Brain
-
 Decoding Fear: KAIST Identifies An Affective Brain Circuit Crucial for Fear Memory Formation by Non-nociceptive Threat Stimulus
Fear memories can form in the brain following exposure to threatening situations such as natural disasters, accidents, or violence. When these memories become excessive or distorted, they can lead to severe mental health disorders, including post-traumatic stress disorder (PTSD), anxiety disorders, and depression. However, the mechanisms underlying fear memory formation triggered by affective pain rather than direct physical pain have remained largely unexplored – until now.
A KAIST research team has identified, for the first time, a brain circuit specifically responsible for forming fear memories in the absence of physical pain, marking a significant advance in understanding how psychological distress is processed and drives fear memory formation in the brain. This discovery opens the door to the development of targeted treatments for trauma-related conditions by addressing the underlying neural pathways.
< Photo 1. (from left) Professor Jin-Hee Han, Dr. Junho Han and Ph.D. Candidate Boin Suh of the Department of Biological Sciences >
KAIST (President Kwang-Hyung Lee) announced on May 15th that the research team led by Professor Jin-Hee Han in the Department of Biological Sciences has identified the pIC-PBN circuit*, a key neural pathway involved in forming fear memories triggered by psychological threats in the absence of sensory pain. This groundbreaking work was conducted through experiments with mice.*pIC–PBN circuit: A newly identified descending neural pathway from the posterior insular cortex (pIC) to the parabrachial nucleus (PBN), specialized for transmitting psychological threat information.
Traditionally, the lateral parabrachial nucleus (PBN) has been recognized as a critical part of the ascending pain pathway, receiving pain signals from the spinal cord. However, this study reveals a previously unknown role for the PBN in processing fear induced by non-painful psychological stimuli, fundamentally changing our understanding of its function in the brain.
This work is considered the first experimental evidence that 'emotional distress' and 'physical pain' are processed through different neural circuits to form fear memories, making it a significant contribution to the field of neuroscience. It clearly demonstrates the existence of a dedicated pathway (pIC-PBN) for transmitting emotional distress.
The study's first author, Dr. Junho Han, shared the personal motivation behind this research: “Our dog, Lego, is afraid of motorcycles. He never actually crashed into one, but ever since having a traumatizing event of having a motorbike almost run into him, just hearing the sound now triggers a fearful response. Humans react similarly – even if you didn’t have a personal experience of being involved in an accident, a near-miss or exposure to alarming media can create lasting fear memories, which may eventually lead to PTSD.”
He continued, “Until now, fear memory research has mainly relied on experimental models involving physical pain. However, much of real-world human fears arise from psychological threats, rather than from direct physical harm. Despite this, little was known about the brain circuits responsible for processing these psychological threats that can drive fear memory formation.”
To investigate this, the research team developed a novel fear conditioning model that utilizes visual threat stimuli instead of electrical shocks. In this model, mice were exposed to a rapidly expanding visual disk on a ceiling screen, simulating the threat of an approaching predator. This approach allowed the team to demonstrate that fear memories can form in response to a non-nociceptive, psychological threat alone, without the need for physical pain.
< Figure 1. Artificial activation of the posterior insular cortex (pIC) to lateral parabrachial nucleus (PBN) neural circuit induces anxiety-like behaviors and fear memory formation in mice. >
Using advanced chemogenetic and optogenetic techniques, the team precisely controlled neuronal activity, revealing that the lateral parabrachial nucleus (PBN) is essential to form fear memories in response to visual threats. They further traced the origin of these signals to the posterior insular cortex (pIC), a region known to process negative emotions and pain, confirming a direct connection between the two areas.
The study also showed that inhibiting the pIC–PBN circuit significantly reduced fear memory formation in response to visual threats, without affecting innate fear responses or physical pain-based learning. Conversely, artificially activating this circuit alone was sufficient to drive fear memory formation, confirming its role as a key pathway for processing psychological threat information.
< Figure 2. Schematic diagram of brain neural circuits transmitting emotional & physical pain threat signals. Visual threat stimuli do not involve physical pain but can create an anxious state and form fear memory through the affective pain signaling pathway. >
Professor Jin-Hee Han commented, “This study lays an important foundation for understanding how emotional distress-based mental disorders, such as PTSD, panic disorder, and anxiety disorder, develop, and opens new possibilities for targeted treatment approaches.”
The findings, authored by Dr. Junho Han (first author), Ph.D. candidate Boin Suh (second author), and Dr. Jin-Hee Han (corresponding author) of the Department of Biological Sciences, were published online in the international journal Science Advances on May 9, 2025.※ Paper Title: A top-down insular cortex circuit crucial for non-nociceptive fear learning. Science Advances (https://doi.org/10.1101/2024.10.14.618356)※ Author Information: Junho Han (first author), Boin Suh (second author), and Jin-Hee Han (corresponding author)
This research was supported by grants from the National Research Foundation of Korea (NRF-2022M3E5E8081183 and NRF-2017M3C7A1031322).
2025.05.15 View 1274
Decoding Fear: KAIST Identifies An Affective Brain Circuit Crucial for Fear Memory Formation by Non-nociceptive Threat Stimulus
Fear memories can form in the brain following exposure to threatening situations such as natural disasters, accidents, or violence. When these memories become excessive or distorted, they can lead to severe mental health disorders, including post-traumatic stress disorder (PTSD), anxiety disorders, and depression. However, the mechanisms underlying fear memory formation triggered by affective pain rather than direct physical pain have remained largely unexplored – until now.
A KAIST research team has identified, for the first time, a brain circuit specifically responsible for forming fear memories in the absence of physical pain, marking a significant advance in understanding how psychological distress is processed and drives fear memory formation in the brain. This discovery opens the door to the development of targeted treatments for trauma-related conditions by addressing the underlying neural pathways.
< Photo 1. (from left) Professor Jin-Hee Han, Dr. Junho Han and Ph.D. Candidate Boin Suh of the Department of Biological Sciences >
KAIST (President Kwang-Hyung Lee) announced on May 15th that the research team led by Professor Jin-Hee Han in the Department of Biological Sciences has identified the pIC-PBN circuit*, a key neural pathway involved in forming fear memories triggered by psychological threats in the absence of sensory pain. This groundbreaking work was conducted through experiments with mice.*pIC–PBN circuit: A newly identified descending neural pathway from the posterior insular cortex (pIC) to the parabrachial nucleus (PBN), specialized for transmitting psychological threat information.
Traditionally, the lateral parabrachial nucleus (PBN) has been recognized as a critical part of the ascending pain pathway, receiving pain signals from the spinal cord. However, this study reveals a previously unknown role for the PBN in processing fear induced by non-painful psychological stimuli, fundamentally changing our understanding of its function in the brain.
This work is considered the first experimental evidence that 'emotional distress' and 'physical pain' are processed through different neural circuits to form fear memories, making it a significant contribution to the field of neuroscience. It clearly demonstrates the existence of a dedicated pathway (pIC-PBN) for transmitting emotional distress.
The study's first author, Dr. Junho Han, shared the personal motivation behind this research: “Our dog, Lego, is afraid of motorcycles. He never actually crashed into one, but ever since having a traumatizing event of having a motorbike almost run into him, just hearing the sound now triggers a fearful response. Humans react similarly – even if you didn’t have a personal experience of being involved in an accident, a near-miss or exposure to alarming media can create lasting fear memories, which may eventually lead to PTSD.”
He continued, “Until now, fear memory research has mainly relied on experimental models involving physical pain. However, much of real-world human fears arise from psychological threats, rather than from direct physical harm. Despite this, little was known about the brain circuits responsible for processing these psychological threats that can drive fear memory formation.”
To investigate this, the research team developed a novel fear conditioning model that utilizes visual threat stimuli instead of electrical shocks. In this model, mice were exposed to a rapidly expanding visual disk on a ceiling screen, simulating the threat of an approaching predator. This approach allowed the team to demonstrate that fear memories can form in response to a non-nociceptive, psychological threat alone, without the need for physical pain.
< Figure 1. Artificial activation of the posterior insular cortex (pIC) to lateral parabrachial nucleus (PBN) neural circuit induces anxiety-like behaviors and fear memory formation in mice. >
Using advanced chemogenetic and optogenetic techniques, the team precisely controlled neuronal activity, revealing that the lateral parabrachial nucleus (PBN) is essential to form fear memories in response to visual threats. They further traced the origin of these signals to the posterior insular cortex (pIC), a region known to process negative emotions and pain, confirming a direct connection between the two areas.
The study also showed that inhibiting the pIC–PBN circuit significantly reduced fear memory formation in response to visual threats, without affecting innate fear responses or physical pain-based learning. Conversely, artificially activating this circuit alone was sufficient to drive fear memory formation, confirming its role as a key pathway for processing psychological threat information.
< Figure 2. Schematic diagram of brain neural circuits transmitting emotional & physical pain threat signals. Visual threat stimuli do not involve physical pain but can create an anxious state and form fear memory through the affective pain signaling pathway. >
Professor Jin-Hee Han commented, “This study lays an important foundation for understanding how emotional distress-based mental disorders, such as PTSD, panic disorder, and anxiety disorder, develop, and opens new possibilities for targeted treatment approaches.”
The findings, authored by Dr. Junho Han (first author), Ph.D. candidate Boin Suh (second author), and Dr. Jin-Hee Han (corresponding author) of the Department of Biological Sciences, were published online in the international journal Science Advances on May 9, 2025.※ Paper Title: A top-down insular cortex circuit crucial for non-nociceptive fear learning. Science Advances (https://doi.org/10.1101/2024.10.14.618356)※ Author Information: Junho Han (first author), Boin Suh (second author), and Jin-Hee Han (corresponding author)
This research was supported by grants from the National Research Foundation of Korea (NRF-2022M3E5E8081183 and NRF-2017M3C7A1031322).
2025.05.15 View 1274 -
 Editing Parkinson's Disease – KAIST Makes World's First Discovery of an Inflammatory RNA Editing Enzyme through Co-work with UCL Researchers
< Professor Minee Choi of the Department of Brain and Cognitive Sciences (top left). Professor Sonia Gandhi (top right) and Professor Klenerman of the University College London (bottom right) >
Parkinson's disease (PD) is a neurodegenerative disorder in which the α-synuclein protein abnormally aggregates within brain cells, causing neuronal damage. Through international collaboration, researchers at KAIST have revealed that RNA editing plays a crucial role in regulating neuroinflammation, a key pathology of Parkinson's disease.
KAIST (represented by President Kwang-Hyung Lee) announced on the 27th of April that a research team led by Professor Minee L. Choi from the Department of Brain and Cognitive Sciences, in collaboration with University College London (UCL) and the Francis Crick Institute, discovered that the RNA editing enzyme ADAR1 plays an important role in controlling immune responses in astrocytes, glial cells that trigger protective reactions in the brain, and demonstrated that this mechanism is critically involved in the progression of Parkinson’s disease.
Professor Choi's research team created a co-culture model composed of astrocytes and neurons derived from stem cells originating from Parkinson's disease patients, in order to study the inflammatory responses of brain immune cells. They then treated the model with α-synuclein aggregates, which are known to cause Parkinson’s disease, and analyzed how the immune cells' inflammatory responses changed.
< Figure 1. Schematic diagram of the inflammatory RNA editing model in Parkinson's disease >
As a result, it was found that early pathological forms of α-synuclein, known as oligomers, activated the Toll-like receptor pathway, which acts as a danger sensor in astrocytes, as well as the interferon response pathway, an immune signaling network that combats viruses and pathogens. During this process, the RNA editing enzyme ADAR1 was expressed and transformed into an isoform with an altered protein structure and function.
Notably, the RNA editing activity of ADAR1, which normally functions to regulate immune responses during viral infections by converting adenosine (A) to inosine (I) through a process known as A-to-I RNA editing, was found to be abnormally focused on genes that cause inflammation rather than operating under normal conditions. This phenomenon was observed not only in the patient-derived neuron models but also in postmortem brain tissues from actual Parkinson’s disease patients.
< Figure 2. Experimental design and inflammatory response induction in astrocytes following treatment with α-synuclein oligomers (abnormally folded protein fragments) >
This directly proves that the dysregulation of RNA editing induces chronic inflammatory responses in astrocytes, ultimately leading to neuronal toxicity and pathological progression.
This study is significant in that it newly identified the regulation of RNA editing within astrocytes as a key mechanism behind neuroinflammatory responses. In particular, it suggests that ADAR1 could serve as a novel genetic target for the treatment of Parkinson’s disease.
It is also noteworthy that the study reflected actual pathological characteristics of patients by utilizing patient-specific induced pluripotent stem cell-based precision models for brain diseases.
Professor Minee L. Choi stated, “This study demonstrates that the regulator of inflammation caused by protein aggregation operates at the new layer of RNA editing, offering a completely different therapeutic strategy from existing approaches to Parkinson's disease treatment." She further emphasized, “RNA editing technology could become an important turning point in the development of therapeutics for neuroinflammation.”
< Figure 3. When treated with α-synuclein oligomers, the causative agent of Parkinson's disease, A-to-I RNA editing is induced to change genetic information by ADAR in patient-derived stem cell-differentiated glial cells, confirming that α-synuclein is likely to be associated with the progression of Parkinson's disease through RNA editing >
This study was published in Science Advances on April 11, with Professor Choi listed as a co-first author.
Paper Title: Astrocytic RNA editing regulates the host immune response to alpha-synuclein, Science Advances Vol.11, Issue 15. (DOI:10.1126/sciadv.adp8504)
Lead Authors: Karishma D’Sa (UCL, Co-First Author), Minee L. Choi (KAIST, Co-First Author), Mina Ryten (UCL, Corresponding Author), Sonia Gandhi (Francis Crick Institute, University of Cambridge, Corresponding Author)
This research was supported by the Brain Research Program and the Excellent Young Researcher Program of the National Research Foundation of Korea, as well as KAIST’s Daekyo Cognitive Enhancement Program.
2025.05.02 View 2307
Editing Parkinson's Disease – KAIST Makes World's First Discovery of an Inflammatory RNA Editing Enzyme through Co-work with UCL Researchers
< Professor Minee Choi of the Department of Brain and Cognitive Sciences (top left). Professor Sonia Gandhi (top right) and Professor Klenerman of the University College London (bottom right) >
Parkinson's disease (PD) is a neurodegenerative disorder in which the α-synuclein protein abnormally aggregates within brain cells, causing neuronal damage. Through international collaboration, researchers at KAIST have revealed that RNA editing plays a crucial role in regulating neuroinflammation, a key pathology of Parkinson's disease.
KAIST (represented by President Kwang-Hyung Lee) announced on the 27th of April that a research team led by Professor Minee L. Choi from the Department of Brain and Cognitive Sciences, in collaboration with University College London (UCL) and the Francis Crick Institute, discovered that the RNA editing enzyme ADAR1 plays an important role in controlling immune responses in astrocytes, glial cells that trigger protective reactions in the brain, and demonstrated that this mechanism is critically involved in the progression of Parkinson’s disease.
Professor Choi's research team created a co-culture model composed of astrocytes and neurons derived from stem cells originating from Parkinson's disease patients, in order to study the inflammatory responses of brain immune cells. They then treated the model with α-synuclein aggregates, which are known to cause Parkinson’s disease, and analyzed how the immune cells' inflammatory responses changed.
< Figure 1. Schematic diagram of the inflammatory RNA editing model in Parkinson's disease >
As a result, it was found that early pathological forms of α-synuclein, known as oligomers, activated the Toll-like receptor pathway, which acts as a danger sensor in astrocytes, as well as the interferon response pathway, an immune signaling network that combats viruses and pathogens. During this process, the RNA editing enzyme ADAR1 was expressed and transformed into an isoform with an altered protein structure and function.
Notably, the RNA editing activity of ADAR1, which normally functions to regulate immune responses during viral infections by converting adenosine (A) to inosine (I) through a process known as A-to-I RNA editing, was found to be abnormally focused on genes that cause inflammation rather than operating under normal conditions. This phenomenon was observed not only in the patient-derived neuron models but also in postmortem brain tissues from actual Parkinson’s disease patients.
< Figure 2. Experimental design and inflammatory response induction in astrocytes following treatment with α-synuclein oligomers (abnormally folded protein fragments) >
This directly proves that the dysregulation of RNA editing induces chronic inflammatory responses in astrocytes, ultimately leading to neuronal toxicity and pathological progression.
This study is significant in that it newly identified the regulation of RNA editing within astrocytes as a key mechanism behind neuroinflammatory responses. In particular, it suggests that ADAR1 could serve as a novel genetic target for the treatment of Parkinson’s disease.
It is also noteworthy that the study reflected actual pathological characteristics of patients by utilizing patient-specific induced pluripotent stem cell-based precision models for brain diseases.
Professor Minee L. Choi stated, “This study demonstrates that the regulator of inflammation caused by protein aggregation operates at the new layer of RNA editing, offering a completely different therapeutic strategy from existing approaches to Parkinson's disease treatment." She further emphasized, “RNA editing technology could become an important turning point in the development of therapeutics for neuroinflammation.”
< Figure 3. When treated with α-synuclein oligomers, the causative agent of Parkinson's disease, A-to-I RNA editing is induced to change genetic information by ADAR in patient-derived stem cell-differentiated glial cells, confirming that α-synuclein is likely to be associated with the progression of Parkinson's disease through RNA editing >
This study was published in Science Advances on April 11, with Professor Choi listed as a co-first author.
Paper Title: Astrocytic RNA editing regulates the host immune response to alpha-synuclein, Science Advances Vol.11, Issue 15. (DOI:10.1126/sciadv.adp8504)
Lead Authors: Karishma D’Sa (UCL, Co-First Author), Minee L. Choi (KAIST, Co-First Author), Mina Ryten (UCL, Corresponding Author), Sonia Gandhi (Francis Crick Institute, University of Cambridge, Corresponding Author)
This research was supported by the Brain Research Program and the Excellent Young Researcher Program of the National Research Foundation of Korea, as well as KAIST’s Daekyo Cognitive Enhancement Program.
2025.05.02 View 2307 -
 KAIST to Collaborate with AT&C to Take Dominance over Dementia
< Photo 1. (From left) KAIST Dean of the College of Natural Sciences Daesoo Kim, KAIST President Kwang Hyung Lee, AT&C Chairman Ki Tae Lee, AT&C CEO Jong-won Lee >
KAIST (President Kwang Hyung Lee) announced on January 9th that it signed a memorandum of understanding for a comprehensive mutual cooperation with AT&C (CEO Jong-won Lee) at its Seoul Dogok Campus to expand research investment and industry-academia cooperation in preparation for the future cutting-edge digital bio era.
Senile dementia is a rapidly increasing brain disease that affects 10% of the elderly population aged 65 and older, and approximately 38% of those aged 85 and older suffer from dementia. Alzheimer's disease is the most common dementia in the elderly and its prevalence has been increasing rapidly in the population of over 40 years of age. However, an effective treatment is yet to be found.
The Korean government is investing a total of KRW 1.1 trillion in dementia R&D projects from 2020 to 2029, with the goal of reducing the rate of increase of dementia patients by 50%. Since it takes a lot of time and money to develop effective and affordable medicinal dementia treatments, it is urgent to work on the development of digital treatments for dementia that can be applied more quickly.
AT&C, a digital healthcare company, has already received approval from the Ministry of Food and Drug Safety (MFDS) for its device for antidepressant treatment based on transcranial magnetic stimulation (TMS) using magnetic fields and is selling it domestically and internationally. In addition, it has developed the first Alzheimer's dementia treatment device in Korea and received MFDS approval for clinical trials. After passing phase 1 to evaluate safety and phase 2 to test efficacy on some patients, it is currently conducting phase 3 clinical trials to test efficacy on a larger group of patients.
This dementia treatment device is equipped with a system that combines non-invasive electronic stimulations (TMS electromagnetic stimulator) and digital therapeutic prescription (cognitive learning programs) to provide precise, automated treatment by applying AI image analysis and robotics technology.
Through this agreement, KAIST and AT&C have agreed to cooperate with each other in the development of innovative digital treatment equipment for brain diseases. Through research collaboration with KAIST, AT&C will be able to develop technology that can be widely applied to Parkinson's disease, stroke, mild cognitive impairment, sleep disorders, etc., and will develop portable equipment that can improve brain function and prevent dementia at home by utilizing KAIST's wearable technology.
To this end, AT&C plans to establish a digital healthcare research center at KAIST by supporting research personnel and research expenses worth approximately 3 billion won with the goal of developing cutting-edge digital equipment within 3 years.
The digital equipment market is expected to grow at a compounded annual growth rate of 22.1% from 2023 to 2033, reaching a market size of $1.9209 trillion by 2033.
< Photo 2. (From left) Dean of the KAIST College of Natural Sciences Daesoo Kim, Professor Young-joon Lee, Professor Minee Choi of the KAIST Department of Brain and Cognitive Sciences, KAIST President Kwang Hyung Lee, Chairman Ki Tae Lee, CEO Jong-won Lee, and Headquarters Director Ki-yong Na of AT&C >
CEO Jong-won Lee said, “AT&C is playing a leading role in the treatment of Alzheimer’s disease using TMS (transcranial magnetic stimulation) technology. Through this agreement with KAIST, we will do our best to create a new paradigm for brain disease treatment and become a platform company that can lead future medical devices and medical technology.”
Former Samsung Electronics Vice Chairman Ki Tae Lee, a strong supporter of this R&D project, said, “Through this agreement with KAIST, we plan to prepare for a new future by combining the technologies AT&C has developed so far with KAIST’s innovative and differentiated technologies.”
KAIST President Kwang Hyung Lee emphasized, “Through this collaboration, KAIST expects to build a world-class digital therapeutics infrastructure for treating brain diseases and contribute greatly to further strengthening Korea’s competitiveness in the biomedical field.”
The signing ceremony was attended by KAIST President Kwang Hyung Lee, the Dean of KAIST College of Natural Sciences Daesoo Kim, AT&C CEO Lee Jong-won, and the current Chairman of AT&C, Ki Tae Lee, former Vice Chairman of Samsung Electronics.
2025.01.09 View 3444
KAIST to Collaborate with AT&C to Take Dominance over Dementia
< Photo 1. (From left) KAIST Dean of the College of Natural Sciences Daesoo Kim, KAIST President Kwang Hyung Lee, AT&C Chairman Ki Tae Lee, AT&C CEO Jong-won Lee >
KAIST (President Kwang Hyung Lee) announced on January 9th that it signed a memorandum of understanding for a comprehensive mutual cooperation with AT&C (CEO Jong-won Lee) at its Seoul Dogok Campus to expand research investment and industry-academia cooperation in preparation for the future cutting-edge digital bio era.
Senile dementia is a rapidly increasing brain disease that affects 10% of the elderly population aged 65 and older, and approximately 38% of those aged 85 and older suffer from dementia. Alzheimer's disease is the most common dementia in the elderly and its prevalence has been increasing rapidly in the population of over 40 years of age. However, an effective treatment is yet to be found.
The Korean government is investing a total of KRW 1.1 trillion in dementia R&D projects from 2020 to 2029, with the goal of reducing the rate of increase of dementia patients by 50%. Since it takes a lot of time and money to develop effective and affordable medicinal dementia treatments, it is urgent to work on the development of digital treatments for dementia that can be applied more quickly.
AT&C, a digital healthcare company, has already received approval from the Ministry of Food and Drug Safety (MFDS) for its device for antidepressant treatment based on transcranial magnetic stimulation (TMS) using magnetic fields and is selling it domestically and internationally. In addition, it has developed the first Alzheimer's dementia treatment device in Korea and received MFDS approval for clinical trials. After passing phase 1 to evaluate safety and phase 2 to test efficacy on some patients, it is currently conducting phase 3 clinical trials to test efficacy on a larger group of patients.
This dementia treatment device is equipped with a system that combines non-invasive electronic stimulations (TMS electromagnetic stimulator) and digital therapeutic prescription (cognitive learning programs) to provide precise, automated treatment by applying AI image analysis and robotics technology.
Through this agreement, KAIST and AT&C have agreed to cooperate with each other in the development of innovative digital treatment equipment for brain diseases. Through research collaboration with KAIST, AT&C will be able to develop technology that can be widely applied to Parkinson's disease, stroke, mild cognitive impairment, sleep disorders, etc., and will develop portable equipment that can improve brain function and prevent dementia at home by utilizing KAIST's wearable technology.
To this end, AT&C plans to establish a digital healthcare research center at KAIST by supporting research personnel and research expenses worth approximately 3 billion won with the goal of developing cutting-edge digital equipment within 3 years.
The digital equipment market is expected to grow at a compounded annual growth rate of 22.1% from 2023 to 2033, reaching a market size of $1.9209 trillion by 2033.
< Photo 2. (From left) Dean of the KAIST College of Natural Sciences Daesoo Kim, Professor Young-joon Lee, Professor Minee Choi of the KAIST Department of Brain and Cognitive Sciences, KAIST President Kwang Hyung Lee, Chairman Ki Tae Lee, CEO Jong-won Lee, and Headquarters Director Ki-yong Na of AT&C >
CEO Jong-won Lee said, “AT&C is playing a leading role in the treatment of Alzheimer’s disease using TMS (transcranial magnetic stimulation) technology. Through this agreement with KAIST, we will do our best to create a new paradigm for brain disease treatment and become a platform company that can lead future medical devices and medical technology.”
Former Samsung Electronics Vice Chairman Ki Tae Lee, a strong supporter of this R&D project, said, “Through this agreement with KAIST, we plan to prepare for a new future by combining the technologies AT&C has developed so far with KAIST’s innovative and differentiated technologies.”
KAIST President Kwang Hyung Lee emphasized, “Through this collaboration, KAIST expects to build a world-class digital therapeutics infrastructure for treating brain diseases and contribute greatly to further strengthening Korea’s competitiveness in the biomedical field.”
The signing ceremony was attended by KAIST President Kwang Hyung Lee, the Dean of KAIST College of Natural Sciences Daesoo Kim, AT&C CEO Lee Jong-won, and the current Chairman of AT&C, Ki Tae Lee, former Vice Chairman of Samsung Electronics.
2025.01.09 View 3444 -
 KAIST Opens Newly Expanded Center for Contemplative Research in Collaboration with Brain and Cognitive Sciences Department
KAIST (represented by President Kwang Hyung Lee) announced on January 2nd that it would hold an opening ceremony for the expanded KAIST Center for Contemplative Research (Director Wan Doo Kim) at the Creativity Learning Building on its Daejeon campus on January 3 (Friday).
Established in 2018 with the mission of "integrating meditation and science for the happiness and prosperity of humanity," the KAIST Center for Contemplative Research has been expanding its scope of research into the neuroscience of meditation and training empathetic educators who will lead the field of meditation science in collaboration with the Brain and Cognitive Sciences Department, which was established in 2022.
Supported by the Plato Academy Foundation and with funding from SK Discovery for the facility’s expansion, the center now occupies an extended space on the 5th floor of the Creativity Learning Center. The new facilities include: ▲ Advanced Research Equipment ▲ Meditation Science Laboratories ▲ VR/XR-Based Meditation Experience Rooms ▲ A Large Digital Art Meditation Hall ▲ Personal Meditation Halls.
Particularly, the center plans to conduct next-generation meditation research using cutting-edge technologies such as: ▲ Brain-Computer Interface Technology ▲ Meditation Wearable Devices ▲ Metaverse-Based Meditation Environments.
The opening ceremony, scheduled for the morning of January 3 (Friday), was attended by key figures, including Plato Academy Foundation Chairman Chang-Won Choi, MindLab CEO Professor Seong-Taek Cho, Bosung Group Vice President Byung-Chul Lee, and KAIST President Kwang Hyung Lee.
The event began with a national moment of silence to honor the victims of the recent Jeju Air passenger accident. It included a progress report by the center director, a lecture by Professor Jaeseung Jeong, panel discussions, and more.
Following a tour of the expanded facilities, the center hosted a 20-minute hands-on meditation science session using *Looxid Labs EEG devices for the first 50 participants.
*Looxid Labs EEG Device: A real-time brainwave measurement device developed by KAIST startup Looxid Labs that enables users to experience efficient and AI-powered data-driven meditation science practice (Looxid Labs website: https://looxidlabs.com/).
During the ceremony, Director of the Center for Contemplative Research Wan Doo Kim presented on "The Mission, Vision, and Future of the KAIST Center for Contemplative Research." Yujin Lee, a combined master’s and doctoral researcher from the Brain and Cognitive Sciences Department, shared insights on "The Latest Trends in Meditation Science Research."
A panel discussion and Q&A session on "The Convergence of Meditation and Brain and Cognitive Sciences" followed featuring Professors Jaeseung Jeong, HyungDong Park (Brain and Cognitive Sciences), and Jiyoung Park (Digital Humanities and Social Sciences).
Director Wan Doo Kim commented, “With this expanded opening, we aim to offer advanced meditation programs integrating brain and cognitive sciences and cutting-edge technology not only to KAIST members but also to the general public interested in meditation. We will continue to dedicate ourselves to interdisciplinary research between meditation and science.”
2025.01.03 View 2671
KAIST Opens Newly Expanded Center for Contemplative Research in Collaboration with Brain and Cognitive Sciences Department
KAIST (represented by President Kwang Hyung Lee) announced on January 2nd that it would hold an opening ceremony for the expanded KAIST Center for Contemplative Research (Director Wan Doo Kim) at the Creativity Learning Building on its Daejeon campus on January 3 (Friday).
Established in 2018 with the mission of "integrating meditation and science for the happiness and prosperity of humanity," the KAIST Center for Contemplative Research has been expanding its scope of research into the neuroscience of meditation and training empathetic educators who will lead the field of meditation science in collaboration with the Brain and Cognitive Sciences Department, which was established in 2022.
Supported by the Plato Academy Foundation and with funding from SK Discovery for the facility’s expansion, the center now occupies an extended space on the 5th floor of the Creativity Learning Center. The new facilities include: ▲ Advanced Research Equipment ▲ Meditation Science Laboratories ▲ VR/XR-Based Meditation Experience Rooms ▲ A Large Digital Art Meditation Hall ▲ Personal Meditation Halls.
Particularly, the center plans to conduct next-generation meditation research using cutting-edge technologies such as: ▲ Brain-Computer Interface Technology ▲ Meditation Wearable Devices ▲ Metaverse-Based Meditation Environments.
The opening ceremony, scheduled for the morning of January 3 (Friday), was attended by key figures, including Plato Academy Foundation Chairman Chang-Won Choi, MindLab CEO Professor Seong-Taek Cho, Bosung Group Vice President Byung-Chul Lee, and KAIST President Kwang Hyung Lee.
The event began with a national moment of silence to honor the victims of the recent Jeju Air passenger accident. It included a progress report by the center director, a lecture by Professor Jaeseung Jeong, panel discussions, and more.
Following a tour of the expanded facilities, the center hosted a 20-minute hands-on meditation science session using *Looxid Labs EEG devices for the first 50 participants.
*Looxid Labs EEG Device: A real-time brainwave measurement device developed by KAIST startup Looxid Labs that enables users to experience efficient and AI-powered data-driven meditation science practice (Looxid Labs website: https://looxidlabs.com/).
During the ceremony, Director of the Center for Contemplative Research Wan Doo Kim presented on "The Mission, Vision, and Future of the KAIST Center for Contemplative Research." Yujin Lee, a combined master’s and doctoral researcher from the Brain and Cognitive Sciences Department, shared insights on "The Latest Trends in Meditation Science Research."
A panel discussion and Q&A session on "The Convergence of Meditation and Brain and Cognitive Sciences" followed featuring Professors Jaeseung Jeong, HyungDong Park (Brain and Cognitive Sciences), and Jiyoung Park (Digital Humanities and Social Sciences).
Director Wan Doo Kim commented, “With this expanded opening, we aim to offer advanced meditation programs integrating brain and cognitive sciences and cutting-edge technology not only to KAIST members but also to the general public interested in meditation. We will continue to dedicate ourselves to interdisciplinary research between meditation and science.”
2025.01.03 View 2671 -
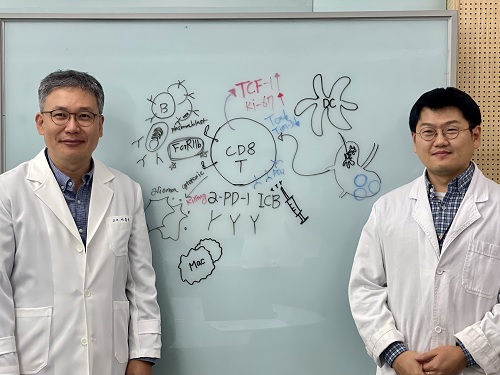 KAIST Unveils New Possibilities for Treating Intractable Brain Tumors
< Photo 1. (From left) Professor Heung Kyu Lee, KAIST Department of Biological Sciences, and Dr. Keun Bon Ku >
Immunotherapy, which enhances the immune system's T cell response to eliminate cancer cells, has emerged as a key approach in cancer treatment. However, in the case of glioblastoma, an aggressive and treatment-resistant brain tumor, numerous clinical trials have failed to confirm their efficacy. Korean researchers have recently analyzed the mechanisms that cause T cell exhaustion, which is characterized by a loss of function or a weakened response following prolonged exposure to antigens in such intractable cancers, identifying key control factors in T cell activation and clarifying the mechanisms that enhance therapeutic effectiveness.
KAIST (represented by President Kwang Hyung Lee) announced on the 6th of November that Professor Heung Kyu Lee’s team from the Department of Biological Sciences, in collaboration with the Korea Research Institute of Chemical Technology (represented by President Young Kuk Lee), has confirmed improved survival rates in a glioblastoma mouse model. By removing the inhibitory Fc gamma receptor (FcγRIIB), the research team was able to restore the responsiveness of cytotoxic T cells to immune checkpoint inhibitors, leading to enhanced anticancer activity.
The research team examined the effect of FcγRIIB, an inhibitory receptor recently found in cytotoxic T cells, on tumor-infiltrating T cells and the therapeutic effectiveness of the anti-PD-1 immune checkpoint inhibitor.
< Figure 1. Study results on improved survival rate due to increased antitumor activity of anti-PD-1 treatment in inhibitory Fc gamma receptor(Fcgr2b) ablation mice with murine glioblastoma. >
Their findings showed that deleting FcγRIIB induced the increase of tumor antigen-specific memory T cells, which helps to suppress exhaustion, enhances stem-like qualities, and reactivates T cell-mediated antitumor immunity, particularly in response to anti-PD-1 treatment. Furthermore, FcγRIIB deletion led to an increase in antigen-specific memory T cells that maintained continuous infiltration into the tumor tissue.
This study presents a new therapeutic target for tumors unresponsive to immune checkpoint inhibitors and demonstrates that combining FcγRIIB inhibition with anti-PD-1 treatment can produce synergistic effects, potentially improving therapeutic outcomes for tumors like glioblastoma, which typically show resistance to anti-PD-1 therapy.
< Figure 2. Overview of the study on the enhanced response to anti-PD-1 therapy for glioblastoma brain tumors upon deletion of the inhibitory Fc gamma receptor (FcγRIIB) in tumor microenvironment. When the inhibitory Fc gamma receptor (FcγRIIB) of cytotoxic T cells is deleted, an increase in tumor-specific memory T cells (Ttsms) was observed. In addition, this T cell subset is identified as originating from the tumor-draining lymph nodes(TdLNs) and leads to persistent infiltration into the tumor tissue. Anti-PD-1 therapy leads to an increased anti-tumor immune response via Ttsms, which is confirmed by increased tumor cell toxicity and increased cell division and decreased cell de-migration indices. Ultimately, the increased cytotoxic T cell immune response leads to an increase in the survival rate of glioblastoma. >
Professor Heung Kyu Lee explained, "This study offers a way to overcome clinical failures in treating brain tumors with immune checkpoint therapy and opens possibilities for broader applications to other intractable cancers. It also highlights the potential of utilizing cytotoxic T cells for tumor cell therapy."
The study, led by Dr. Keun Bon Ku of KAIST (currently a senior researcher at the Korea Research Institute of Chemical Technology's Center for Infectious Disease Diagnosis and Prevention), along with Chae Won Kim, Yumin Kim, Byeong Hoon Kang, Jeongwoo La, In Kang, Won Hyung Park, Stephen Ahn, and Sung Ki Lee, was published online on October 26 in the Journal for ImmunoTherapy of Cancer, an international journal in tumor immunology and therapy from the Society for Immunotherapy of Cancer. (Paper title: “Inhibitory Fcγ receptor deletion enhances CD8 T cell stemness increasing anti-PD-1 therapy responsiveness against glioblastoma,” http://dx.doi.org/10.1136/jitc-2024-009449).
This research received support from the National Research Foundation of Korea, the Bio & Medical Technology Development Program, and the Samsung Science & Technology Foundation.
2024.11.15 View 4104
KAIST Unveils New Possibilities for Treating Intractable Brain Tumors
< Photo 1. (From left) Professor Heung Kyu Lee, KAIST Department of Biological Sciences, and Dr. Keun Bon Ku >
Immunotherapy, which enhances the immune system's T cell response to eliminate cancer cells, has emerged as a key approach in cancer treatment. However, in the case of glioblastoma, an aggressive and treatment-resistant brain tumor, numerous clinical trials have failed to confirm their efficacy. Korean researchers have recently analyzed the mechanisms that cause T cell exhaustion, which is characterized by a loss of function or a weakened response following prolonged exposure to antigens in such intractable cancers, identifying key control factors in T cell activation and clarifying the mechanisms that enhance therapeutic effectiveness.
KAIST (represented by President Kwang Hyung Lee) announced on the 6th of November that Professor Heung Kyu Lee’s team from the Department of Biological Sciences, in collaboration with the Korea Research Institute of Chemical Technology (represented by President Young Kuk Lee), has confirmed improved survival rates in a glioblastoma mouse model. By removing the inhibitory Fc gamma receptor (FcγRIIB), the research team was able to restore the responsiveness of cytotoxic T cells to immune checkpoint inhibitors, leading to enhanced anticancer activity.
The research team examined the effect of FcγRIIB, an inhibitory receptor recently found in cytotoxic T cells, on tumor-infiltrating T cells and the therapeutic effectiveness of the anti-PD-1 immune checkpoint inhibitor.
< Figure 1. Study results on improved survival rate due to increased antitumor activity of anti-PD-1 treatment in inhibitory Fc gamma receptor(Fcgr2b) ablation mice with murine glioblastoma. >
Their findings showed that deleting FcγRIIB induced the increase of tumor antigen-specific memory T cells, which helps to suppress exhaustion, enhances stem-like qualities, and reactivates T cell-mediated antitumor immunity, particularly in response to anti-PD-1 treatment. Furthermore, FcγRIIB deletion led to an increase in antigen-specific memory T cells that maintained continuous infiltration into the tumor tissue.
This study presents a new therapeutic target for tumors unresponsive to immune checkpoint inhibitors and demonstrates that combining FcγRIIB inhibition with anti-PD-1 treatment can produce synergistic effects, potentially improving therapeutic outcomes for tumors like glioblastoma, which typically show resistance to anti-PD-1 therapy.
< Figure 2. Overview of the study on the enhanced response to anti-PD-1 therapy for glioblastoma brain tumors upon deletion of the inhibitory Fc gamma receptor (FcγRIIB) in tumor microenvironment. When the inhibitory Fc gamma receptor (FcγRIIB) of cytotoxic T cells is deleted, an increase in tumor-specific memory T cells (Ttsms) was observed. In addition, this T cell subset is identified as originating from the tumor-draining lymph nodes(TdLNs) and leads to persistent infiltration into the tumor tissue. Anti-PD-1 therapy leads to an increased anti-tumor immune response via Ttsms, which is confirmed by increased tumor cell toxicity and increased cell division and decreased cell de-migration indices. Ultimately, the increased cytotoxic T cell immune response leads to an increase in the survival rate of glioblastoma. >
Professor Heung Kyu Lee explained, "This study offers a way to overcome clinical failures in treating brain tumors with immune checkpoint therapy and opens possibilities for broader applications to other intractable cancers. It also highlights the potential of utilizing cytotoxic T cells for tumor cell therapy."
The study, led by Dr. Keun Bon Ku of KAIST (currently a senior researcher at the Korea Research Institute of Chemical Technology's Center for Infectious Disease Diagnosis and Prevention), along with Chae Won Kim, Yumin Kim, Byeong Hoon Kang, Jeongwoo La, In Kang, Won Hyung Park, Stephen Ahn, and Sung Ki Lee, was published online on October 26 in the Journal for ImmunoTherapy of Cancer, an international journal in tumor immunology and therapy from the Society for Immunotherapy of Cancer. (Paper title: “Inhibitory Fcγ receptor deletion enhances CD8 T cell stemness increasing anti-PD-1 therapy responsiveness against glioblastoma,” http://dx.doi.org/10.1136/jitc-2024-009449).
This research received support from the National Research Foundation of Korea, the Bio & Medical Technology Development Program, and the Samsung Science & Technology Foundation.
2024.11.15 View 4104 -
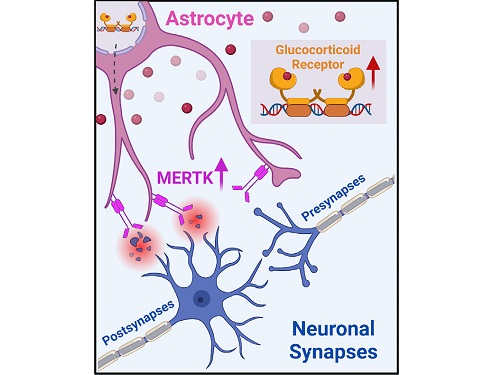 A KAIST research team identifies a cause of mental diseases induced by childhood abuse
Childhood neglect and/or abuse can induce extreme stress that significantly changes neural networks and functions during growth. This can lead to mental illnesses, including depression and schizophrenia, but the exact mechanism and means to control it were yet to be discovered.
On August 1, a KAIST research team led by Professor Won-Suk Chung from the Department of Biological Sciences announced the identification of excessive synapse removal mediated by astrocytes as the cause of mental diseases induced by childhood abuse trauma. Their research was published in Immunity, a top international journal in the field of immunology.
The research team discovered that the excessive astrocyte-mediated removal of excitatory synapses in the brain in response to stress hormones is a cause of mental diseases induced by childhood neglect and abuse. Clinical data have previously shown that high levels of stress can lead to various mental diseases, but the exact mechanism has been unknown. The results of this research therefore are expected to be widely applied to the prevention and treatment of such diseases.
The research team clinically screened an FDA-approved drug to uncover the mechanism that regulates the phagocytotic role of astrocytes, in which they capture external substances and eliminate them. As a result, the team found that synthetic glucocorticoids, namely stress hormones, enhanced astrocyte-mediated phagocytosis to an abnormal level. Glucocorticoids play essential roles in processes that maintain life, such as carbohydrate metabolism and anti-inflammation, but are also secreted in response to external stimuli such as stress, allowing the body to respond appropriately. However, excessive and long-term exposure to glucocorticoids caused by chronic stress can lead to various mental diseases including depression, cognitive disorders, and anxiety.
< Figure 1. Results of screening for compounds that increase astrocyte phagocytosis
(A) Discovered that synthetic glucocorticoid (stress hormone) increases the phagocytosis of astrocytes through screening of FDA-approved clinical compounds. (B-C) When treated with stress hormones, the phagocytosis of astrocytes is greatly increased, but this phenomenon is strongly suppressed by the GR antagonist (Mifepristone). CORT: corticosterone (stress hormone), Eplerenone: mineralocorticoid receptor (MR) antagonist, Mifepristone: glucocorticoid receptor (GR) antagonist >
To understand the changes in astrocyte functions caused by childhood stress, the research team used mice models with early social deprivation, and discovered that stress hormones bind to the glucocorticoid receptors (GRs) of astrocytes. This significantly increased the expression of Mer tyrosine kinase (MERK), which plays an essential role in astrocyte phagocytosis. Surprisingly, out of the various neurons in the cerebral cortex, astrocytes would eliminate only the excitatory synapses of specific neurons. The team found that this builds abnormal neural networks, which can lead to complex behavioral abnormalities such as social deficiencies and depression in adulthood.
The team also observed that microglia, which also play an important role in cerebral immunity, did not contribute to synapse removal in the mice models with early social deprivation. This confirms that the response to stress hormones during childhood is specifically astrocyte-mediated.
To find out whether these results are also applicable in humans, the research team used a brain organoid grown from human-induced pluripotent stem cells to observe human responses to stress hormones. The team observed that the stress hormones induced astrocyte GRs and phagocyte activation in the human brain organoid as well, and confirmed that the astrocytes subsequently eliminated excessive amounts of excitatory synapses. By showing that mice and humans both showed the same synapse control mechanism in response to stress, the team suggested that this discovery is applicable to mental disorders in humans.
< Figure 2. A schematic diagram of the study published in Immunity. Excessive stress hormone secretion in childhood increases the expression of the MERTK phagocytic receptor through the glucocorticoid receptor (GR) of astrocytes, resulting in excessive elimination of excitatory synapses. Excessive synaptic elimination by astrocytes during brain development causes permanent damage to brain circuits, resulting in abnormal neural activity in the adult brain and psychiatric behaviors such as depression and anti-social tendencies. >
Prof. Won-Suk Chung said, “Until now, we did not know the exact mechanism for how childhood stress caused brain diseases. This research was the first to show that the excessive phagocytosis of astrocytes could be an important cause of such diseases.” He added, “In the future, controlling the immune response of astrocytes will be used as a fundamental target for understanding and treating brain diseases.”
This research, written by co-first authors Youkyeong Byun (Ph.D. candidate) and Nam-Shik Kim (post-doctoral associate) from the KAIST Department of Biological Sciences, was published in the internationally renowned journal Immunity, a sister magazine of Cell and one of the best journal in the field of immunology, on July 31 under the title "Stress induces behavioral abnormalities by increasing expression of phagocytic receptor MERTK in astrocytes to promote synapse phagocytosis."
This work was supported by a National Research Foundation of Korea grant, the Korea Health Industry Development Institute (KHIDI), and the Korea Dementia Research Center (KDRC).
2023.08.04 View 7206
A KAIST research team identifies a cause of mental diseases induced by childhood abuse
Childhood neglect and/or abuse can induce extreme stress that significantly changes neural networks and functions during growth. This can lead to mental illnesses, including depression and schizophrenia, but the exact mechanism and means to control it were yet to be discovered.
On August 1, a KAIST research team led by Professor Won-Suk Chung from the Department of Biological Sciences announced the identification of excessive synapse removal mediated by astrocytes as the cause of mental diseases induced by childhood abuse trauma. Their research was published in Immunity, a top international journal in the field of immunology.
The research team discovered that the excessive astrocyte-mediated removal of excitatory synapses in the brain in response to stress hormones is a cause of mental diseases induced by childhood neglect and abuse. Clinical data have previously shown that high levels of stress can lead to various mental diseases, but the exact mechanism has been unknown. The results of this research therefore are expected to be widely applied to the prevention and treatment of such diseases.
The research team clinically screened an FDA-approved drug to uncover the mechanism that regulates the phagocytotic role of astrocytes, in which they capture external substances and eliminate them. As a result, the team found that synthetic glucocorticoids, namely stress hormones, enhanced astrocyte-mediated phagocytosis to an abnormal level. Glucocorticoids play essential roles in processes that maintain life, such as carbohydrate metabolism and anti-inflammation, but are also secreted in response to external stimuli such as stress, allowing the body to respond appropriately. However, excessive and long-term exposure to glucocorticoids caused by chronic stress can lead to various mental diseases including depression, cognitive disorders, and anxiety.
< Figure 1. Results of screening for compounds that increase astrocyte phagocytosis
(A) Discovered that synthetic glucocorticoid (stress hormone) increases the phagocytosis of astrocytes through screening of FDA-approved clinical compounds. (B-C) When treated with stress hormones, the phagocytosis of astrocytes is greatly increased, but this phenomenon is strongly suppressed by the GR antagonist (Mifepristone). CORT: corticosterone (stress hormone), Eplerenone: mineralocorticoid receptor (MR) antagonist, Mifepristone: glucocorticoid receptor (GR) antagonist >
To understand the changes in astrocyte functions caused by childhood stress, the research team used mice models with early social deprivation, and discovered that stress hormones bind to the glucocorticoid receptors (GRs) of astrocytes. This significantly increased the expression of Mer tyrosine kinase (MERK), which plays an essential role in astrocyte phagocytosis. Surprisingly, out of the various neurons in the cerebral cortex, astrocytes would eliminate only the excitatory synapses of specific neurons. The team found that this builds abnormal neural networks, which can lead to complex behavioral abnormalities such as social deficiencies and depression in adulthood.
The team also observed that microglia, which also play an important role in cerebral immunity, did not contribute to synapse removal in the mice models with early social deprivation. This confirms that the response to stress hormones during childhood is specifically astrocyte-mediated.
To find out whether these results are also applicable in humans, the research team used a brain organoid grown from human-induced pluripotent stem cells to observe human responses to stress hormones. The team observed that the stress hormones induced astrocyte GRs and phagocyte activation in the human brain organoid as well, and confirmed that the astrocytes subsequently eliminated excessive amounts of excitatory synapses. By showing that mice and humans both showed the same synapse control mechanism in response to stress, the team suggested that this discovery is applicable to mental disorders in humans.
< Figure 2. A schematic diagram of the study published in Immunity. Excessive stress hormone secretion in childhood increases the expression of the MERTK phagocytic receptor through the glucocorticoid receptor (GR) of astrocytes, resulting in excessive elimination of excitatory synapses. Excessive synaptic elimination by astrocytes during brain development causes permanent damage to brain circuits, resulting in abnormal neural activity in the adult brain and psychiatric behaviors such as depression and anti-social tendencies. >
Prof. Won-Suk Chung said, “Until now, we did not know the exact mechanism for how childhood stress caused brain diseases. This research was the first to show that the excessive phagocytosis of astrocytes could be an important cause of such diseases.” He added, “In the future, controlling the immune response of astrocytes will be used as a fundamental target for understanding and treating brain diseases.”
This research, written by co-first authors Youkyeong Byun (Ph.D. candidate) and Nam-Shik Kim (post-doctoral associate) from the KAIST Department of Biological Sciences, was published in the internationally renowned journal Immunity, a sister magazine of Cell and one of the best journal in the field of immunology, on July 31 under the title "Stress induces behavioral abnormalities by increasing expression of phagocytic receptor MERTK in astrocytes to promote synapse phagocytosis."
This work was supported by a National Research Foundation of Korea grant, the Korea Health Industry Development Institute (KHIDI), and the Korea Dementia Research Center (KDRC).
2023.08.04 View 7206 -
 The cause of disability in aged brain meningeal membranes identified
Due to the increase in average age, studies on changes in the brain following general aging process without serious brain diseases have also become an issue that requires in-depth studies. Regarding aging research, as aging progresses, ‘sugar’ accumulates in the body, and the accumulated sugar becomes a causative agent for various diseases such as aging-related inflammation and vascular disease. In the end, “surplus” sugar molecules attach to various proteins in the body and interfere with their functions.
KAIST (President Kwang Hyung Lee), a joint research team of Professor Pilnam Kim and Professor Yong Jeong of the Department of Bio and Brain Engineering, revealed on the 15th that it was confirmed that the function of being the “front line of defense” for the cerebrocortex of the brain meninges, the layers of membranes that surrounds the brain, is hindered when 'sugar' begins to build up on them as aging progresses.
Professor Kim's research team confirmed excessive accumulation of sugar molecules in the meninges of the elderly and also confirmed that sugar accumulation occurs mouse models in accordance with certain age levels. The meninges are thin membranes that surround the brain and exist at the boundary between the cerebrospinal fluid and the cortex and play an important role in protecting the brain. In this study, it was revealed that the dysfunction of these brain membranes caused by aging is induced by 'excess' sugar in the brain. In particular, as the meningeal membrane becomes thinner and stickier due to aging, a new paradigm has been provided for the discovery of the principle of the decrease in material exchange between the cerebrospinal fluid and the cerebral cortex.
This research was conducted by the Ph.D. candidate Hyo Min Kim and Dr. Shinheun Kim as the co-first authors to be published online on February 28th in the international journal, Aging Cell. (Paper Title: Glycation-mediated tissue-level remodeling of brain meningeal membrane by aging)
The meninges, which are in direct contact with the cerebrospinal fluid, are mainly composed of collagen, an extracellular matrix (ECM) protein, and are composed of fibroblasts, which are cells that produce this protein. The cells that come in contact with collagen proteins that are attached with sugar have a low collagen production function, while the meningeal membrane continuously thins and collapses as the expression of collagen degrading enzymes increases.
Studies on the relationship between excess sugar molecules accumulation in the brain due to continued sugar intake and the degeneration of neurons and brain diseases have been continuously conducted. However, this study was the first to identify meningeal degeneration and dysfunction caused by glucose accumulation with the focus on the meninges itself, and the results are expected to present new ideas for research into approach towards discoveries of new treatments for brain disease.
Researcher Hyomin Kim, the first author, introduced the research results as “an interesting study that identified changes in the barriers of the brain due to aging through a convergent approach, starting from the human brain and utilizing an animal model with a biomimetic meningeal model”.
Professor Pilnam Kim's research team is conducting research and development to remove sugar that accumulated throughout the human body, including the meninges. Advanced glycation end products, which are waste products formed when proteins and sugars meet in the human body, are partially removed by macrophages. However, glycated products bound to extracellular matrix proteins such as collagen are difficult to remove naturally. Through the KAIST-Ceragem Research Center, this research team is developing a healthcare medical device to remove 'sugar residue' in the body.
This study was carried out with the National Research Foundation of Korea's collective research support.
Figure 1. Schematic diagram of proposed mechanism showing aging‐related ECM remodeling through meningeal fibroblasts on the brain leptomeninges. Meningeal fibroblasts in the young brain showed dynamic COL1A1 synthetic and COL1‐interactive function on the collagen membrane. They showed ITGB1‐mediated adhesion on the COL1‐composed leptomeningeal membrane and induction of COL1A1 synthesis for maintaining the collagen membrane. With aging, meningeal fibroblasts showed depletion of COL1A1 synthetic function and altered cell–matrix interaction.
Figure 2. Representative rat meningeal images observed in the study. Compared to young rats, it was confirmed that type 1 collagen (COL1) decreased along with the accumulation of glycated end products (AGE) in the brain membrane of aged rats, and the activity of integrin beta 1 (ITGB1), a representative receptor corresponding to cell-collagen interaction. Instead, it was observed that the activity of discoidin domain receptor 2 (DDR2), one of the tyrosine kinases, increased.
Figure 3. Substance flux through the brain membrane decreases with aging. It was confirmed that the degree of adsorption of fluorescent substances contained in cerebrospinal fluid (CSF) to the brain membrane increased and the degree of entry into the periphery of the cerebral blood vessels decreased in the aged rats. In this study, only the influx into the brain was confirmed during the entry and exit of substances, but the degree of outflow will also be confirmed through future studies.
2023.03.15 View 8270
The cause of disability in aged brain meningeal membranes identified
Due to the increase in average age, studies on changes in the brain following general aging process without serious brain diseases have also become an issue that requires in-depth studies. Regarding aging research, as aging progresses, ‘sugar’ accumulates in the body, and the accumulated sugar becomes a causative agent for various diseases such as aging-related inflammation and vascular disease. In the end, “surplus” sugar molecules attach to various proteins in the body and interfere with their functions.
KAIST (President Kwang Hyung Lee), a joint research team of Professor Pilnam Kim and Professor Yong Jeong of the Department of Bio and Brain Engineering, revealed on the 15th that it was confirmed that the function of being the “front line of defense” for the cerebrocortex of the brain meninges, the layers of membranes that surrounds the brain, is hindered when 'sugar' begins to build up on them as aging progresses.
Professor Kim's research team confirmed excessive accumulation of sugar molecules in the meninges of the elderly and also confirmed that sugar accumulation occurs mouse models in accordance with certain age levels. The meninges are thin membranes that surround the brain and exist at the boundary between the cerebrospinal fluid and the cortex and play an important role in protecting the brain. In this study, it was revealed that the dysfunction of these brain membranes caused by aging is induced by 'excess' sugar in the brain. In particular, as the meningeal membrane becomes thinner and stickier due to aging, a new paradigm has been provided for the discovery of the principle of the decrease in material exchange between the cerebrospinal fluid and the cerebral cortex.
This research was conducted by the Ph.D. candidate Hyo Min Kim and Dr. Shinheun Kim as the co-first authors to be published online on February 28th in the international journal, Aging Cell. (Paper Title: Glycation-mediated tissue-level remodeling of brain meningeal membrane by aging)
The meninges, which are in direct contact with the cerebrospinal fluid, are mainly composed of collagen, an extracellular matrix (ECM) protein, and are composed of fibroblasts, which are cells that produce this protein. The cells that come in contact with collagen proteins that are attached with sugar have a low collagen production function, while the meningeal membrane continuously thins and collapses as the expression of collagen degrading enzymes increases.
Studies on the relationship between excess sugar molecules accumulation in the brain due to continued sugar intake and the degeneration of neurons and brain diseases have been continuously conducted. However, this study was the first to identify meningeal degeneration and dysfunction caused by glucose accumulation with the focus on the meninges itself, and the results are expected to present new ideas for research into approach towards discoveries of new treatments for brain disease.
Researcher Hyomin Kim, the first author, introduced the research results as “an interesting study that identified changes in the barriers of the brain due to aging through a convergent approach, starting from the human brain and utilizing an animal model with a biomimetic meningeal model”.
Professor Pilnam Kim's research team is conducting research and development to remove sugar that accumulated throughout the human body, including the meninges. Advanced glycation end products, which are waste products formed when proteins and sugars meet in the human body, are partially removed by macrophages. However, glycated products bound to extracellular matrix proteins such as collagen are difficult to remove naturally. Through the KAIST-Ceragem Research Center, this research team is developing a healthcare medical device to remove 'sugar residue' in the body.
This study was carried out with the National Research Foundation of Korea's collective research support.
Figure 1. Schematic diagram of proposed mechanism showing aging‐related ECM remodeling through meningeal fibroblasts on the brain leptomeninges. Meningeal fibroblasts in the young brain showed dynamic COL1A1 synthetic and COL1‐interactive function on the collagen membrane. They showed ITGB1‐mediated adhesion on the COL1‐composed leptomeningeal membrane and induction of COL1A1 synthesis for maintaining the collagen membrane. With aging, meningeal fibroblasts showed depletion of COL1A1 synthetic function and altered cell–matrix interaction.
Figure 2. Representative rat meningeal images observed in the study. Compared to young rats, it was confirmed that type 1 collagen (COL1) decreased along with the accumulation of glycated end products (AGE) in the brain membrane of aged rats, and the activity of integrin beta 1 (ITGB1), a representative receptor corresponding to cell-collagen interaction. Instead, it was observed that the activity of discoidin domain receptor 2 (DDR2), one of the tyrosine kinases, increased.
Figure 3. Substance flux through the brain membrane decreases with aging. It was confirmed that the degree of adsorption of fluorescent substances contained in cerebrospinal fluid (CSF) to the brain membrane increased and the degree of entry into the periphery of the cerebral blood vessels decreased in the aged rats. In this study, only the influx into the brain was confirmed during the entry and exit of substances, but the degree of outflow will also be confirmed through future studies.
2023.03.15 View 8270 -
 Professor Jae-Woong Jeong Receives Hyonwoo KAIST Academic Award
Professor Jae-Woong Jeong from the School of Electrical Engineering was selected for the Hyonwoo KAIST Academic Award, funded by the HyonWoo Cultural Foundation (Chairman Soo-il Kwak, honorary professor at Seoul National University Business School).
The Hyonwoo KAIST Academic Award, presented for the first time in 2021, is an award newly founded by the donations of Chairman Soo-il Kwak of the HyonWoo Cultural Foundation, who aims to reward excellent KAIST scholars who have made outstanding academic achievements.
Every year, through the strict evaluations of the selection committee of the HyonWoo Cultural Foundation and the faculty reward recommendation board, KAIST will choose one faculty member that may represent the school with their excellent academic achievement, and reward them with a plaque and 100 million won.
Professor Jae-Woong Jeong, the winner of this year’s award, developed the first IoT-based wireless remote brain neural network control system to overcome brain diseases, and has been leading the field. The research was published in 2021 in Nature Biomedical Engineering, one of world’s best scientific journals, and has been recognized as a novel technology that suggested a new vision for the automation of brain research and disease treatment. This study, led by Professor Jeong’s research team, was part of the KAIST College of Engineering Global Initiative Interdisciplinary Research Project, and was jointly studied by Washington University School of Medicine through an international research collaboration. The technology was introduced more than 60 times through both domestic and international media, including Medical Xpress, MBC News, and Maeil Business News.
Professor Jeong has also developed a wirelessly chargeable soft machine for brain transplants, and the results were published in Nature Communications. He thereby opened a new paradigm for implantable semi-permanent devices for transplants, and is making unprecedented research achievements.
2022.06.13 View 8382
Professor Jae-Woong Jeong Receives Hyonwoo KAIST Academic Award
Professor Jae-Woong Jeong from the School of Electrical Engineering was selected for the Hyonwoo KAIST Academic Award, funded by the HyonWoo Cultural Foundation (Chairman Soo-il Kwak, honorary professor at Seoul National University Business School).
The Hyonwoo KAIST Academic Award, presented for the first time in 2021, is an award newly founded by the donations of Chairman Soo-il Kwak of the HyonWoo Cultural Foundation, who aims to reward excellent KAIST scholars who have made outstanding academic achievements.
Every year, through the strict evaluations of the selection committee of the HyonWoo Cultural Foundation and the faculty reward recommendation board, KAIST will choose one faculty member that may represent the school with their excellent academic achievement, and reward them with a plaque and 100 million won.
Professor Jae-Woong Jeong, the winner of this year’s award, developed the first IoT-based wireless remote brain neural network control system to overcome brain diseases, and has been leading the field. The research was published in 2021 in Nature Biomedical Engineering, one of world’s best scientific journals, and has been recognized as a novel technology that suggested a new vision for the automation of brain research and disease treatment. This study, led by Professor Jeong’s research team, was part of the KAIST College of Engineering Global Initiative Interdisciplinary Research Project, and was jointly studied by Washington University School of Medicine through an international research collaboration. The technology was introduced more than 60 times through both domestic and international media, including Medical Xpress, MBC News, and Maeil Business News.
Professor Jeong has also developed a wirelessly chargeable soft machine for brain transplants, and the results were published in Nature Communications. He thereby opened a new paradigm for implantable semi-permanent devices for transplants, and is making unprecedented research achievements.
2022.06.13 View 8382 -
 Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 12448
Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 12448 -
 Scientists Develop Wireless Networks that Allow Brain Circuits to Be Controlled Remotely through the Internet
Wireless implantable devices and IoT could manipulate the brains of animals from anywhere around the world due to their minimalistic hardware, low setup cost, ease of use, and customizable versatility
A new study shows that researchers can remotely control the brain circuits of numerous animals simultaneously and independently through the internet. The scientists believe this newly developed technology can speed up brain research and various neuroscience studies to uncover basic brain functions as well as the underpinnings of various neuropsychiatric and neurological disorders.
A multidisciplinary team of researchers at KAIST, Washington University in St. Louis, and the University of Colorado, Boulder, created a wireless ecosystem with its own wireless implantable devices and Internet of Things (IoT) infrastructure to enable high-throughput neuroscience experiments over the internet. This innovative technology could enable scientists to manipulate the brains of animals from anywhere around the world. The study was published in the journal Nature Biomedical Engineering on November 25
“This novel technology is highly versatile and adaptive. It can remotely control numerous neural implants and laboratory tools in real-time or in a scheduled way without direct human interactions,” said Professor Jae-Woong Jeong of the School of Electrical Engineering at KAIST and a senior author of the study. “These wireless neural devices and equipment integrated with IoT technology have enormous potential for science and medicine.”
The wireless ecosystem only requires a mini-computer that can be purchased for under $45, which connects to the internet and communicates with wireless multifunctional brain probes or other types of conventional laboratory equipment using IoT control modules. By optimally integrating the versatility and modular construction of both unique IoT hardware and software within a single ecosystem, this wireless technology offers new applications that have not been demonstrated before by a single standalone technology. This includes, but is not limited to minimalistic hardware, global remote access, selective and scheduled experiments, customizable automation, and high-throughput scalability.
“As long as researchers have internet access, they are able to trigger, customize, stop, validate, and store the outcomes of large experiments at any time and from anywhere in the world. They can remotely perform large-scale neuroscience experiments in animals deployed in multiple countries,” said one of the lead authors, Dr. Raza Qazi, a researcher with KAIST and the University of Colorado, Boulder. “The low cost of this system allows it to be easily adopted and can further fuel innovation across many laboratories,” Dr. Qazi added.
One of the significant advantages of this IoT neurotechnology is its ability to be mass deployed across the globe due to its minimalistic hardware, low setup cost, ease of use, and customizable versatility. Scientists across the world can quickly implement this technology within their existing laboratories with minimal budget concerns to achieve globally remote access, scalable experimental automation, or both, thus potentially reducing the time needed to unravel various neuroscientific challenges such as those associated with intractable neurological conditions.
Another senior author on the study, Professor Jordan McCall from the Department of Anesthesiology and Center for Clinical Pharmacology at Washington University in St. Louis, said this technology has the potential to change how basic neuroscience studies are performed. “One of the biggest limitations when trying to understand how the mammalian brain works is that we have to study these functions in unnatural conditions. This technology brings us one step closer to performing important studies without direct human interaction with the study subjects.”
The ability to remotely schedule experiments moves toward automating these types of experiments. Dr. Kyle Parker, an instructor at Washington University in St. Louis and another lead author on the study added, “This experimental automation can potentially help us reduce the number of animals used in biomedical research by reducing the variability introduced by various experimenters. This is especially important given our moral imperative to seek research designs that enable this reduction.”
The researchers believe this wireless technology may open new opportunities for many applications including brain research, pharmaceuticals, and telemedicine to treat diseases in the brain and other organs remotely. This remote automation technology could become even more valuable when many labs need to shut down, such as during the height of the COVID-19 pandemic.
This work was supported by grants from the KAIST Global Singularity Research Program, the National Research Foundation of Korea, the United States National Institute of Health, and Oak Ridge Associated Universities.
-PublicationRaza Qazi, Kyle Parker, Choong Yeon Kim, Jordan McCall, Jae-Woong Jeong et al. “Scalable and modular wireless-network infrastructure for large-scale behavioral neuroscience,” Nature Biomedical Engineering, November 25 2021 (doi.org/10.1038/s41551-021-00814-w)
-ProfileProfessor Jae-Woong JeongBio-Integrated Electronics and Systems LabSchool of Electrical EngineeringKAIST
2021.11.29 View 15621
Scientists Develop Wireless Networks that Allow Brain Circuits to Be Controlled Remotely through the Internet
Wireless implantable devices and IoT could manipulate the brains of animals from anywhere around the world due to their minimalistic hardware, low setup cost, ease of use, and customizable versatility
A new study shows that researchers can remotely control the brain circuits of numerous animals simultaneously and independently through the internet. The scientists believe this newly developed technology can speed up brain research and various neuroscience studies to uncover basic brain functions as well as the underpinnings of various neuropsychiatric and neurological disorders.
A multidisciplinary team of researchers at KAIST, Washington University in St. Louis, and the University of Colorado, Boulder, created a wireless ecosystem with its own wireless implantable devices and Internet of Things (IoT) infrastructure to enable high-throughput neuroscience experiments over the internet. This innovative technology could enable scientists to manipulate the brains of animals from anywhere around the world. The study was published in the journal Nature Biomedical Engineering on November 25
“This novel technology is highly versatile and adaptive. It can remotely control numerous neural implants and laboratory tools in real-time or in a scheduled way without direct human interactions,” said Professor Jae-Woong Jeong of the School of Electrical Engineering at KAIST and a senior author of the study. “These wireless neural devices and equipment integrated with IoT technology have enormous potential for science and medicine.”
The wireless ecosystem only requires a mini-computer that can be purchased for under $45, which connects to the internet and communicates with wireless multifunctional brain probes or other types of conventional laboratory equipment using IoT control modules. By optimally integrating the versatility and modular construction of both unique IoT hardware and software within a single ecosystem, this wireless technology offers new applications that have not been demonstrated before by a single standalone technology. This includes, but is not limited to minimalistic hardware, global remote access, selective and scheduled experiments, customizable automation, and high-throughput scalability.
“As long as researchers have internet access, they are able to trigger, customize, stop, validate, and store the outcomes of large experiments at any time and from anywhere in the world. They can remotely perform large-scale neuroscience experiments in animals deployed in multiple countries,” said one of the lead authors, Dr. Raza Qazi, a researcher with KAIST and the University of Colorado, Boulder. “The low cost of this system allows it to be easily adopted and can further fuel innovation across many laboratories,” Dr. Qazi added.
One of the significant advantages of this IoT neurotechnology is its ability to be mass deployed across the globe due to its minimalistic hardware, low setup cost, ease of use, and customizable versatility. Scientists across the world can quickly implement this technology within their existing laboratories with minimal budget concerns to achieve globally remote access, scalable experimental automation, or both, thus potentially reducing the time needed to unravel various neuroscientific challenges such as those associated with intractable neurological conditions.
Another senior author on the study, Professor Jordan McCall from the Department of Anesthesiology and Center for Clinical Pharmacology at Washington University in St. Louis, said this technology has the potential to change how basic neuroscience studies are performed. “One of the biggest limitations when trying to understand how the mammalian brain works is that we have to study these functions in unnatural conditions. This technology brings us one step closer to performing important studies without direct human interaction with the study subjects.”
The ability to remotely schedule experiments moves toward automating these types of experiments. Dr. Kyle Parker, an instructor at Washington University in St. Louis and another lead author on the study added, “This experimental automation can potentially help us reduce the number of animals used in biomedical research by reducing the variability introduced by various experimenters. This is especially important given our moral imperative to seek research designs that enable this reduction.”
The researchers believe this wireless technology may open new opportunities for many applications including brain research, pharmaceuticals, and telemedicine to treat diseases in the brain and other organs remotely. This remote automation technology could become even more valuable when many labs need to shut down, such as during the height of the COVID-19 pandemic.
This work was supported by grants from the KAIST Global Singularity Research Program, the National Research Foundation of Korea, the United States National Institute of Health, and Oak Ridge Associated Universities.
-PublicationRaza Qazi, Kyle Parker, Choong Yeon Kim, Jordan McCall, Jae-Woong Jeong et al. “Scalable and modular wireless-network infrastructure for large-scale behavioral neuroscience,” Nature Biomedical Engineering, November 25 2021 (doi.org/10.1038/s41551-021-00814-w)
-ProfileProfessor Jae-Woong JeongBio-Integrated Electronics and Systems LabSchool of Electrical EngineeringKAIST
2021.11.29 View 15621 -
 Two Researchers Designated as SUHF Fellows
Professor Taeyun Ku from the Graduate School of Medical Science and Engineering and Professor Hanseul Yang from the Department of Biological Sciences were nominated as 2021 fellows of the Suh Kyungbae Foundation (SUHF).
SUHF selected three young promising scientists from 53 researchers who are less than five years into their careers. A panel of judges comprised of scholars from home and abroad made the final selection based on the candidates’ innovativeness and power to influence. Professor You-Bong Hyun from Seoul National University also won the fellowship.
Professor Ku’s main topic is opto-connectomics. He will study ways to visualize the complex brain network using innovative technology that transforms neurons into optical elements.
Professor Yang will research the possibility of helping patients recover from skin diseases or injuries without scars by studying spiny mouse genes.
SUHF was established by Amorepacific Group Chairman Suh Kyungbae in 2016 with 300 billion KRW of his private funds. Under the vision of ‘contributing to humanity by supporting innovative discoveries of bioscience researchers,’ the foundation supports promising Korean scientists who pioneer new fields of research in biological sciences.
From 2017 to this year, SUHF has selected 20 promising scientists in the field of biological sciences. Selected scientists are provided with up to KRW 500 million each year for five years. The foundation has provided a total of KRW 48.5 billion in research funds to date.
2021.09.15 View 8908
Two Researchers Designated as SUHF Fellows
Professor Taeyun Ku from the Graduate School of Medical Science and Engineering and Professor Hanseul Yang from the Department of Biological Sciences were nominated as 2021 fellows of the Suh Kyungbae Foundation (SUHF).
SUHF selected three young promising scientists from 53 researchers who are less than five years into their careers. A panel of judges comprised of scholars from home and abroad made the final selection based on the candidates’ innovativeness and power to influence. Professor You-Bong Hyun from Seoul National University also won the fellowship.
Professor Ku’s main topic is opto-connectomics. He will study ways to visualize the complex brain network using innovative technology that transforms neurons into optical elements.
Professor Yang will research the possibility of helping patients recover from skin diseases or injuries without scars by studying spiny mouse genes.
SUHF was established by Amorepacific Group Chairman Suh Kyungbae in 2016 with 300 billion KRW of his private funds. Under the vision of ‘contributing to humanity by supporting innovative discoveries of bioscience researchers,’ the foundation supports promising Korean scientists who pioneer new fields of research in biological sciences.
From 2017 to this year, SUHF has selected 20 promising scientists in the field of biological sciences. Selected scientists are provided with up to KRW 500 million each year for five years. The foundation has provided a total of KRW 48.5 billion in research funds to date.
2021.09.15 View 8908 -
 A Mechanism Underlying Most Common Cause of Epileptic Seizures Revealed
An interdisciplinary study shows that neurons carrying somatic mutations in MTOR can lead to focal epileptogenesis via non-cell-autonomous hyperexcitability of nearby nonmutated neurons
During fetal development, cells should migrate to the outer edge of the brain to form critical connections for information transfer and regulation in the body. When even a few cells fail to move to the correct location, the neurons become disorganized and this results in focal cortical dysplasia. This condition is the most common cause of seizures that cannot be controlled with medication in children and the second most common cause in adults.
Now, an interdisciplinary team studying neurogenetics, neural networks, and neurophysiology at KAIST has revealed how dysfunctions in even a small percentage of cells can cause disorder across the entire brain. They published their results on June 28 in Annals of Neurology.
The work builds on a previous finding, also by a KAIST scientists, who found that focal cortical dysplasia was caused by mutations in the cells involved in mTOR, a pathway that regulates signaling between neurons in the brain.
“Only 1 to 2% of neurons carrying mutations in the mTOR signaling pathway that regulates cell signaling in the brain have been found to include seizures in animal models of focal cortical dysplasia,” said Professor Jong-Woo Sohn from the Department of Biological Sciences. “The main challenge of this study was to explain how nearby non-mutated neurons are hyperexcitable.”
Initially, the researchers hypothesized that the mutated cells affected the number of excitatory and inhibitory synapses in all neurons, mutated or not. These neural gates can trigger or halt activity, respectively, in other neurons. Seizures are a result of extreme activity, called hyperexcitability. If the mutated cells upend the balance and result in more excitatory cells, the researchers thought, it made sense that the cells would be more susceptible to hyperexcitability and, as a result, seizures.
“Contrary to our expectations, the synaptic input balance was not changed in either the mutated or non-mutated neurons,” said Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering. “We turned our attention to a protein overproduced by mutated neurons.”
The protein is adenosine kinase, which lowers the concentration of adenosine. This naturally occurring compound is an anticonvulsant and works to relax vessels. In mice engineered to have focal cortical dysplasia, the researchers injected adenosine to replace the levels lowered by the protein. It worked and the neurons became less excitable.
“We demonstrated that augmentation of adenosine signaling could attenuate the excitability of non-mutated neurons,” said Professor Se-Bum Paik from the Department of Bio and Brain Engineering.
The effect on the non-mutated neurons was the surprising part, according to Paik. “The seizure-triggering hyperexcitability originated not in the mutation-carrying neurons, but instead in the nearby non-mutated neurons,” he said.
The mutated neurons excreted more adenosine kinase, reducing the adenosine levels in the local environment of all the cells. With less adenosine, the non-mutated neurons became hyperexcitable, leading to seizures.
“While we need further investigate into the relationship between the concentration of adenosine and the increased excitation of nearby neurons, our results support the medical use of drugs to activate adenosine signaling as a possible treatment pathway for focal cortical dysplasia,” Professor Lee said.
The Suh Kyungbae Foundation, the Korea Health Technology Research and Development Project, the Ministry of Health & Welfare, and the National Research Foundation in Korea funded this work.
-Publication:Koh, H.Y., Jang, J., Ju, S.H., Kim, R., Cho, G.-B., Kim, D.S., Sohn, J.-W., Paik, S.-B. and Lee, J.H. (2021), ‘Non–Cell Autonomous Epileptogenesis in Focal Cortical Dysplasia’ Annals of Neurology, 90: 285 299. (https://doi.org/10.1002/ana.26149)
-ProfileProfessor Jeong Ho Lee Translational Neurogenetics Labhttps://tnl.kaist.ac.kr/ Graduate School of Medical Science and Engineering KAIST
Professor Se-Bum Paik Visual System and Neural Network Laboratory http://vs.kaist.ac.kr/ Department of Bio and Brain EngineeringKAIST
Professor Jong-Woo Sohn Laboratory for Neurophysiology, https://sites.google.com/site/sohnlab2014/home Department of Biological SciencesKAIST
Dr. Hyun Yong Koh Translational Neurogenetics LabGraduate School of Medical Science and EngineeringKAIST
Dr. Jaeson Jang Ph.D.Visual System and Neural Network LaboratoryDepartment of Bio and Brain Engineering KAIST
Sang Hyeon Ju M.D.Laboratory for NeurophysiologyDepartment of Biological SciencesKAIST
2021.08.26 View 13661
A Mechanism Underlying Most Common Cause of Epileptic Seizures Revealed
An interdisciplinary study shows that neurons carrying somatic mutations in MTOR can lead to focal epileptogenesis via non-cell-autonomous hyperexcitability of nearby nonmutated neurons
During fetal development, cells should migrate to the outer edge of the brain to form critical connections for information transfer and regulation in the body. When even a few cells fail to move to the correct location, the neurons become disorganized and this results in focal cortical dysplasia. This condition is the most common cause of seizures that cannot be controlled with medication in children and the second most common cause in adults.
Now, an interdisciplinary team studying neurogenetics, neural networks, and neurophysiology at KAIST has revealed how dysfunctions in even a small percentage of cells can cause disorder across the entire brain. They published their results on June 28 in Annals of Neurology.
The work builds on a previous finding, also by a KAIST scientists, who found that focal cortical dysplasia was caused by mutations in the cells involved in mTOR, a pathway that regulates signaling between neurons in the brain.
“Only 1 to 2% of neurons carrying mutations in the mTOR signaling pathway that regulates cell signaling in the brain have been found to include seizures in animal models of focal cortical dysplasia,” said Professor Jong-Woo Sohn from the Department of Biological Sciences. “The main challenge of this study was to explain how nearby non-mutated neurons are hyperexcitable.”
Initially, the researchers hypothesized that the mutated cells affected the number of excitatory and inhibitory synapses in all neurons, mutated or not. These neural gates can trigger or halt activity, respectively, in other neurons. Seizures are a result of extreme activity, called hyperexcitability. If the mutated cells upend the balance and result in more excitatory cells, the researchers thought, it made sense that the cells would be more susceptible to hyperexcitability and, as a result, seizures.
“Contrary to our expectations, the synaptic input balance was not changed in either the mutated or non-mutated neurons,” said Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering. “We turned our attention to a protein overproduced by mutated neurons.”
The protein is adenosine kinase, which lowers the concentration of adenosine. This naturally occurring compound is an anticonvulsant and works to relax vessels. In mice engineered to have focal cortical dysplasia, the researchers injected adenosine to replace the levels lowered by the protein. It worked and the neurons became less excitable.
“We demonstrated that augmentation of adenosine signaling could attenuate the excitability of non-mutated neurons,” said Professor Se-Bum Paik from the Department of Bio and Brain Engineering.
The effect on the non-mutated neurons was the surprising part, according to Paik. “The seizure-triggering hyperexcitability originated not in the mutation-carrying neurons, but instead in the nearby non-mutated neurons,” he said.
The mutated neurons excreted more adenosine kinase, reducing the adenosine levels in the local environment of all the cells. With less adenosine, the non-mutated neurons became hyperexcitable, leading to seizures.
“While we need further investigate into the relationship between the concentration of adenosine and the increased excitation of nearby neurons, our results support the medical use of drugs to activate adenosine signaling as a possible treatment pathway for focal cortical dysplasia,” Professor Lee said.
The Suh Kyungbae Foundation, the Korea Health Technology Research and Development Project, the Ministry of Health & Welfare, and the National Research Foundation in Korea funded this work.
-Publication:Koh, H.Y., Jang, J., Ju, S.H., Kim, R., Cho, G.-B., Kim, D.S., Sohn, J.-W., Paik, S.-B. and Lee, J.H. (2021), ‘Non–Cell Autonomous Epileptogenesis in Focal Cortical Dysplasia’ Annals of Neurology, 90: 285 299. (https://doi.org/10.1002/ana.26149)
-ProfileProfessor Jeong Ho Lee Translational Neurogenetics Labhttps://tnl.kaist.ac.kr/ Graduate School of Medical Science and Engineering KAIST
Professor Se-Bum Paik Visual System and Neural Network Laboratory http://vs.kaist.ac.kr/ Department of Bio and Brain EngineeringKAIST
Professor Jong-Woo Sohn Laboratory for Neurophysiology, https://sites.google.com/site/sohnlab2014/home Department of Biological SciencesKAIST
Dr. Hyun Yong Koh Translational Neurogenetics LabGraduate School of Medical Science and EngineeringKAIST
Dr. Jaeson Jang Ph.D.Visual System and Neural Network LaboratoryDepartment of Bio and Brain Engineering KAIST
Sang Hyeon Ju M.D.Laboratory for NeurophysiologyDepartment of Biological SciencesKAIST
2021.08.26 View 13661