAs for fundamental and applied biological and biotechnological studies.
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012-C1AAA001-2012M1A2A2026556) and the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) of the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea.
2013.10.31 View 10190 -
 Distinguished Professor Sang Yup Lee appointed as an advisor for Shanghai Jiao Tong University in China
In recognition of his outstanding accomplishments in the area of bioengineering, specializing in metabolic engineering, Sang Yup Lee, a distinguished professor of Chemical & Biomolecular Engineering at KAIST, was assigned as an advisory professor for the bioengineering department at Shanghai Jiao Tong University in China for five years from August 2013 to July 2018.
Together with Peking University and Tsinghua University, Shanghai Jiao Tong University is one of the top three universities in China.
The advisory professors carry out collaborated research programs in special areas and provide advice on education and research issues.
Professor Lee, a specialist in metabolic engineering, has initiated systems metabolic engineering which integrates metabolic engineering, systems biology, and synthetic biology and has applied it to various chemical production systems to develop bio fuel and many eco-friendly chemical production processes. Recently, he received the Marvin J. Johnson Award from the American Chemistry Society, the Charles Thom Award from the American Society for Industrial Microbiology, as well as the Amgen Biochemical Engineering Award. As a global leader in the area of bioengineering, Professor Lee is a member of the Korean Academy of Science & Technology, the National Academy of Engineering of Korea, the US National Academy of Engineering, and is the chairman of the Global Agenda Council on Biotechnology at the World Economic Forum.
2013.10.31 View 9371
Distinguished Professor Sang Yup Lee appointed as an advisor for Shanghai Jiao Tong University in China
In recognition of his outstanding accomplishments in the area of bioengineering, specializing in metabolic engineering, Sang Yup Lee, a distinguished professor of Chemical & Biomolecular Engineering at KAIST, was assigned as an advisory professor for the bioengineering department at Shanghai Jiao Tong University in China for five years from August 2013 to July 2018.
Together with Peking University and Tsinghua University, Shanghai Jiao Tong University is one of the top three universities in China.
The advisory professors carry out collaborated research programs in special areas and provide advice on education and research issues.
Professor Lee, a specialist in metabolic engineering, has initiated systems metabolic engineering which integrates metabolic engineering, systems biology, and synthetic biology and has applied it to various chemical production systems to develop bio fuel and many eco-friendly chemical production processes. Recently, he received the Marvin J. Johnson Award from the American Chemistry Society, the Charles Thom Award from the American Society for Industrial Microbiology, as well as the Amgen Biochemical Engineering Award. As a global leader in the area of bioengineering, Professor Lee is a member of the Korean Academy of Science & Technology, the National Academy of Engineering of Korea, the US National Academy of Engineering, and is the chairman of the Global Agenda Council on Biotechnology at the World Economic Forum.
2013.10.31 View 9371 -
 First Prize in the 2013 International Military Science and Technology Contest
Professor James R. Morrison and his students of the Industrial and Systems Engineering Department at KAIST were awarded the first prize in the 2013 International Military Science and Technology Contest organized by the Defense Acquisition Program Administration held in COEX from July 11 to 14.
The research group, Byungduk Song (Ph.D candidate), Jonghoe Kim (Ph.D candidate), Hyolin Park (MS candidate) and Professor James R. Morrison, received the first prize with their paper entitled “Automated and persistent UAV system for a complementary method for border patrol and target tracking.”
The Defense Acquisition Program Administration is the host of the annual contest which aims to contribute to the future of the defense industry and to expand technology exchange between private institutes and the military through the coordination of defense technology and advanced technology from industrial and educational cooperation.Professor Morrison’s team received the honor of the first-place prize out of 56 competitors from within Korea and 7 from overseas in the field of Synthetic New Technology/Academic Thesis.
2013.10.31 View 9589
First Prize in the 2013 International Military Science and Technology Contest
Professor James R. Morrison and his students of the Industrial and Systems Engineering Department at KAIST were awarded the first prize in the 2013 International Military Science and Technology Contest organized by the Defense Acquisition Program Administration held in COEX from July 11 to 14.
The research group, Byungduk Song (Ph.D candidate), Jonghoe Kim (Ph.D candidate), Hyolin Park (MS candidate) and Professor James R. Morrison, received the first prize with their paper entitled “Automated and persistent UAV system for a complementary method for border patrol and target tracking.”
The Defense Acquisition Program Administration is the host of the annual contest which aims to contribute to the future of the defense industry and to expand technology exchange between private institutes and the military through the coordination of defense technology and advanced technology from industrial and educational cooperation.Professor Morrison’s team received the honor of the first-place prize out of 56 competitors from within Korea and 7 from overseas in the field of Synthetic New Technology/Academic Thesis.
2013.10.31 View 9589 -
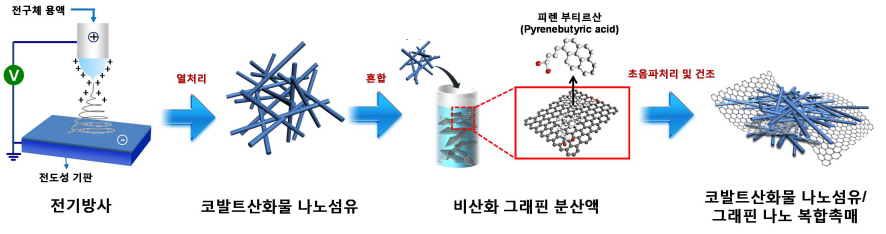 Core Technology for Lithium Air Secondary Battery Developed
KAIST-Kyonggi University joint research team developed composite catalyst out of nano fiber and graphene
Five times improvement in capacity compared to lithium-ion secondary battery, driving 800 km at maximum
The core technology for lithium air secondary battery, the next generation high capacity battery, has been developed.
A research team formed by KAIST Department of Materials Science’s Professors Il-Doo Kim and Seokwoo Jeon, and Kyonggi University Department of Materials Science’s Professor Yong-Joon Park has created a lithium air secondary battery, with five times greater storage than the lithium-ion secondary battery, by developing a nano fiber-graphene composite catalyst. The research results are published in the August 8th online edition of Nano Letters.
A cathode of a lithium-ion battery consists of graphite and an anode of the battery consists of a lithium transition metal oxide. Lithium-ion batteries are widely used in mobile phones and laptops. However, lithium-ion batteries cannot support electric vehicles, providing energy for only 160 kilometers on one full charge. The lithium air secondary battery just developed by the research team uses lithium on the cathode and oxygen on the anode. It is earning a popular acknowledgement among the next generation secondary battery research community for having lightweight mass and high energy density.
However, lithium-ion batteries remain difficult to commercialize because of their short lifespan. Lithium and oxygen meet up to form lithium oxide (Li2O2) at discharge, and decompose again at charge. In a traditional lithium air battery, this cycle does not occur smoothly and results in high resistance, thereby reducing the lifespan of the battery. It is thus essential to develop high efficiency catalyst that facilitates the formation and decomposition of lithium oxides.
The research team used electric radiation to develop a nano composite catalyst by mixing cobalt oxide nano fiber and graphene. The performance of the battery has been maximized by settling nonoxidative graphene, which has high specific surface area and electrical conductivity, on catalyst active cobalt oxide nano fiber. Applying the nano composite catalyst on both poles of the lithium air battery resulted in an improved lifespan of over 80 recharge cycles with capacity greater than 100mAh/g, five times greater than a lithium ion battery. The newly discovered charge-discharge property is the highest among the reported performances of the lithium air battery so far.
The lithium air battery is cheap to make, as the main materials are metal oxide and graphene. “There are yet more issues to resolve such as stability, but we will collaborate with other organizations to open up the era of electronic vehicles,” said Professor Il-Doo Kim. “We hope to contribute to vitalizing the fields of next generation lithium air battery by leading nanocatalyst synthesis technology, one of the core materials in the fields of secondary battery,” Professor Kim spoke of his aspiration.
The graduate students participated in the research are Won-Hee Ryu, a postdoctorate at KAIST Department of Materials Science, Sungho Song, a PhD candidate at KAIST Department of Materials Science, and Taek-Han Yoon, a graduate student at Kyonggi University.
Picture I: Schematic Diagram of Lithium Air Battery Made of Nano Composite Catalysts
Picture II: Images of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts
Picture III: Images of Manufacturing Process of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts for Lithium Air Battery
2013.10.18 View 12454
Core Technology for Lithium Air Secondary Battery Developed
KAIST-Kyonggi University joint research team developed composite catalyst out of nano fiber and graphene
Five times improvement in capacity compared to lithium-ion secondary battery, driving 800 km at maximum
The core technology for lithium air secondary battery, the next generation high capacity battery, has been developed.
A research team formed by KAIST Department of Materials Science’s Professors Il-Doo Kim and Seokwoo Jeon, and Kyonggi University Department of Materials Science’s Professor Yong-Joon Park has created a lithium air secondary battery, with five times greater storage than the lithium-ion secondary battery, by developing a nano fiber-graphene composite catalyst. The research results are published in the August 8th online edition of Nano Letters.
A cathode of a lithium-ion battery consists of graphite and an anode of the battery consists of a lithium transition metal oxide. Lithium-ion batteries are widely used in mobile phones and laptops. However, lithium-ion batteries cannot support electric vehicles, providing energy for only 160 kilometers on one full charge. The lithium air secondary battery just developed by the research team uses lithium on the cathode and oxygen on the anode. It is earning a popular acknowledgement among the next generation secondary battery research community for having lightweight mass and high energy density.
However, lithium-ion batteries remain difficult to commercialize because of their short lifespan. Lithium and oxygen meet up to form lithium oxide (Li2O2) at discharge, and decompose again at charge. In a traditional lithium air battery, this cycle does not occur smoothly and results in high resistance, thereby reducing the lifespan of the battery. It is thus essential to develop high efficiency catalyst that facilitates the formation and decomposition of lithium oxides.
The research team used electric radiation to develop a nano composite catalyst by mixing cobalt oxide nano fiber and graphene. The performance of the battery has been maximized by settling nonoxidative graphene, which has high specific surface area and electrical conductivity, on catalyst active cobalt oxide nano fiber. Applying the nano composite catalyst on both poles of the lithium air battery resulted in an improved lifespan of over 80 recharge cycles with capacity greater than 100mAh/g, five times greater than a lithium ion battery. The newly discovered charge-discharge property is the highest among the reported performances of the lithium air battery so far.
The lithium air battery is cheap to make, as the main materials are metal oxide and graphene. “There are yet more issues to resolve such as stability, but we will collaborate with other organizations to open up the era of electronic vehicles,” said Professor Il-Doo Kim. “We hope to contribute to vitalizing the fields of next generation lithium air battery by leading nanocatalyst synthesis technology, one of the core materials in the fields of secondary battery,” Professor Kim spoke of his aspiration.
The graduate students participated in the research are Won-Hee Ryu, a postdoctorate at KAIST Department of Materials Science, Sungho Song, a PhD candidate at KAIST Department of Materials Science, and Taek-Han Yoon, a graduate student at Kyonggi University.
Picture I: Schematic Diagram of Lithium Air Battery Made of Nano Composite Catalysts
Picture II: Images of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts
Picture III: Images of Manufacturing Process of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts for Lithium Air Battery
2013.10.18 View 12454 -
 Nanowire Made of Diverse Materials May Become Marketable
- Technology to commercialize nanowire developed after 2 years of industrial-academic joint research -
- 2 million strands of 50nm-width, 20 cm-length nanowire mass producible in 2 hours –
A South Korean joint industrial-academic research team has developed the technology to put forward the commercialization of nanowire that is only a few nanometers wide. It is expected to be applied in various fields such as semiconductors, high performance sensors, and biodevices.
In cooperation with LG Innotek and the National Nanofab center, Professor Jun-Bo Yoon, from KAIST Department of Electrical Engineering, developed the technology to mass produce nanowire at any length with various materials. The research results are published on the online edition of Nano Letters on July 30th.
Nanowire has a long linear structure with its width at 100 nanometers at maximum. It is a multifunctional material that has yet undiscovered thermal, electric, and mechanical properties. Nanowire is highly acclaimed as a cutting-edge material with unique nano-level properties that can be applied in semiconductors, energy, biodevices, and optic devices.
Previously, nanowires had an extremely low synthesis rate that required three or four days to grow few millimeters. It was therefore difficult to produce the desired products using nanowires. Moreover, nanowires needed to be evenly arranged for practical application, but the traditional technology required complex post-treatment, not to mention the arrangement was not immaculate.
The research team applied semiconductor process instead of chemical synthesis to resolve these issues. The team first formed a pattern greater that of the target frequency by using a photo-engraving process on a silicon wafer board whose diameter was 20 centimeters, then repeatedly reduced the frequency to produce 100 nm ultrafine linear grid pattern. Based on this pattern, the research team applied the sputtering process to mass-produce nanowires in perfect shapes of 50 nm width and 20 cm maximum length.
The new technology requires neither a lengthy synthesis process nor post-cleaning to attain a perfectly aligned state. Thus, academic and industrial circles consider the technology has high possibilities for commercialization.
“The significance is in resolving the issues in traditional technology, such as low productivity, long manufacturing time, restrictions in material synthesis, and nanowire alignment,” commented Professor Yoon on this research. “Nanowires have not been widely applied in the industry, but this technology will bring forward the commercialization of high performance semiconductors, optic devices, and biodevices that make use of nanowires.”
2013.10.18 View 9856
Nanowire Made of Diverse Materials May Become Marketable
- Technology to commercialize nanowire developed after 2 years of industrial-academic joint research -
- 2 million strands of 50nm-width, 20 cm-length nanowire mass producible in 2 hours –
A South Korean joint industrial-academic research team has developed the technology to put forward the commercialization of nanowire that is only a few nanometers wide. It is expected to be applied in various fields such as semiconductors, high performance sensors, and biodevices.
In cooperation with LG Innotek and the National Nanofab center, Professor Jun-Bo Yoon, from KAIST Department of Electrical Engineering, developed the technology to mass produce nanowire at any length with various materials. The research results are published on the online edition of Nano Letters on July 30th.
Nanowire has a long linear structure with its width at 100 nanometers at maximum. It is a multifunctional material that has yet undiscovered thermal, electric, and mechanical properties. Nanowire is highly acclaimed as a cutting-edge material with unique nano-level properties that can be applied in semiconductors, energy, biodevices, and optic devices.
Previously, nanowires had an extremely low synthesis rate that required three or four days to grow few millimeters. It was therefore difficult to produce the desired products using nanowires. Moreover, nanowires needed to be evenly arranged for practical application, but the traditional technology required complex post-treatment, not to mention the arrangement was not immaculate.
The research team applied semiconductor process instead of chemical synthesis to resolve these issues. The team first formed a pattern greater that of the target frequency by using a photo-engraving process on a silicon wafer board whose diameter was 20 centimeters, then repeatedly reduced the frequency to produce 100 nm ultrafine linear grid pattern. Based on this pattern, the research team applied the sputtering process to mass-produce nanowires in perfect shapes of 50 nm width and 20 cm maximum length.
The new technology requires neither a lengthy synthesis process nor post-cleaning to attain a perfectly aligned state. Thus, academic and industrial circles consider the technology has high possibilities for commercialization.
“The significance is in resolving the issues in traditional technology, such as low productivity, long manufacturing time, restrictions in material synthesis, and nanowire alignment,” commented Professor Yoon on this research. “Nanowires have not been widely applied in the industry, but this technology will bring forward the commercialization of high performance semiconductors, optic devices, and biodevices that make use of nanowires.”
2013.10.18 View 9856 -
 Prof. Jiyun Lee receives the U.S. FAA Achievement Award
- Ensures the safe operation of aircraft by monitoring ionospheric changes caused by solar storms.
KAIST’s Aerospace Engineering Department’s Professor Jiyun Lee has received an award from the U.S. Federal Aviation Administration (FAA), in recognition of her work for developing a Global Positioning System (GPS) reinforcing system and improving Satellite Navigation technology.
A GPS reinforcing system provides real-time GPS location and integrity information within 1m ranges to enable the accurate and safe navigation of aircraft. However, when the sun reaches Solar Max, the amount of total electron increases rapidly in the ionosphere. This also increases the possibility of the position error of navigation using the GPS reinforcing system. In order to solve this problem, Professor Lee built an Ionosphere Danger model that monitors the changes in the ionosphere due to solar storm. The model has been implemented into original monitoring software that secures the safety of the GPS reinforcing system user. The research results were published in July 2012 in Radio Science, one of the most prestigious international journals in the field of geophysical studies.
The FAA Technical Center successfully verified Professor Lee’s software and the software is currently being distributed and used by major institutions around the globe, including Eurocontrol. It is expected that the software will be standardized after consultations with international organizations in the recent future.
Professor Lee said, “Satellite navigation is the core of future navigation technology. Since its utilization has been extended to aviation, marine, transportation, telecommunications, finance and other major national infrastructures, it is crucial to ensure the performance and reliability of the system … In the future, cooperation between nations will help to develop the worldwide service of the GPS reinforcing system.”
2013.10.12 View 9686
Prof. Jiyun Lee receives the U.S. FAA Achievement Award
- Ensures the safe operation of aircraft by monitoring ionospheric changes caused by solar storms.
KAIST’s Aerospace Engineering Department’s Professor Jiyun Lee has received an award from the U.S. Federal Aviation Administration (FAA), in recognition of her work for developing a Global Positioning System (GPS) reinforcing system and improving Satellite Navigation technology.
A GPS reinforcing system provides real-time GPS location and integrity information within 1m ranges to enable the accurate and safe navigation of aircraft. However, when the sun reaches Solar Max, the amount of total electron increases rapidly in the ionosphere. This also increases the possibility of the position error of navigation using the GPS reinforcing system. In order to solve this problem, Professor Lee built an Ionosphere Danger model that monitors the changes in the ionosphere due to solar storm. The model has been implemented into original monitoring software that secures the safety of the GPS reinforcing system user. The research results were published in July 2012 in Radio Science, one of the most prestigious international journals in the field of geophysical studies.
The FAA Technical Center successfully verified Professor Lee’s software and the software is currently being distributed and used by major institutions around the globe, including Eurocontrol. It is expected that the software will be standardized after consultations with international organizations in the recent future.
Professor Lee said, “Satellite navigation is the core of future navigation technology. Since its utilization has been extended to aviation, marine, transportation, telecommunications, finance and other major national infrastructures, it is crucial to ensure the performance and reliability of the system … In the future, cooperation between nations will help to develop the worldwide service of the GPS reinforcing system.”
2013.10.12 View 9686