Fuel+cells
-
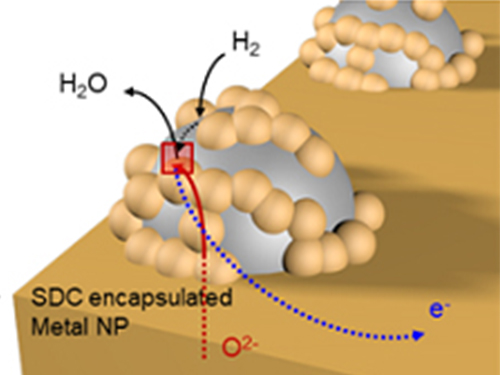 Unravelling Inherent Electrocatalysis to Improve the Performance of Hydrogen Fuel Cells
(Figure 1. Electrode structure for the precise evaluation of the metal nanoparticles’ electrochemical catalytic characteristics at a high temperature.)
A KAIST team presented an ideal electrode design to enhance the performance of high-temperature fuel cells. The new analytical platform with advanced nanoscale patterning method quantitatively revealed the electrochemical value of metal nanoparticles dispersed on the oxide electrode, thus leading to electrode design directions that can be used in a variety of eco-friendly energy technologies.
The team, working under Professor WooChul Jung and Professor Sang Ouk Kim at the Department of Materials Science and Engineering, described an accurate analysis of the reactivity of oxide electrodes boosted by metal nanoparticles, where all particles participate in the reaction. They identified how the metal catalysts activate hydrogen electro-oxidation on the ceria-based electrode surface and quantify how rapidly the reaction rate increases with the proper choice of metals.
Metal nanoparticles with diameters of 10 nanometers or less have become a key component in high-performance heterogeneous catalysts, primarily serving as a catalytic activator. Recent experimental and theoretical findings suggest that the optimization of the chemical nature at the metal and support interfaces is essential for performance improvement.
However, the high cost associated with cell fabrication and operation as well as poorer stability of metal nanoparticles at high temperatures have been a long-standing challenge. To solve this problem, the team utilized a globally recognized metal nano patterning technology that uses block copolymer self-assembled nano templates and succeeded in uniformly synthesizing metal particles 10 nanometers in size on the surface of oxide fuel cell electrodes. They also developed a technology to accurately analyze the catalyst characteristics of single particles at high temperatures and maximize the performance of a fuel cell with minimal catalyst use.
The research team confirmed that platinum, which is a commonly used metal catalyst, could boost fuel cell performance by as much as 21 times even at an amount of 300 nanograms, which only costs about 0.015 KRW.
The team quantitatively identified and compared the characteristics of widely used metal catalysts other than platinum, such as palladium, gold, and cobalt, and also elucidated the precise principle of catalyst performance through theoretical analysis.
(Figure 2. Comparison of the electrochemical catalytic characteristics for various 10nm metal nanoparticles (platinum, palladium, cobalt, gold) at a high temperature.)
Professor Jung said, "We have broken the conventional methods of increasing the amount of catalyst which have deemed inefficient and expensive. Our results suggest a clear idea for high performance fuel cells using very small amounts of nanoparticles. This technology can be applied to many different industrial fields, advancing the commercialization of eco-friendly energy technologies such as fuel cells that generate electricity and electrolytic cells that produce hydrogen from water.”
The research has been published as the cover article of Nature Nanotechnology in the March issue. This research was carried out with support from the Nano-Material Technology Development Program through the National Research Foundation of Korea.
2019.03.28 View 31191
Unravelling Inherent Electrocatalysis to Improve the Performance of Hydrogen Fuel Cells
(Figure 1. Electrode structure for the precise evaluation of the metal nanoparticles’ electrochemical catalytic characteristics at a high temperature.)
A KAIST team presented an ideal electrode design to enhance the performance of high-temperature fuel cells. The new analytical platform with advanced nanoscale patterning method quantitatively revealed the electrochemical value of metal nanoparticles dispersed on the oxide electrode, thus leading to electrode design directions that can be used in a variety of eco-friendly energy technologies.
The team, working under Professor WooChul Jung and Professor Sang Ouk Kim at the Department of Materials Science and Engineering, described an accurate analysis of the reactivity of oxide electrodes boosted by metal nanoparticles, where all particles participate in the reaction. They identified how the metal catalysts activate hydrogen electro-oxidation on the ceria-based electrode surface and quantify how rapidly the reaction rate increases with the proper choice of metals.
Metal nanoparticles with diameters of 10 nanometers or less have become a key component in high-performance heterogeneous catalysts, primarily serving as a catalytic activator. Recent experimental and theoretical findings suggest that the optimization of the chemical nature at the metal and support interfaces is essential for performance improvement.
However, the high cost associated with cell fabrication and operation as well as poorer stability of metal nanoparticles at high temperatures have been a long-standing challenge. To solve this problem, the team utilized a globally recognized metal nano patterning technology that uses block copolymer self-assembled nano templates and succeeded in uniformly synthesizing metal particles 10 nanometers in size on the surface of oxide fuel cell electrodes. They also developed a technology to accurately analyze the catalyst characteristics of single particles at high temperatures and maximize the performance of a fuel cell with minimal catalyst use.
The research team confirmed that platinum, which is a commonly used metal catalyst, could boost fuel cell performance by as much as 21 times even at an amount of 300 nanograms, which only costs about 0.015 KRW.
The team quantitatively identified and compared the characteristics of widely used metal catalysts other than platinum, such as palladium, gold, and cobalt, and also elucidated the precise principle of catalyst performance through theoretical analysis.
(Figure 2. Comparison of the electrochemical catalytic characteristics for various 10nm metal nanoparticles (platinum, palladium, cobalt, gold) at a high temperature.)
Professor Jung said, "We have broken the conventional methods of increasing the amount of catalyst which have deemed inefficient and expensive. Our results suggest a clear idea for high performance fuel cells using very small amounts of nanoparticles. This technology can be applied to many different industrial fields, advancing the commercialization of eco-friendly energy technologies such as fuel cells that generate electricity and electrolytic cells that produce hydrogen from water.”
The research has been published as the cover article of Nature Nanotechnology in the March issue. This research was carried out with support from the Nano-Material Technology Development Program through the National Research Foundation of Korea.
2019.03.28 View 31191 -
 A New Efficient Oxide Coating Technology to Improve Fuel Cells
A new efficient oxide coating technology that can be applied in less than five minutes could lead to dramatic improvements in the lifetime and performance of fuel cells. The fundamental principle behind this approach is maximizing the oxygen reduction reaction site of a platinum thin-film electrode, preventing the electrodes from aggregating at high temperatures.
Fuel cells have emerged as a clean electricity generation system that does not pollute the air. In particular, solid oxide fuel cells (SOFCs) are beginning to gain a great deal of attention due to their higher power generation efficiency compared to other fuel cells. It is also advantageous to use other power sources than expensive hydrogen fuel.
However, the high costs and insufficient lifetimes caused by high temperatures needed to operate the solid oxide fuel cells have remained significant challenges to commercialization.
Recently, attempts to lower the operating temperature (< 600°C) of these devices by introducing thin-film processes have drew attention of researchers, with the resulting products known as thin-film-based solid oxide fuel cells.
In order to create enhanced device performance at lower temperatures, the research team, led by Professor WooChul Jung in the Department of Materials Science and Engineering, applied and developed oxide coating technology to maximize the oxygen reduction reaction sites of a platinum thin-film electrode and to prevent platinum electrodes from thermal aggregating.
The team succeeded in over-coating a platinum electrode with a new coating material called praseodymium-doped ceria (Pr,Ce)O2-, which has high conductivity for both electrons and oxygen ions and excellent catalytic properties for oxygen reduction reactions. As a result, electrode resistance was reduced by more than 1000 times, creating the potential for these electrodes to be used in high-temperature electrochemical cells.
In addition, they proposed that the high performance of thin-film-based oxide fuel cells’ oxygen electrodes could be realized through the nano-structuring of (Pr,Ce)O2-δ without any platinum.
Professor Jung said, “The electrode coating technology used in this study is of great technical value because of the utilization of affordable and mass-produced electrochemical deposition.” He added, “In the future, this technology will be feasible for replacing platinum electrodes in thin-film-based oxide fuel cells, and we expect that the affordable prices of this fuel cell will eventually boost market competitiveness.”
This research was described in Advanced Energy Materials in July and was featured as the Inside Front Cover and video abstract. It was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Korea Electric Power Corporation (KEPCO) Research Institute.
Figure 1. The change of electrode activity with and without overcoated (Pr,Ce)O2-δ nanostructures.
2018.07.18 View 8955
A New Efficient Oxide Coating Technology to Improve Fuel Cells
A new efficient oxide coating technology that can be applied in less than five minutes could lead to dramatic improvements in the lifetime and performance of fuel cells. The fundamental principle behind this approach is maximizing the oxygen reduction reaction site of a platinum thin-film electrode, preventing the electrodes from aggregating at high temperatures.
Fuel cells have emerged as a clean electricity generation system that does not pollute the air. In particular, solid oxide fuel cells (SOFCs) are beginning to gain a great deal of attention due to their higher power generation efficiency compared to other fuel cells. It is also advantageous to use other power sources than expensive hydrogen fuel.
However, the high costs and insufficient lifetimes caused by high temperatures needed to operate the solid oxide fuel cells have remained significant challenges to commercialization.
Recently, attempts to lower the operating temperature (< 600°C) of these devices by introducing thin-film processes have drew attention of researchers, with the resulting products known as thin-film-based solid oxide fuel cells.
In order to create enhanced device performance at lower temperatures, the research team, led by Professor WooChul Jung in the Department of Materials Science and Engineering, applied and developed oxide coating technology to maximize the oxygen reduction reaction sites of a platinum thin-film electrode and to prevent platinum electrodes from thermal aggregating.
The team succeeded in over-coating a platinum electrode with a new coating material called praseodymium-doped ceria (Pr,Ce)O2-, which has high conductivity for both electrons and oxygen ions and excellent catalytic properties for oxygen reduction reactions. As a result, electrode resistance was reduced by more than 1000 times, creating the potential for these electrodes to be used in high-temperature electrochemical cells.
In addition, they proposed that the high performance of thin-film-based oxide fuel cells’ oxygen electrodes could be realized through the nano-structuring of (Pr,Ce)O2-δ without any platinum.
Professor Jung said, “The electrode coating technology used in this study is of great technical value because of the utilization of affordable and mass-produced electrochemical deposition.” He added, “In the future, this technology will be feasible for replacing platinum electrodes in thin-film-based oxide fuel cells, and we expect that the affordable prices of this fuel cell will eventually boost market competitiveness.”
This research was described in Advanced Energy Materials in July and was featured as the Inside Front Cover and video abstract. It was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Korea Electric Power Corporation (KEPCO) Research Institute.
Figure 1. The change of electrode activity with and without overcoated (Pr,Ce)O2-δ nanostructures.
2018.07.18 View 8955 -
 Lifespan of Fuel Cells Maximized Using Small Amount of Metals
(Professor Jung (far right) and his team)
Fuel cells are key future energy technology that is emerging as eco-friendly and renewable energy sources. In particular, solid oxide fuel cells composed of ceramic materials gain increasing attention for their ability to directly convert various forms of fuel such as biomass, LNG, and LPG to electric energy.
KAIST researchers described a new technique to improve chemical stability of electrode materials which can extend the lifespan by employing a very little amount of metals.
The core factor that determines the performance of solid oxide fuel cells is the cathode at which the reduction reaction of oxygen occurs. Conventionally, oxides with perovskite structure (ABO3) are used in cathodes. However, despite the high performance of perovskite oxides at initial operation, the performance decreases with time, limiting their long-term use. In particular, the condition of high temperature oxidation state required for cathode operation leads to surface segregation phenomenon, in which second phases such as strontium oxide (SrOx) accumulate on the surface of oxides, resulting in decrease in electrode performance. The detailed mechanism of this phenomenon and a way to effectively inhibit it has not been suggested.
Using computational chemistry and experimental data, Professor WooChul Jung’s team at the Department of Materials Science and Engineering observed that local compressive states around the Sr atoms in a perovskite electrode lattice weakened the Sr-O bond strength, which in turn promote strontium segregation. The team identified local changes in strain distribution in perovskite oxide as the main cause of segregation on strontium surface. Based on these findings, the team doped different sizes of metals in oxides to control the extent of lattice strain in cathode material and effectively inhibited strontium segregation.
Professor Jung said, “This technology can be implemented by adding a small amount of metal atoms during material synthesis, without any additional process.” He continued, “I hope this technology will be useful in developing high-durable perovskite oxide electrode in the future.”
The study co-led by Professor Jung and Professor Jeong Woo Han at Department of Chemical Engineering, University of Seoul was featured as the cover of Energy and Environmental Science in the first issue of 2018.
(Figure1.Correlation between the extent of lattice strain in electrode, strontium segregation, and electrode reaction.)
(Figure 2. Cathode surface of solid oxide fuel cell stabilized using the developed technology)
2018.01.18 View 8840
Lifespan of Fuel Cells Maximized Using Small Amount of Metals
(Professor Jung (far right) and his team)
Fuel cells are key future energy technology that is emerging as eco-friendly and renewable energy sources. In particular, solid oxide fuel cells composed of ceramic materials gain increasing attention for their ability to directly convert various forms of fuel such as biomass, LNG, and LPG to electric energy.
KAIST researchers described a new technique to improve chemical stability of electrode materials which can extend the lifespan by employing a very little amount of metals.
The core factor that determines the performance of solid oxide fuel cells is the cathode at which the reduction reaction of oxygen occurs. Conventionally, oxides with perovskite structure (ABO3) are used in cathodes. However, despite the high performance of perovskite oxides at initial operation, the performance decreases with time, limiting their long-term use. In particular, the condition of high temperature oxidation state required for cathode operation leads to surface segregation phenomenon, in which second phases such as strontium oxide (SrOx) accumulate on the surface of oxides, resulting in decrease in electrode performance. The detailed mechanism of this phenomenon and a way to effectively inhibit it has not been suggested.
Using computational chemistry and experimental data, Professor WooChul Jung’s team at the Department of Materials Science and Engineering observed that local compressive states around the Sr atoms in a perovskite electrode lattice weakened the Sr-O bond strength, which in turn promote strontium segregation. The team identified local changes in strain distribution in perovskite oxide as the main cause of segregation on strontium surface. Based on these findings, the team doped different sizes of metals in oxides to control the extent of lattice strain in cathode material and effectively inhibited strontium segregation.
Professor Jung said, “This technology can be implemented by adding a small amount of metal atoms during material synthesis, without any additional process.” He continued, “I hope this technology will be useful in developing high-durable perovskite oxide electrode in the future.”
The study co-led by Professor Jung and Professor Jeong Woo Han at Department of Chemical Engineering, University of Seoul was featured as the cover of Energy and Environmental Science in the first issue of 2018.
(Figure1.Correlation between the extent of lattice strain in electrode, strontium segregation, and electrode reaction.)
(Figure 2. Cathode surface of solid oxide fuel cell stabilized using the developed technology)
2018.01.18 View 8840