Electrocatalysis
-
 Tungsten Suboxide Improves the Efficiency of Platinum in Hydrogen Production
< PhD Candidate Jinkyu Park and Professor Jinwoo Lee >
Researchers presented a new strategy for enhancing catalytic activity using tungsten suboxide as a single-atom catalyst (SAC). This strategy, which significantly improves hydrogen evolution reaction (HER) in metal platinum (pt) by 16.3 times, sheds light on the development of new electrochemical catalyst technologies.
Hydrogen has been touted as a promising alternative to fossil fuels. However, most of the conventional industrial hydrogen production methods come with environmental issues, releasing significant amounts of carbon dioxide and greenhouse gases.
Electrochemical water splitting is considered a potential approach for clean hydrogen production. Pt is one of the most commonly used catalysts to improve HER performance in electrochemical water splitting, but the high cost and scarcity of Pt remain key obstacles to mass commercial applications.
SACs, where all metal species are individually dispersed on a desired support material, have been identified as one way to reduce the amount of Pt usage, as they offer the maximum number of surface exposed Pt atoms.
Inspired by earlier studies, which mainly focused on SACs supported by carbon-based materials, a KAIST research team led by Professor Jinwoo Lee from the Department of Chemical and Biomolecular Engineering investigated the influence of support materials on the performance of SACs.
Professor Lee and his researchers suggested mesoporous tungsten suboxide as a new support material for atomically dispersed Pt, as this was expected to provide high electronic conductivity and have a synergetic effect with Pt.
They compared the performance of single-atom Pt supported by carbon and tungsten suboxide respectively. The results revealed that the support effect occurred with tungsten suboxide, in which the mass activity of a single-atom Pt supported by tungsten suboxide was 2.1 times greater than that of single-atom Pt supported by carbon, and 16.3 times higher than that of Pt nanoparticles supported by carbon.
The team indicated a change in the electronic structure of Pt via charge transfer from tungsten suboxide to Pt. This phenomenon was reported as a result of strong metal-support interaction between Pt and tungsten suboxide.
HER performance can be improved not only by changing the electronic structure of the supported metal, but also by inducing another support effect, the spillover effect, the research group reported. Hydrogen spillover is a phenomenon where adsorbed hydrogen migrates from one surface to another, and it occurs more easily as the Pt size becomes smaller.
The researchers compared the performance of single-atom Pt and Pt nanoparticles supported by tungsten suboxide. The single-atom Pt supported by tungsten suboxide exhibited a higher degree of hydrogen spillover phenomenon, which enhanced the Pt mass activity for hydrogen evolution up to 10.7 times compared to Pt nanoparticles supported by tungsten suboxide.
Professor Lee said, “Choosing the right support material is important for improving electrocatalysis in hydrogen production. The tungsten suboxide catalyst we used to support Pt in our study implies that interactions between the well-matched metal and support can drastically enhance the efficiency of the process.”
This research was supported by the Ministry of Science and ICT and introduced in the International Edition of the German journal Angewandte Chemie.
Figure. Schematic representation of hydrogen evolution reaction (HER) of pseudo single-atom Pt supported by tungsten suboxide
-Publication
Jinkyu Park, Dr. Seonggyu Lee, Hee-Eun Kim, Ara Cho, Seongbeen Kim, Dr. Youngjin Ye, Prof. Jeong Woo Han, Prof. Hyunjoo Lee, Dr. Jong Hyun Jang, and Prof. Jinwoo Lee. 2019. Investigation of the Support Effect in Atomically Dispersed Pt on WO3−x for Utilization of Pt in the Hydrogen Evolution Reaction. International Edition of Angewandte Chemie. Volume No. 58. Issue No. 45. 6 pages. https://doi.org/10.1002/anie.201908122
-ProfileProfessor Jinwoo LeeConvergence of Energy and Nano Science Laboratoryhttp://cens.kaist.ac.kr
Department of Chemical and Biomolecular EngineeringKAIST
2019.10.28 View 23034
Tungsten Suboxide Improves the Efficiency of Platinum in Hydrogen Production
< PhD Candidate Jinkyu Park and Professor Jinwoo Lee >
Researchers presented a new strategy for enhancing catalytic activity using tungsten suboxide as a single-atom catalyst (SAC). This strategy, which significantly improves hydrogen evolution reaction (HER) in metal platinum (pt) by 16.3 times, sheds light on the development of new electrochemical catalyst technologies.
Hydrogen has been touted as a promising alternative to fossil fuels. However, most of the conventional industrial hydrogen production methods come with environmental issues, releasing significant amounts of carbon dioxide and greenhouse gases.
Electrochemical water splitting is considered a potential approach for clean hydrogen production. Pt is one of the most commonly used catalysts to improve HER performance in electrochemical water splitting, but the high cost and scarcity of Pt remain key obstacles to mass commercial applications.
SACs, where all metal species are individually dispersed on a desired support material, have been identified as one way to reduce the amount of Pt usage, as they offer the maximum number of surface exposed Pt atoms.
Inspired by earlier studies, which mainly focused on SACs supported by carbon-based materials, a KAIST research team led by Professor Jinwoo Lee from the Department of Chemical and Biomolecular Engineering investigated the influence of support materials on the performance of SACs.
Professor Lee and his researchers suggested mesoporous tungsten suboxide as a new support material for atomically dispersed Pt, as this was expected to provide high electronic conductivity and have a synergetic effect with Pt.
They compared the performance of single-atom Pt supported by carbon and tungsten suboxide respectively. The results revealed that the support effect occurred with tungsten suboxide, in which the mass activity of a single-atom Pt supported by tungsten suboxide was 2.1 times greater than that of single-atom Pt supported by carbon, and 16.3 times higher than that of Pt nanoparticles supported by carbon.
The team indicated a change in the electronic structure of Pt via charge transfer from tungsten suboxide to Pt. This phenomenon was reported as a result of strong metal-support interaction between Pt and tungsten suboxide.
HER performance can be improved not only by changing the electronic structure of the supported metal, but also by inducing another support effect, the spillover effect, the research group reported. Hydrogen spillover is a phenomenon where adsorbed hydrogen migrates from one surface to another, and it occurs more easily as the Pt size becomes smaller.
The researchers compared the performance of single-atom Pt and Pt nanoparticles supported by tungsten suboxide. The single-atom Pt supported by tungsten suboxide exhibited a higher degree of hydrogen spillover phenomenon, which enhanced the Pt mass activity for hydrogen evolution up to 10.7 times compared to Pt nanoparticles supported by tungsten suboxide.
Professor Lee said, “Choosing the right support material is important for improving electrocatalysis in hydrogen production. The tungsten suboxide catalyst we used to support Pt in our study implies that interactions between the well-matched metal and support can drastically enhance the efficiency of the process.”
This research was supported by the Ministry of Science and ICT and introduced in the International Edition of the German journal Angewandte Chemie.
Figure. Schematic representation of hydrogen evolution reaction (HER) of pseudo single-atom Pt supported by tungsten suboxide
-Publication
Jinkyu Park, Dr. Seonggyu Lee, Hee-Eun Kim, Ara Cho, Seongbeen Kim, Dr. Youngjin Ye, Prof. Jeong Woo Han, Prof. Hyunjoo Lee, Dr. Jong Hyun Jang, and Prof. Jinwoo Lee. 2019. Investigation of the Support Effect in Atomically Dispersed Pt on WO3−x for Utilization of Pt in the Hydrogen Evolution Reaction. International Edition of Angewandte Chemie. Volume No. 58. Issue No. 45. 6 pages. https://doi.org/10.1002/anie.201908122
-ProfileProfessor Jinwoo LeeConvergence of Energy and Nano Science Laboratoryhttp://cens.kaist.ac.kr
Department of Chemical and Biomolecular EngineeringKAIST
2019.10.28 View 23034 -
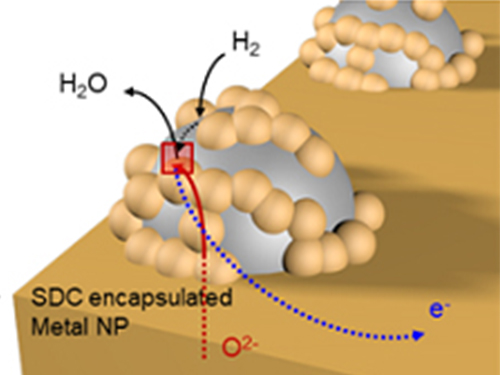 Unravelling Inherent Electrocatalysis to Improve the Performance of Hydrogen Fuel Cells
(Figure 1. Electrode structure for the precise evaluation of the metal nanoparticles’ electrochemical catalytic characteristics at a high temperature.)
A KAIST team presented an ideal electrode design to enhance the performance of high-temperature fuel cells. The new analytical platform with advanced nanoscale patterning method quantitatively revealed the electrochemical value of metal nanoparticles dispersed on the oxide electrode, thus leading to electrode design directions that can be used in a variety of eco-friendly energy technologies.
The team, working under Professor WooChul Jung and Professor Sang Ouk Kim at the Department of Materials Science and Engineering, described an accurate analysis of the reactivity of oxide electrodes boosted by metal nanoparticles, where all particles participate in the reaction. They identified how the metal catalysts activate hydrogen electro-oxidation on the ceria-based electrode surface and quantify how rapidly the reaction rate increases with the proper choice of metals.
Metal nanoparticles with diameters of 10 nanometers or less have become a key component in high-performance heterogeneous catalysts, primarily serving as a catalytic activator. Recent experimental and theoretical findings suggest that the optimization of the chemical nature at the metal and support interfaces is essential for performance improvement.
However, the high cost associated with cell fabrication and operation as well as poorer stability of metal nanoparticles at high temperatures have been a long-standing challenge. To solve this problem, the team utilized a globally recognized metal nano patterning technology that uses block copolymer self-assembled nano templates and succeeded in uniformly synthesizing metal particles 10 nanometers in size on the surface of oxide fuel cell electrodes. They also developed a technology to accurately analyze the catalyst characteristics of single particles at high temperatures and maximize the performance of a fuel cell with minimal catalyst use.
The research team confirmed that platinum, which is a commonly used metal catalyst, could boost fuel cell performance by as much as 21 times even at an amount of 300 nanograms, which only costs about 0.015 KRW.
The team quantitatively identified and compared the characteristics of widely used metal catalysts other than platinum, such as palladium, gold, and cobalt, and also elucidated the precise principle of catalyst performance through theoretical analysis.
(Figure 2. Comparison of the electrochemical catalytic characteristics for various 10nm metal nanoparticles (platinum, palladium, cobalt, gold) at a high temperature.)
Professor Jung said, "We have broken the conventional methods of increasing the amount of catalyst which have deemed inefficient and expensive. Our results suggest a clear idea for high performance fuel cells using very small amounts of nanoparticles. This technology can be applied to many different industrial fields, advancing the commercialization of eco-friendly energy technologies such as fuel cells that generate electricity and electrolytic cells that produce hydrogen from water.”
The research has been published as the cover article of Nature Nanotechnology in the March issue. This research was carried out with support from the Nano-Material Technology Development Program through the National Research Foundation of Korea.
2019.03.28 View 31183
Unravelling Inherent Electrocatalysis to Improve the Performance of Hydrogen Fuel Cells
(Figure 1. Electrode structure for the precise evaluation of the metal nanoparticles’ electrochemical catalytic characteristics at a high temperature.)
A KAIST team presented an ideal electrode design to enhance the performance of high-temperature fuel cells. The new analytical platform with advanced nanoscale patterning method quantitatively revealed the electrochemical value of metal nanoparticles dispersed on the oxide electrode, thus leading to electrode design directions that can be used in a variety of eco-friendly energy technologies.
The team, working under Professor WooChul Jung and Professor Sang Ouk Kim at the Department of Materials Science and Engineering, described an accurate analysis of the reactivity of oxide electrodes boosted by metal nanoparticles, where all particles participate in the reaction. They identified how the metal catalysts activate hydrogen electro-oxidation on the ceria-based electrode surface and quantify how rapidly the reaction rate increases with the proper choice of metals.
Metal nanoparticles with diameters of 10 nanometers or less have become a key component in high-performance heterogeneous catalysts, primarily serving as a catalytic activator. Recent experimental and theoretical findings suggest that the optimization of the chemical nature at the metal and support interfaces is essential for performance improvement.
However, the high cost associated with cell fabrication and operation as well as poorer stability of metal nanoparticles at high temperatures have been a long-standing challenge. To solve this problem, the team utilized a globally recognized metal nano patterning technology that uses block copolymer self-assembled nano templates and succeeded in uniformly synthesizing metal particles 10 nanometers in size on the surface of oxide fuel cell electrodes. They also developed a technology to accurately analyze the catalyst characteristics of single particles at high temperatures and maximize the performance of a fuel cell with minimal catalyst use.
The research team confirmed that platinum, which is a commonly used metal catalyst, could boost fuel cell performance by as much as 21 times even at an amount of 300 nanograms, which only costs about 0.015 KRW.
The team quantitatively identified and compared the characteristics of widely used metal catalysts other than platinum, such as palladium, gold, and cobalt, and also elucidated the precise principle of catalyst performance through theoretical analysis.
(Figure 2. Comparison of the electrochemical catalytic characteristics for various 10nm metal nanoparticles (platinum, palladium, cobalt, gold) at a high temperature.)
Professor Jung said, "We have broken the conventional methods of increasing the amount of catalyst which have deemed inefficient and expensive. Our results suggest a clear idea for high performance fuel cells using very small amounts of nanoparticles. This technology can be applied to many different industrial fields, advancing the commercialization of eco-friendly energy technologies such as fuel cells that generate electricity and electrolytic cells that produce hydrogen from water.”
The research has been published as the cover article of Nature Nanotechnology in the March issue. This research was carried out with support from the Nano-Material Technology Development Program through the National Research Foundation of Korea.
2019.03.28 View 31183