NMR
-
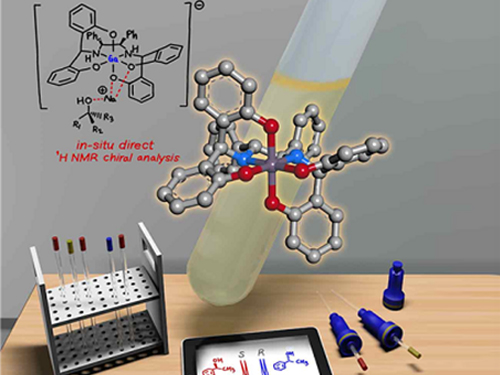 Gallium-Based Solvating Agent Efficiently Analyzes Optically Active Alcohols
A KAIST research team has developed a gallium-based metal complex enabling the rapid chiral analysis of alcohols. A team working under Professor Hyunwoo Kim reported the efficient new alcohol analysis method using nuclear magnetic resonance (NMR) spectroscopy in iScience.
Enantiopure chiral alcohols are ubiquitous in nature and widely utilized as pharmaceuticals. This importance of chirality in synthetic and medicinal chemistry has advanced the search for rapid and facile methods to determine the enantiomeric purities of compounds. To date, chiral analysis has been performed using high-performance liquid chromatography (HPLC) with chiral columns.
Along with the HPLC technique, chiral analysis using NMR spectroscopy has gained tremendous attention as an alternative to traditionally employed chromatographic methods due to its simplicity and rapid detection for real-time measurement. However, this method carries drawbacks such as line-broadening, narrow substrate scope, and poor resolution. Thus, compared with popular methods of chromatographic analysis, NMR spectroscopy is infrequently used for chiral analysis.
In principle, a chiral solvating agent is additionally required for the NMR measurement of chiral alcohols to obtain two distinct signals. However, NMR analysis of chiral alcohols has been challenging due to weak binding interactions with chiral solvating agents. To overcome the intrinsic difficulty of relatively weak molecular interactions that are common for alcohols, many researchers have used multifunctional alcohols to enhance interactions with solvating agents.
Instead, the KAIST team successfully varied the physical properties of metal complexes to induce stronger interactions with alcohols rather than the strategy of using multifunctional analytes, in the hopes of developing a universal chiral solvating agent for alcohols. Compared to the current method of chiral analysis used in the pharmaceutical industry, alcohols that do not possess chromophores can also be directly analyzed with the gallium complexes.
Professor Kim said that this method could be a complementary chiral analysis technique at the industry level in the near future. He added that since the developed gallium complex can determine enantiomeric excess within minutes, it can be further utilized to monitor asymmetric synthesis. This feature will benefit a large number of researchers in the organic chemistry community, as well as the pharmaceutical industry.
(Figure: Schematic view of the in-situ direct 1H NMR chiral analysis.)
-Profile:
Professor Hyunwoo Kim
Department of Chemistry
KAIST
http://mdos.kaist.ac.kr
hwk34@kaist.ac.kr
For more on this article,
please go to https://doi.org/10.1016/j.isci2019.07051
2019.11.14 View 11354
Gallium-Based Solvating Agent Efficiently Analyzes Optically Active Alcohols
A KAIST research team has developed a gallium-based metal complex enabling the rapid chiral analysis of alcohols. A team working under Professor Hyunwoo Kim reported the efficient new alcohol analysis method using nuclear magnetic resonance (NMR) spectroscopy in iScience.
Enantiopure chiral alcohols are ubiquitous in nature and widely utilized as pharmaceuticals. This importance of chirality in synthetic and medicinal chemistry has advanced the search for rapid and facile methods to determine the enantiomeric purities of compounds. To date, chiral analysis has been performed using high-performance liquid chromatography (HPLC) with chiral columns.
Along with the HPLC technique, chiral analysis using NMR spectroscopy has gained tremendous attention as an alternative to traditionally employed chromatographic methods due to its simplicity and rapid detection for real-time measurement. However, this method carries drawbacks such as line-broadening, narrow substrate scope, and poor resolution. Thus, compared with popular methods of chromatographic analysis, NMR spectroscopy is infrequently used for chiral analysis.
In principle, a chiral solvating agent is additionally required for the NMR measurement of chiral alcohols to obtain two distinct signals. However, NMR analysis of chiral alcohols has been challenging due to weak binding interactions with chiral solvating agents. To overcome the intrinsic difficulty of relatively weak molecular interactions that are common for alcohols, many researchers have used multifunctional alcohols to enhance interactions with solvating agents.
Instead, the KAIST team successfully varied the physical properties of metal complexes to induce stronger interactions with alcohols rather than the strategy of using multifunctional analytes, in the hopes of developing a universal chiral solvating agent for alcohols. Compared to the current method of chiral analysis used in the pharmaceutical industry, alcohols that do not possess chromophores can also be directly analyzed with the gallium complexes.
Professor Kim said that this method could be a complementary chiral analysis technique at the industry level in the near future. He added that since the developed gallium complex can determine enantiomeric excess within minutes, it can be further utilized to monitor asymmetric synthesis. This feature will benefit a large number of researchers in the organic chemistry community, as well as the pharmaceutical industry.
(Figure: Schematic view of the in-situ direct 1H NMR chiral analysis.)
-Profile:
Professor Hyunwoo Kim
Department of Chemistry
KAIST
http://mdos.kaist.ac.kr
hwk34@kaist.ac.kr
For more on this article,
please go to https://doi.org/10.1016/j.isci2019.07051
2019.11.14 View 11354 -
 KAIST Develops New Technique for Chiral Activity in Molecules
Professor Hyunwoo Kim of the Chemistry Department and his research team have developed a technique that can easily analyze the optical activity of charged compounds by using nuclear magnetic resonance (NMR) spectroscopy. The research finding entitled “H NMR Chiral Analysis of Charged Molecules via Ion Pairing with Aluminum Complexes” was published online in the October 19th issue of The Journal of the American Chemical Society.
The technique relies on observation of the behavior of optical isomers. Molecules with the same composition that are mirror images of each other are optical isomers. For example, the building blocks of all living organisms, amino acids, are a single optical isomer. In our bodies, optical isomers bring different physiological changes due to their distinct optical activities. Therefore, controlling and analyzing the optical activities are critical when developing a new drug.
High-performance liquid chromatography (HPLC) is the de facto standard of analyzing the optical activity of a compound. However, HPLC is very expensive that many laboratories can’t afford to have. In addition, with the machine, one analysis may take 30 minutes to one hour to complete. It lacks in signal sensitivity and chemical decomposition, and the application is limited to nonpolar compounds.
Usually adopted in analyzing the structure of a chemical compound, NMR spectroscopy requires only one to five minutes per single analysis. Since it is essential for analyzing the molecular structure, many chemistry labs have NMR equipment. However, until this technique was invented, no other research team had reported an effective way of using the NMR spectroscopy to decompose the signal of chiral activity of a compound.
The research team uses negatively-charged metal compounds in NMR spectroscopy. The technique employs negatively-charged metal compounds which bond ionically to positively- and negatively-charged optical compounds. As a result, the NMR spectroscopy can distinguish the signal from chiral activity. Not only can it analyze various chemicals without structural constraints, but it can also be used for both nonpolar and polar solvents.
As many compounds for new drugs have functional groups, which can be charged, this analysis method can be directly employed in the development process of drugs. Professor Kim said, “A revolutionary analysis method has been developed using simple chemical principles. I hope that our method will be applied to the development of new medicine.”
This research was sponsored by the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science and the Supercomputing Research Center of KAIST.
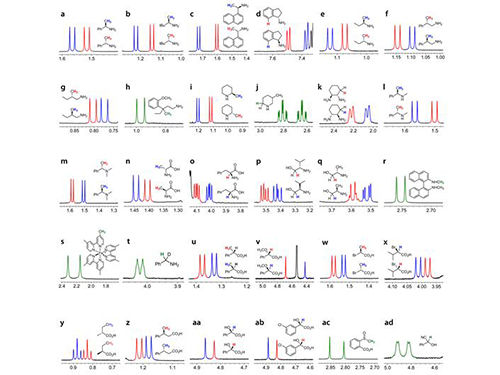
Picture 1: Separations of NMR Signals of Chemicals due to Interaction with Metal Compounds
Picture 2: Separations of NMR Signals in Different Chemicals
2015.11.20 View 11927
KAIST Develops New Technique for Chiral Activity in Molecules
Professor Hyunwoo Kim of the Chemistry Department and his research team have developed a technique that can easily analyze the optical activity of charged compounds by using nuclear magnetic resonance (NMR) spectroscopy. The research finding entitled “H NMR Chiral Analysis of Charged Molecules via Ion Pairing with Aluminum Complexes” was published online in the October 19th issue of The Journal of the American Chemical Society.
The technique relies on observation of the behavior of optical isomers. Molecules with the same composition that are mirror images of each other are optical isomers. For example, the building blocks of all living organisms, amino acids, are a single optical isomer. In our bodies, optical isomers bring different physiological changes due to their distinct optical activities. Therefore, controlling and analyzing the optical activities are critical when developing a new drug.
High-performance liquid chromatography (HPLC) is the de facto standard of analyzing the optical activity of a compound. However, HPLC is very expensive that many laboratories can’t afford to have. In addition, with the machine, one analysis may take 30 minutes to one hour to complete. It lacks in signal sensitivity and chemical decomposition, and the application is limited to nonpolar compounds.
Usually adopted in analyzing the structure of a chemical compound, NMR spectroscopy requires only one to five minutes per single analysis. Since it is essential for analyzing the molecular structure, many chemistry labs have NMR equipment. However, until this technique was invented, no other research team had reported an effective way of using the NMR spectroscopy to decompose the signal of chiral activity of a compound.
The research team uses negatively-charged metal compounds in NMR spectroscopy. The technique employs negatively-charged metal compounds which bond ionically to positively- and negatively-charged optical compounds. As a result, the NMR spectroscopy can distinguish the signal from chiral activity. Not only can it analyze various chemicals without structural constraints, but it can also be used for both nonpolar and polar solvents.
As many compounds for new drugs have functional groups, which can be charged, this analysis method can be directly employed in the development process of drugs. Professor Kim said, “A revolutionary analysis method has been developed using simple chemical principles. I hope that our method will be applied to the development of new medicine.”
This research was sponsored by the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science and the Supercomputing Research Center of KAIST.
Picture 1: Separations of NMR Signals of Chemicals due to Interaction with Metal Compounds
Picture 2: Separations of NMR Signals in Different Chemicals
2015.11.20 View 11927