electrolyte
-
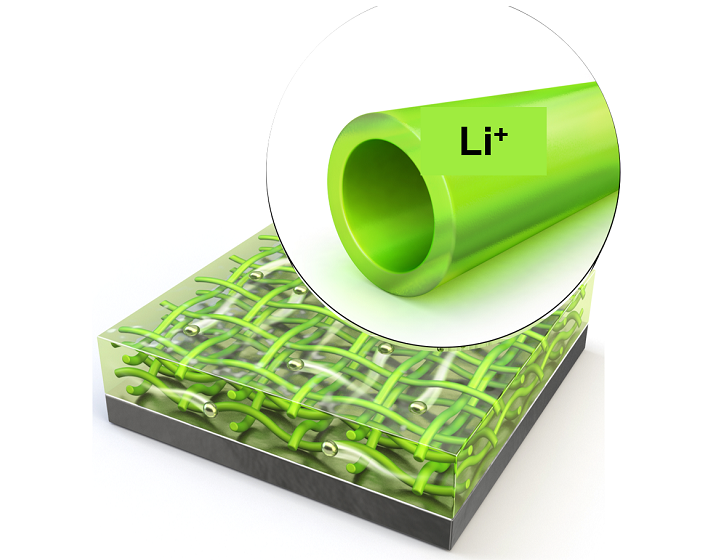 KAIST Extends Lithium Metal Battery Lifespan by 750% Using Water
Lithium metal, a next-generation anode material, has been highlighted for overcoming the performance limitations of commercial batteries. However, issues inherent to lithium metal have caused shortened battery lifespans and increased fire risks. KAIST researchers have achieved a world-class breakthrough by extending the lifespan of lithium metal anodes by approximately 750% only using water.
KAIST (represented by President Kwang Hyung Lee) announced on the 2nd of December that Professor Il-Doo Kim from the Department of Materials Science and Engineering, in collaboration with Professor Jiyoung Lee from Ajou University, successfully stabilized lithium growth and significantly enhanced the lifespan of next-generation lithium metal batteries using eco-friendly hollow nanofibers as protective layers.
Conventional protective layer technologies, which involve applying a surface coating onto lithium metal in order to create an artificial interface with the electrolyte, have relied on toxic processes and expensive materials, with limited improvements in the lifespan of lithium metal anodes.
< Figure 1. Schematic illustration of the fabrication process of the newly developed protective membrane by eco-friendly electrospinning process using water >
To address these limitations, Professor Kim’s team proposed a hollow nanofiber protective layer capable of controlling lithium-ion growth through both physical and chemical means. This protective layer was manufactured through an environmentally friendly electrospinning process* using guar gum** extracted from plants as the primary material and utilizing water as the sole solvent.
*Electrospinning process: A method where polymer solutions are subjected to an electric field, producing continuous fibers with diameters ranging from tens of nanometers to several micrometers.
**Guar gum: A natural polymer extracted from guar beans, composed mainly of monosaccharides. Its oxidized functional groups regulate interactions with lithium ions.
< Figure 2. Physical and chemical control of Lithium dendrite by the newly developed protective membrane >
The nanofiber protective layer effectively controlled reversible chemical reactions between the electrolyte and lithium ions. The hollow spaces within the fibers suppressed the random accumulation of lithium ions on the metal surface, stabilizing the interface between the lithium metal surface and the electrolyte.
< Figure 3. Performance of Lithium metal battery full cells with the newly developed protective membrane >
As a result, the lithium metal anodes with this protective layer demonstrated approximately a 750% increase in lifespan compared to conventional lithium metal anodes. The battery retained 93.3% of its capacity even after 300 charge-discharge cycles, achieving world-class performance.
The researchers also verified that this natural protective layer decomposes entirely within about a month in soil, proving its eco-friendly nature throughout the manufacturing and disposal process.
< Figure 4. Excellent decomposition rate of the newly developed protective membrane >
Professor Il-Doo Kim explained, “By leveraging both physical and chemical protective functions, we were able to guide reversible reactions between lithium metal and the electrolyte more effectively and suppress dendrite growth, resulting in lithium metal anodes with unprecedented lifespan characteristics.”
He added, “As the environmental burden caused by battery production and disposal becomes a pressing issue due to surging battery demand, this water-based manufacturing method with biodegradable properties will significantly contribute to the commercialization of next-generation eco-friendly batteries.”
This study was led by Dr. Jiyoung Lee (now a professor in the Department of Chemical Engineering at Ajou University) and Dr. Hyunsub Song (currently at Samsung Electronics), both graduates of KAIST’s Department of Materials Science and Engineering. The findings were published as a front cover article in Advanced Materials, Volume 36, Issue 47, on November 21.
(Paper title: “Overcoming Chemical and Mechanical Instabilities in Lithium Metal Anodes with Sustainable and Eco-Friendly Artificial SEI Layer”)
The research was supported by the KAIST-LG Energy Solution Frontier Research Lab (FRL), the Alchemist Project funded by the Ministry of Trade, Industry and Energy, and the Top-Tier Research Support Program from the Ministry of Science and ICT.
2024.12.12 View 7634
KAIST Extends Lithium Metal Battery Lifespan by 750% Using Water
Lithium metal, a next-generation anode material, has been highlighted for overcoming the performance limitations of commercial batteries. However, issues inherent to lithium metal have caused shortened battery lifespans and increased fire risks. KAIST researchers have achieved a world-class breakthrough by extending the lifespan of lithium metal anodes by approximately 750% only using water.
KAIST (represented by President Kwang Hyung Lee) announced on the 2nd of December that Professor Il-Doo Kim from the Department of Materials Science and Engineering, in collaboration with Professor Jiyoung Lee from Ajou University, successfully stabilized lithium growth and significantly enhanced the lifespan of next-generation lithium metal batteries using eco-friendly hollow nanofibers as protective layers.
Conventional protective layer technologies, which involve applying a surface coating onto lithium metal in order to create an artificial interface with the electrolyte, have relied on toxic processes and expensive materials, with limited improvements in the lifespan of lithium metal anodes.
< Figure 1. Schematic illustration of the fabrication process of the newly developed protective membrane by eco-friendly electrospinning process using water >
To address these limitations, Professor Kim’s team proposed a hollow nanofiber protective layer capable of controlling lithium-ion growth through both physical and chemical means. This protective layer was manufactured through an environmentally friendly electrospinning process* using guar gum** extracted from plants as the primary material and utilizing water as the sole solvent.
*Electrospinning process: A method where polymer solutions are subjected to an electric field, producing continuous fibers with diameters ranging from tens of nanometers to several micrometers.
**Guar gum: A natural polymer extracted from guar beans, composed mainly of monosaccharides. Its oxidized functional groups regulate interactions with lithium ions.
< Figure 2. Physical and chemical control of Lithium dendrite by the newly developed protective membrane >
The nanofiber protective layer effectively controlled reversible chemical reactions between the electrolyte and lithium ions. The hollow spaces within the fibers suppressed the random accumulation of lithium ions on the metal surface, stabilizing the interface between the lithium metal surface and the electrolyte.
< Figure 3. Performance of Lithium metal battery full cells with the newly developed protective membrane >
As a result, the lithium metal anodes with this protective layer demonstrated approximately a 750% increase in lifespan compared to conventional lithium metal anodes. The battery retained 93.3% of its capacity even after 300 charge-discharge cycles, achieving world-class performance.
The researchers also verified that this natural protective layer decomposes entirely within about a month in soil, proving its eco-friendly nature throughout the manufacturing and disposal process.
< Figure 4. Excellent decomposition rate of the newly developed protective membrane >
Professor Il-Doo Kim explained, “By leveraging both physical and chemical protective functions, we were able to guide reversible reactions between lithium metal and the electrolyte more effectively and suppress dendrite growth, resulting in lithium metal anodes with unprecedented lifespan characteristics.”
He added, “As the environmental burden caused by battery production and disposal becomes a pressing issue due to surging battery demand, this water-based manufacturing method with biodegradable properties will significantly contribute to the commercialization of next-generation eco-friendly batteries.”
This study was led by Dr. Jiyoung Lee (now a professor in the Department of Chemical Engineering at Ajou University) and Dr. Hyunsub Song (currently at Samsung Electronics), both graduates of KAIST’s Department of Materials Science and Engineering. The findings were published as a front cover article in Advanced Materials, Volume 36, Issue 47, on November 21.
(Paper title: “Overcoming Chemical and Mechanical Instabilities in Lithium Metal Anodes with Sustainable and Eco-Friendly Artificial SEI Layer”)
The research was supported by the KAIST-LG Energy Solution Frontier Research Lab (FRL), the Alchemist Project funded by the Ministry of Trade, Industry and Energy, and the Top-Tier Research Support Program from the Ministry of Science and ICT.
2024.12.12 View 7634 -
 KAIST Develops a Multifunctional Structural Battery Capable of Energy Storage and Load Support
Structural batteries are used in industries such as eco-friendly, energy-based automobiles, mobility, and aerospace, and they must simultaneously meet the requirements of high energy density for energy storage and high load-bearing capacity. Conventional structural battery technology has struggled to enhance both functions concurrently. However, KAIST researchers have succeeded in developing foundational technology to address this issue.
< Photo 1. (From left) Professor Seong Su Kim, PhD candidates Sangyoon Bae and Su Hyun Lim of the Department of Mechanical Engineering >
< Photo 2. (From left) Professor Seong Su Kim and Master's Graduate Mohamad A. Raja of KAIST Department of Mechanical Engineering >
KAIST (represented by President Kwang Hyung Lee) announced on the 19th of November that Professor Seong Su Kim's team from the Department of Mechanical Engineering has developed a thin, uniform, high-density, multifunctional structural carbon fiber composite battery* capable of supporting loads, and that is free from fire risks while offering high energy density.
*Multifunctional structural batteries: Refers to the ability of each material in the composite to simultaneously serve as a load-bearing structure and an energy storage element.
Early structural batteries involved embedding commercial lithium-ion batteries into layered composite materials. These batteries suffered from low integration of their mechanical and electrochemical properties, leading to challenges in material processing, assembly, and design optimization, making commercialization difficult.
To overcome these challenges, Professor Kim's team explored the concept of "energy-storing composite materials," focusing on interface and curing properties, which are critical in traditional composite design. This led to the development of high-density multifunctional structural carbon fiber composite batteries that maximize multifunctionality.
The team analyzed the curing mechanisms of epoxy resin, known for its strong mechanical properties, combined with ionic liquid and carbonate electrolyte-based solid polymer electrolytes. By controlling temperature and pressure, they were able to optimize the curing process.
The newly developed structural battery was manufactured through vacuum compression molding, increasing the volume fraction of carbon fibers—serving as both electrodes and current collectors—by over 160% compared to previous carbon-fiber-based batteries.
This greatly increased the contact area between electrodes and electrolytes, resulting in a high-density structural battery with improved electrochemical performance. Furthermore, the team effectively controlled air bubbles within the structural battery during the curing process, simultaneously enhancing the battery's mechanical properties.
Professor Seong Su Kim, the lead researcher, explained, “We proposed a framework for designing solid polymer electrolytes, a core material for high-stiffness, ultra-thin structural batteries, from both material and structural perspectives. These material-based structural batteries can serve as internal components in cars, drones, airplanes, and robots, significantly extending their operating time with a single charge. This represents a foundational technology for next-generation multifunctional energy storage applications.”
< Figure 2. Supplementary cover of ACS Applied Materials & Interfaces >
Mohamad A. Raja, a master’s graduate of KAIST’s Department of Mechanical Engineering, participated as the first author of this research, which was published in the prestigious journal ACS Applied Materials & Interfaces on September 10. The paper was recognized for its excellence and selected as a supplementary cover article. (Paper title: “Thin, Uniform, and Highly Packed Multifunctional Structural Carbon Fiber Composite Battery Lamina Informed by Solid Polymer Electrolyte Cure Kinetics.” https://doi.org/10.1021/acsami.4c08698)
This research was supported by the National Research Foundation of Korea’s Mid-Career Researcher Program and the National Semiconductor Research Laboratory Development Program.
2024.11.27 View 7068
KAIST Develops a Multifunctional Structural Battery Capable of Energy Storage and Load Support
Structural batteries are used in industries such as eco-friendly, energy-based automobiles, mobility, and aerospace, and they must simultaneously meet the requirements of high energy density for energy storage and high load-bearing capacity. Conventional structural battery technology has struggled to enhance both functions concurrently. However, KAIST researchers have succeeded in developing foundational technology to address this issue.
< Photo 1. (From left) Professor Seong Su Kim, PhD candidates Sangyoon Bae and Su Hyun Lim of the Department of Mechanical Engineering >
< Photo 2. (From left) Professor Seong Su Kim and Master's Graduate Mohamad A. Raja of KAIST Department of Mechanical Engineering >
KAIST (represented by President Kwang Hyung Lee) announced on the 19th of November that Professor Seong Su Kim's team from the Department of Mechanical Engineering has developed a thin, uniform, high-density, multifunctional structural carbon fiber composite battery* capable of supporting loads, and that is free from fire risks while offering high energy density.
*Multifunctional structural batteries: Refers to the ability of each material in the composite to simultaneously serve as a load-bearing structure and an energy storage element.
Early structural batteries involved embedding commercial lithium-ion batteries into layered composite materials. These batteries suffered from low integration of their mechanical and electrochemical properties, leading to challenges in material processing, assembly, and design optimization, making commercialization difficult.
To overcome these challenges, Professor Kim's team explored the concept of "energy-storing composite materials," focusing on interface and curing properties, which are critical in traditional composite design. This led to the development of high-density multifunctional structural carbon fiber composite batteries that maximize multifunctionality.
The team analyzed the curing mechanisms of epoxy resin, known for its strong mechanical properties, combined with ionic liquid and carbonate electrolyte-based solid polymer electrolytes. By controlling temperature and pressure, they were able to optimize the curing process.
The newly developed structural battery was manufactured through vacuum compression molding, increasing the volume fraction of carbon fibers—serving as both electrodes and current collectors—by over 160% compared to previous carbon-fiber-based batteries.
This greatly increased the contact area between electrodes and electrolytes, resulting in a high-density structural battery with improved electrochemical performance. Furthermore, the team effectively controlled air bubbles within the structural battery during the curing process, simultaneously enhancing the battery's mechanical properties.
Professor Seong Su Kim, the lead researcher, explained, “We proposed a framework for designing solid polymer electrolytes, a core material for high-stiffness, ultra-thin structural batteries, from both material and structural perspectives. These material-based structural batteries can serve as internal components in cars, drones, airplanes, and robots, significantly extending their operating time with a single charge. This represents a foundational technology for next-generation multifunctional energy storage applications.”
< Figure 2. Supplementary cover of ACS Applied Materials & Interfaces >
Mohamad A. Raja, a master’s graduate of KAIST’s Department of Mechanical Engineering, participated as the first author of this research, which was published in the prestigious journal ACS Applied Materials & Interfaces on September 10. The paper was recognized for its excellence and selected as a supplementary cover article. (Paper title: “Thin, Uniform, and Highly Packed Multifunctional Structural Carbon Fiber Composite Battery Lamina Informed by Solid Polymer Electrolyte Cure Kinetics.” https://doi.org/10.1021/acsami.4c08698)
This research was supported by the National Research Foundation of Korea’s Mid-Career Researcher Program and the National Semiconductor Research Laboratory Development Program.
2024.11.27 View 7068 -
 A Study Shows Reactive Electrolyte Additives Improve Lithium Metal Battery Performance
Stable electrode-electrolyte interfaces constructed by fluorine- and nitrogen-donating ionic additives provide an opportunity to improve high-performance lithium metal batteries
A research team showed that electrolyte additives increase the lifetime of lithium metal batteries and remarkably improve the performance of fast charging and discharging. Professor Nam-Soon Choi’s team from the Department of Chemical and Biomolecular Engineering at KAIST hierarchized the solid electrolyte interphase to make a dual-layer structure and showed groundbreaking run times for lithium metal batteries.
The team applied two electrolyte additives that have different reduction and adsorption properties to improve the functionality of the dual-layer solid electrolyte interphase. In addition, the team has confirmed that the structural stability of the nickel-rich cathode was achieved through the formation of a thin protective layer on the cathode. This study was reported in Energy Storage Materials.
Securing high-energy-density lithium metal batteries with a long lifespan and fast charging performance is vital for realizing their ubiquitous use as superior power sources for electric vehicles. Lithium metal batteries comprise a lithium metal anode that delivers 10 times higher capacity than the graphite anodes in lithium-ion batteries. Therefore, lithium metal is an indispensable anode material for realizing high-energy rechargeable batteries. However, undesirable reactions among the electrolytes with lithium metal anodes can reduce the power and this remains an impediment to achieving a longer battery lifespan. Previous studies only focused on the formation of the solid electrolyte interphase on the surface of the lithium metal anode.
The team designed a way to create a dual-layer solid electrolyte interphase to resolve the instability of the lithium metal anode by using electrolyte additives, depending on their electron accepting ability and adsorption tendencies. This hierarchical structure of the solid electrolyte interphase on the lithium metal anode has the potential to be further applied to lithium-alloy anodes, lithium storage structures, and anode-free technology to meet market expectations for electrolyte technology.
The batteries with lithium metal anodes and nickel-rich cathodes represented 80.9% of the initial capacity after 600 cycles and achieved a high Coulombic efficiency of 99.94%. These remarkable results contributed to the development of protective dual-layer solid electrolyte interphase technology for lithium metal anodes.
Professor Choi said that the research suggests a new direction for the development of electrolyte additives to regulate the unstable lithium metal anode-electrolyte interface, the biggest hurdle in research on lithium metal batteries.
She added that anode-free secondary battery technology is expected to be a game changer in the secondary battery market and electrolyte additive technology will contribute to the enhancement of anode-free secondary batteries through the stabilization of lithium metal anodes.
This research was funded by the Technology Development Program to Solve Climate Change of the National Research Foundation in Korea funded by the Ministry of Science, ICT & Future Planning and the Technology Innovation Program funded by the Ministry of Trade, Industry & Energy, and Hyundai Motor Company.
- PublicationSaehun Kim, Sung O Park, Min-Young Lee, Jeong-A Lee, Imanuel Kristanto, Tae Kyung Lee, Daeyeon Hwang, Juyoung Kim, Tae-Ung Wi, Hyun-Wook Lee, Sang Kyu Kwak, and NamSoon Choi, “Stable electrode-electrolyte interfaces constructed by fluorine- and nitrogen-donating ionic additives for high-performance lithium metal batteries,” Energy Storage Materials,45, 1-13 (2022), (doi: https://doi.org/10.1016/j.ensm.2021.10.031)
- ProfileProfessor Nam-Soon ChoiEnergy Materials LaboratoryDepartment of Chemical and Biomolecular EngineeringKAIST
2021.12.16 View 11088
A Study Shows Reactive Electrolyte Additives Improve Lithium Metal Battery Performance
Stable electrode-electrolyte interfaces constructed by fluorine- and nitrogen-donating ionic additives provide an opportunity to improve high-performance lithium metal batteries
A research team showed that electrolyte additives increase the lifetime of lithium metal batteries and remarkably improve the performance of fast charging and discharging. Professor Nam-Soon Choi’s team from the Department of Chemical and Biomolecular Engineering at KAIST hierarchized the solid electrolyte interphase to make a dual-layer structure and showed groundbreaking run times for lithium metal batteries.
The team applied two electrolyte additives that have different reduction and adsorption properties to improve the functionality of the dual-layer solid electrolyte interphase. In addition, the team has confirmed that the structural stability of the nickel-rich cathode was achieved through the formation of a thin protective layer on the cathode. This study was reported in Energy Storage Materials.
Securing high-energy-density lithium metal batteries with a long lifespan and fast charging performance is vital for realizing their ubiquitous use as superior power sources for electric vehicles. Lithium metal batteries comprise a lithium metal anode that delivers 10 times higher capacity than the graphite anodes in lithium-ion batteries. Therefore, lithium metal is an indispensable anode material for realizing high-energy rechargeable batteries. However, undesirable reactions among the electrolytes with lithium metal anodes can reduce the power and this remains an impediment to achieving a longer battery lifespan. Previous studies only focused on the formation of the solid electrolyte interphase on the surface of the lithium metal anode.
The team designed a way to create a dual-layer solid electrolyte interphase to resolve the instability of the lithium metal anode by using electrolyte additives, depending on their electron accepting ability and adsorption tendencies. This hierarchical structure of the solid electrolyte interphase on the lithium metal anode has the potential to be further applied to lithium-alloy anodes, lithium storage structures, and anode-free technology to meet market expectations for electrolyte technology.
The batteries with lithium metal anodes and nickel-rich cathodes represented 80.9% of the initial capacity after 600 cycles and achieved a high Coulombic efficiency of 99.94%. These remarkable results contributed to the development of protective dual-layer solid electrolyte interphase technology for lithium metal anodes.
Professor Choi said that the research suggests a new direction for the development of electrolyte additives to regulate the unstable lithium metal anode-electrolyte interface, the biggest hurdle in research on lithium metal batteries.
She added that anode-free secondary battery technology is expected to be a game changer in the secondary battery market and electrolyte additive technology will contribute to the enhancement of anode-free secondary batteries through the stabilization of lithium metal anodes.
This research was funded by the Technology Development Program to Solve Climate Change of the National Research Foundation in Korea funded by the Ministry of Science, ICT & Future Planning and the Technology Innovation Program funded by the Ministry of Trade, Industry & Energy, and Hyundai Motor Company.
- PublicationSaehun Kim, Sung O Park, Min-Young Lee, Jeong-A Lee, Imanuel Kristanto, Tae Kyung Lee, Daeyeon Hwang, Juyoung Kim, Tae-Ung Wi, Hyun-Wook Lee, Sang Kyu Kwak, and NamSoon Choi, “Stable electrode-electrolyte interfaces constructed by fluorine- and nitrogen-donating ionic additives for high-performance lithium metal batteries,” Energy Storage Materials,45, 1-13 (2022), (doi: https://doi.org/10.1016/j.ensm.2021.10.031)
- ProfileProfessor Nam-Soon ChoiEnergy Materials LaboratoryDepartment of Chemical and Biomolecular EngineeringKAIST
2021.12.16 View 11088