IBS
-
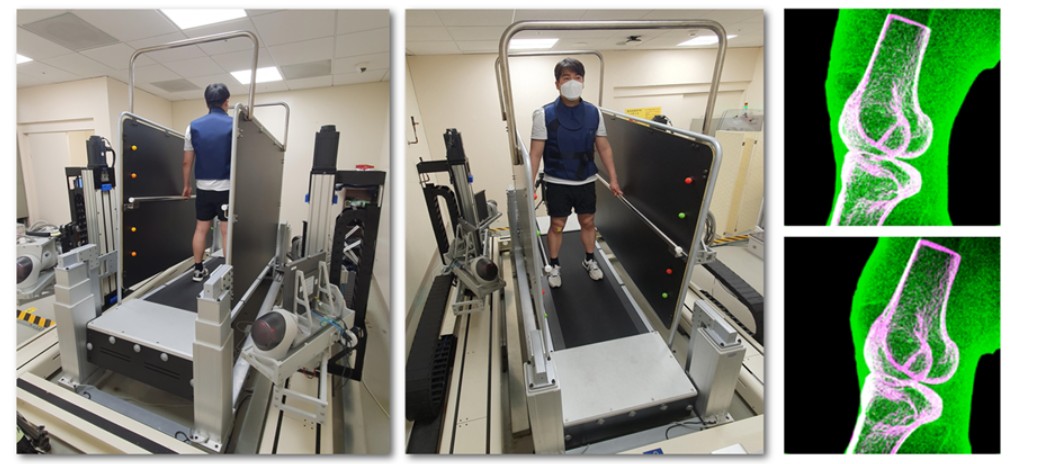 Prof. Seungbum Koo’s Team Receives Clinical Biomechanics Award at the 30th International Society of Biomechanics Conference
<(From Left) Ph.D candidate Jeongseok Oh from KAIST, Dr. Seungwoo Yoon from KAIST, Prof.Joon-Ho Wang from Samsung Medical Center, Prof.Seungbum Koo from KAIST>
Professor Seungbum Koo’s research team received the Clinical Biomechanics Award at the 30th International Society of Biomechanics (ISB) Conference, held in July 2025 in Stockholm, Sweden. The Plenary Lecture was delivered by first author and Ph.D. candidate Jeongseok Oh. This research was conducted in collaboration with Professor Joon-Ho Wang’s team at Samsung Medical Center.
Residual Translational and Rotational Kinematics After Combined ACL and Anterolateral Ligament Reconstruction During Walking
Jeongseok Oh, Seungwoo Yoon, Joon-Ho Wang, Seungbum Koo
The study analyzed gait-related knee joint motion using high-speed biplane X-ray imaging and three-dimensional kinematic reconstruction in 10 healthy individuals and 10 patients who underwent ACL reconstruction with ALL augmentation. The patient group showed excessive anterior translation and internal rotation, suggesting incomplete restoration of normal joint kinematics post-surgery. These findings provide mechanistic insight into the early onset of knee osteoarthritis often reported in this population.'
The ISB conference, held biennially for over 60 years, is the largest international biomechanics meeting. This year, it hosted 1,600 researchers from 46 countries and featured over 1,400 presentations. The Clinical Biomechanics Award is given to one outstanding study selected from five top-rated abstracts invited for full manuscript review. The winning paper is published in Clinical Biomechanics, and the award includes a monetary prize and a Plenary Lecture opportunity.
From 2019 to 2023, Koo and Wang’s teams developed a system with support from the Samsung Future Technology Development Program to track knee motion in real time during treadmill walking, using high-speed biplane X-rays and custom three-dimensional reconstruction software. This system, along with proprietary software that precisely reconstructs the three-dimensional motion of joints, was approved for clinical trials by the Ministry of Food and Drug Safety and installed at Samsung Medical Center. It is being used to quantitatively analyze abnormal joint motion patterns in patients with knee ligament injuries and those who have undergone knee surgery.
Additionally, Jeongseok Oh was named one of five finalists for the David Winter Young Investigator Award, presenting his work during the award session. This award recognizes promising young researchers in biomechanics worldwide.
2025.08.10 View 156
Prof. Seungbum Koo’s Team Receives Clinical Biomechanics Award at the 30th International Society of Biomechanics Conference
<(From Left) Ph.D candidate Jeongseok Oh from KAIST, Dr. Seungwoo Yoon from KAIST, Prof.Joon-Ho Wang from Samsung Medical Center, Prof.Seungbum Koo from KAIST>
Professor Seungbum Koo’s research team received the Clinical Biomechanics Award at the 30th International Society of Biomechanics (ISB) Conference, held in July 2025 in Stockholm, Sweden. The Plenary Lecture was delivered by first author and Ph.D. candidate Jeongseok Oh. This research was conducted in collaboration with Professor Joon-Ho Wang’s team at Samsung Medical Center.
Residual Translational and Rotational Kinematics After Combined ACL and Anterolateral Ligament Reconstruction During Walking
Jeongseok Oh, Seungwoo Yoon, Joon-Ho Wang, Seungbum Koo
The study analyzed gait-related knee joint motion using high-speed biplane X-ray imaging and three-dimensional kinematic reconstruction in 10 healthy individuals and 10 patients who underwent ACL reconstruction with ALL augmentation. The patient group showed excessive anterior translation and internal rotation, suggesting incomplete restoration of normal joint kinematics post-surgery. These findings provide mechanistic insight into the early onset of knee osteoarthritis often reported in this population.'
The ISB conference, held biennially for over 60 years, is the largest international biomechanics meeting. This year, it hosted 1,600 researchers from 46 countries and featured over 1,400 presentations. The Clinical Biomechanics Award is given to one outstanding study selected from five top-rated abstracts invited for full manuscript review. The winning paper is published in Clinical Biomechanics, and the award includes a monetary prize and a Plenary Lecture opportunity.
From 2019 to 2023, Koo and Wang’s teams developed a system with support from the Samsung Future Technology Development Program to track knee motion in real time during treadmill walking, using high-speed biplane X-rays and custom three-dimensional reconstruction software. This system, along with proprietary software that precisely reconstructs the three-dimensional motion of joints, was approved for clinical trials by the Ministry of Food and Drug Safety and installed at Samsung Medical Center. It is being used to quantitatively analyze abnormal joint motion patterns in patients with knee ligament injuries and those who have undergone knee surgery.
Additionally, Jeongseok Oh was named one of five finalists for the David Winter Young Investigator Award, presenting his work during the award session. This award recognizes promising young researchers in biomechanics worldwide.
2025.08.10 View 156 -
 A KAIST Research Team Develops a Smart Color-Changing Flexible Battery with Ultra-high Efficiency
With the rapid growth of the smart and wearable electronic devices market, smart next-generation energy storage systems that have energy storage functions as well as additional color-changing properties are receiving a great deal of attention. However, existing electrochromic devices have low electrical conductivity, leading to low efficiency in electron and ion mobility, and low storage capacities. Such batteries have therefore been limited to use in flexible and wearable devices.
On August 21, a joint research team led by Professor Il-Doo Kim from the KAIST Department of Materials Science and Engineering (DMSE) and Professor Tae Gwang Yun from the Myongji University Department of Materials Science and Engineering announced the development of a smart electrochromic Zn-ion battery that can visually represent its charging and discharging processes using an electrochromic polymer anode incorporated with a “π-bridge spacer”, which increases electron and ion mobility efficiency.
Batteries topped with electrochromic properties are groundbreaking inventions that can visually represent their charged and discharged states using colors, and can be used as display devices that cut down energy consumption for indoor cooling by controlling solar absorbance. The research team successfully built a flexible and electrochromic smart Zn-ion battery that can maintain its excellent electrochromic and electrochemical properties, even under long-term exposure to the atmosphere and mechanical deformations.
< Figure 1. Electrochromic zinc ion battery whose anode is made of a polymer that turns dark blue when charged and transparent when discharged. >
To maximize the efficiency of electron and ion mobility, the team modelled and synthesized the first π-bridge spacer-incorporated polymer anode in the world. π-bonds can improve the mobility of electrons within a structure to speed up ion movement and maximize ion adsorption efficiency, which improves its energy storage capacity.
In anode-based batteries with a π-bridge spacer, the spacer provides room for quicker ion movement. This allows fast charging, an improved zinc-ion discharging capacity of 110 mAh/g, which is 40% greater than previously reported, and a 30% increase in electrochromic function that switches from dark blue to transparent when the device is charged/discharged. In addition, should the transparent flexible battery technology be applied to smart windows, they would display darker colors during the day while they absorb solar energy, and function as a futuristic energy storage technique that can block out UV radiation and replace curtains.
< Figure 2. A schematic diagram of the structure of the electrochromic polymer with π-π spacer and the operation of a smart flexible battery using this cathode material. >
< Figure 3. (A) Density Functional Theory (DFT) theory-based atomic and electronic structure analysis. (B) Comparison of rate characteristics for polymers with and without π-bridge spacers. (C) Electrochemical performance comparison graph with previously reported zinc ion batteries. The anode material, which has an electron donor-acceptor structure with a built-in π-bridge spacer, shows better electrochemical performance and electrochromic properties than existing zinc ion batteries and electrochromic devices. >
Professor Il-Doo Kim said, “We have developed a polymer incorporated with a π-bridge spacer and successfully built a smart Zn-ion battery with excellent electrochromic efficiency and high energy storage capacity.” He added, “This technique goes beyond the existing concept of batteries that are used simply as energy storage devices, and we expect this technology to be used as a futuristic energy storage system that accelerates innovation in smart batteries and wearable technologies.”
This research, co-first authored by the alums of KAIST Departments of Material Sciences of Engineering, Professor Tae Gwang Yun of Myongji University, Dr. Jiyoung Lee, a post-doctoral associate at Northwestern University, and Professor Han Seul Kim at Chungbuk National University, was published as an inside cover article for Advanced Materials on August 3 under the title, “A π-Bridge Spacer Embedded Electron Donor-Acceptor Polymer for Flexible Electrochromic Zn-Ion Batteries”.
< Figure 4. Advanced Materials Inside Cover (August Issue) >
This research was supported by the Nanomaterial Technology Development Project under the Korean Ministry of Science and ICT, the Nano and Material Technology Development Project under the National Research Foundation of Korea, the Successive Academic Generation Development Project under the Korean Ministry of Education, and the Alchemist Project under the Korean Ministry of Trade, Industry & Energy.
2023.09.01 View 10417
A KAIST Research Team Develops a Smart Color-Changing Flexible Battery with Ultra-high Efficiency
With the rapid growth of the smart and wearable electronic devices market, smart next-generation energy storage systems that have energy storage functions as well as additional color-changing properties are receiving a great deal of attention. However, existing electrochromic devices have low electrical conductivity, leading to low efficiency in electron and ion mobility, and low storage capacities. Such batteries have therefore been limited to use in flexible and wearable devices.
On August 21, a joint research team led by Professor Il-Doo Kim from the KAIST Department of Materials Science and Engineering (DMSE) and Professor Tae Gwang Yun from the Myongji University Department of Materials Science and Engineering announced the development of a smart electrochromic Zn-ion battery that can visually represent its charging and discharging processes using an electrochromic polymer anode incorporated with a “π-bridge spacer”, which increases electron and ion mobility efficiency.
Batteries topped with electrochromic properties are groundbreaking inventions that can visually represent their charged and discharged states using colors, and can be used as display devices that cut down energy consumption for indoor cooling by controlling solar absorbance. The research team successfully built a flexible and electrochromic smart Zn-ion battery that can maintain its excellent electrochromic and electrochemical properties, even under long-term exposure to the atmosphere and mechanical deformations.
< Figure 1. Electrochromic zinc ion battery whose anode is made of a polymer that turns dark blue when charged and transparent when discharged. >
To maximize the efficiency of electron and ion mobility, the team modelled and synthesized the first π-bridge spacer-incorporated polymer anode in the world. π-bonds can improve the mobility of electrons within a structure to speed up ion movement and maximize ion adsorption efficiency, which improves its energy storage capacity.
In anode-based batteries with a π-bridge spacer, the spacer provides room for quicker ion movement. This allows fast charging, an improved zinc-ion discharging capacity of 110 mAh/g, which is 40% greater than previously reported, and a 30% increase in electrochromic function that switches from dark blue to transparent when the device is charged/discharged. In addition, should the transparent flexible battery technology be applied to smart windows, they would display darker colors during the day while they absorb solar energy, and function as a futuristic energy storage technique that can block out UV radiation and replace curtains.
< Figure 2. A schematic diagram of the structure of the electrochromic polymer with π-π spacer and the operation of a smart flexible battery using this cathode material. >
< Figure 3. (A) Density Functional Theory (DFT) theory-based atomic and electronic structure analysis. (B) Comparison of rate characteristics for polymers with and without π-bridge spacers. (C) Electrochemical performance comparison graph with previously reported zinc ion batteries. The anode material, which has an electron donor-acceptor structure with a built-in π-bridge spacer, shows better electrochemical performance and electrochromic properties than existing zinc ion batteries and electrochromic devices. >
Professor Il-Doo Kim said, “We have developed a polymer incorporated with a π-bridge spacer and successfully built a smart Zn-ion battery with excellent electrochromic efficiency and high energy storage capacity.” He added, “This technique goes beyond the existing concept of batteries that are used simply as energy storage devices, and we expect this technology to be used as a futuristic energy storage system that accelerates innovation in smart batteries and wearable technologies.”
This research, co-first authored by the alums of KAIST Departments of Material Sciences of Engineering, Professor Tae Gwang Yun of Myongji University, Dr. Jiyoung Lee, a post-doctoral associate at Northwestern University, and Professor Han Seul Kim at Chungbuk National University, was published as an inside cover article for Advanced Materials on August 3 under the title, “A π-Bridge Spacer Embedded Electron Donor-Acceptor Polymer for Flexible Electrochromic Zn-Ion Batteries”.
< Figure 4. Advanced Materials Inside Cover (August Issue) >
This research was supported by the Nanomaterial Technology Development Project under the Korean Ministry of Science and ICT, the Nano and Material Technology Development Project under the National Research Foundation of Korea, the Successive Academic Generation Development Project under the Korean Ministry of Education, and the Alchemist Project under the Korean Ministry of Trade, Industry & Energy.
2023.09.01 View 10417 -
 Professor Jae Kyoung Kim to Lead a New Mathematical Biology Research Group at IBS
Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences was appointed as the third Chief Investigator (CI) of the Pioneer Research Center (PRC) for Mathematical and Computational Sciences at the Institute for Basic Science (IBS). Professor Kim will launch and lead a new research group that will be devoted to resolving various biological conundrums from a mathematical perspective. His appointment began on March 1, 2021.
Professor Kim, a rising researcher in the field of mathematical biology, has received attention from both the mathematical and biological communities at the international level. Professor Kim puts novel and unremitting efforts into understanding biological systems such as cell-to-cell interactions mathematically and designing mathematical models for identifying causes of diseases and developing therapeutic medicines.
Through active joint research with biologists, mathematician Kim has addressed many challenges that have remained unsolved in biology and published papers in a number of leading international journals in related fields. His notable works based on mathematical modelling include having designed a biological circuit that can maintain a stable circadian rhythm (Science, 2015) and unveiling the principles of how the biological clock in the body maintains a steady speed for the first time in over 60 years (Molecular Cell, 2015). Recently, through a joint research project with Pfizer, Professor Kim identified what causes the differences between animal and clinical test results during drug development explaining why drugs have different efficacies in different people (Molecular Systems Biology, 2019).
The new IBS biomedical mathematics research group led by Professor Kim will further investigate the causes of unstable circadian rhythms and sleeping patterns. The team will aim to present a new paradigm in treatments for sleep disorders.
Professor Kim said, “We are all so familiar with sleep behaviors, but the exact mechanisms behind how such behaviors occur are still unknown. Through cooperation with biomedical scientists, our group will do its best to discover the complicated, fundamental mechanisms of sleep, and investigate the causes and cures of sleep disorders.”
Every year, the IBS selects young and promising researchers and appoints them as CIs. A maximum of five selected CIs can form each independent research group within the IBS PRC, and receive research funds of 1 billion to 1.5 billion KRW over five years.
(END)
2021.03.18 View 12037
Professor Jae Kyoung Kim to Lead a New Mathematical Biology Research Group at IBS
Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences was appointed as the third Chief Investigator (CI) of the Pioneer Research Center (PRC) for Mathematical and Computational Sciences at the Institute for Basic Science (IBS). Professor Kim will launch and lead a new research group that will be devoted to resolving various biological conundrums from a mathematical perspective. His appointment began on March 1, 2021.
Professor Kim, a rising researcher in the field of mathematical biology, has received attention from both the mathematical and biological communities at the international level. Professor Kim puts novel and unremitting efforts into understanding biological systems such as cell-to-cell interactions mathematically and designing mathematical models for identifying causes of diseases and developing therapeutic medicines.
Through active joint research with biologists, mathematician Kim has addressed many challenges that have remained unsolved in biology and published papers in a number of leading international journals in related fields. His notable works based on mathematical modelling include having designed a biological circuit that can maintain a stable circadian rhythm (Science, 2015) and unveiling the principles of how the biological clock in the body maintains a steady speed for the first time in over 60 years (Molecular Cell, 2015). Recently, through a joint research project with Pfizer, Professor Kim identified what causes the differences between animal and clinical test results during drug development explaining why drugs have different efficacies in different people (Molecular Systems Biology, 2019).
The new IBS biomedical mathematics research group led by Professor Kim will further investigate the causes of unstable circadian rhythms and sleeping patterns. The team will aim to present a new paradigm in treatments for sleep disorders.
Professor Kim said, “We are all so familiar with sleep behaviors, but the exact mechanisms behind how such behaviors occur are still unknown. Through cooperation with biomedical scientists, our group will do its best to discover the complicated, fundamental mechanisms of sleep, and investigate the causes and cures of sleep disorders.”
Every year, the IBS selects young and promising researchers and appoints them as CIs. A maximum of five selected CIs can form each independent research group within the IBS PRC, and receive research funds of 1 billion to 1.5 billion KRW over five years.
(END)
2021.03.18 View 12037 -
 Simple Molecular Reagents to Treat Alzheimer’s Disease
- Researchers report minimalistic principles for designing small molecules with multiple reactivities against dementia. -
Sometimes the most complex problems actually have very simple solutions. A group of South Korean researchers reported an efficient and effective redox-based strategy for incorporating multiple functions into simple molecular reagents against neurodegenerative disorders. The team developed redox-active aromatic molecular reagents with a simple structural composition that can simultaneously target and modulate various pathogenic factors in complex neurodegenerative disorders such as Alzheimer’s disease.
Alzheimer’s disease is one of the most prevalent neurodegenerative disorders, affecting one in ten people over the age of 65. Early-onset dementia also increasingly affects younger people.
A number of pathogenic elements such as reactive oxygen species, amyloid-beta, and metal ions have been suggested as potential causes of Alzheimer’s disease. Each element itself can lead to Alzheimer’s disease, but interactions between them may also aggravate the patient’s condition or interfere with the appropriate clinical care.
For example, when interacting with amyloid-beta, metal ions foster the aggregation and accumulation of amyloid-beta peptides that can induce oxidative stress and toxicity in the brain and lead to neurodegeneration.
Because these pathogenic factors of Alzheimer’s disease are intertwined, developing therapeutic agents that are capable of simultaneously regulating metal ion dyshomeostasis, amyloid-beta agglutination, and oxidative stress responses remains a key to halting the progression of the disease.
A research team led by Professor Mi Hee Lim from the Department of Chemistry at KAIST demonstrated the feasibility of structure-mechanism-based molecular design for controlling a molecule’s chemical reactivity toward the various pathological factors of Alzheimer’s disease by tuning the redox properties of the molecule.
This study, featured as the ‘ACS Editors’ Choice’ in the May 6th issue of the Journal of the American Chemical Society (JACS), was conducted in conjunction with KAIST Professor Mu-Hyun Baik’s group and Professor Joo-Young Lee’s group at the Asan Medical Center.
Professor Lim and her collaborators rationally designed and generated 10 compact aromatic molecules presenting a range of redox potentials by adjusting the electronic distribution of the phenyl, phenylene, or pyridyl moiety to impart redox-dependent reactivities against the multiple pathogenic factors in Alzheimer’s disease.
During the team’s biochemical and biophysical studies, these designed molecular reagents displayed redox-dependent reactivities against numerous desirable targets that are associated with Alzheimer’s disease such as free radicals, metal-free amyloid-beta, and metal-bound amyloid-beta.
Further mechanistic results revealed that the redox properties of these designed molecular reagents were essential for their function. The team demonstrated that these reagents engaged in oxidative reactions with metal-free and metal-bound amyloid-beta and led to chemical modifications. The products of such oxidative transformations were observed to form covalent adducts with amyloid-beta and alter its aggregation.
Moreover, the administration of the most promising candidate molecule significantly attenuated the amyloid pathology in the brains of Alzheimer’s disease transgenic mice and improved their cognitive defects.
Professor Lim said, “This strategy is straightforward, time-saving, and cost-effective, and its effect is significant. We are excited to help enable the advancement of new therapeutic agents for neurodegenerative disorders, which can improve the lives of so many patients.”
This work was supported by the National Research Foundation (NRF) of Korea, the Institute for Basic Science (IBS), and the Asan Institute for Life Sciences.
Image credit: Professor Mi Hee Lim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of the news coverage of this paper only.
Publication:
Kim, M. et al. (2020) ‘Minimalistic Principles for Designing Small Molecules with Multiple Reactivities against Pathological Factors in Dementia.’ Journal of the American Chemical Society (JACS), Volume 142, Issue 18, pp.8183-8193. Available online at https://doi.org/10.1021/jacs.9b13100
Profile:
Mi Hee Lim
Professor
miheelim@kaist.ac.kr
http://sites.google.com/site/miheelimlab
Lim Laboratory
Department of Chemistry
KAIST
Profile:
Mu-Hyun Baik
Professor
mbaik2805@kaist.ac.kr
https://baik-laboratory.com/
Baik Laboratory
Department of Chemistry
KAIST
Profile:
Joo-Yong Lee
Professor
jlee@amc.seoul.kr
Asan Institute for Life Sciences
Asan Medical Center
(END)
2020.05.11 View 19107
Simple Molecular Reagents to Treat Alzheimer’s Disease
- Researchers report minimalistic principles for designing small molecules with multiple reactivities against dementia. -
Sometimes the most complex problems actually have very simple solutions. A group of South Korean researchers reported an efficient and effective redox-based strategy for incorporating multiple functions into simple molecular reagents against neurodegenerative disorders. The team developed redox-active aromatic molecular reagents with a simple structural composition that can simultaneously target and modulate various pathogenic factors in complex neurodegenerative disorders such as Alzheimer’s disease.
Alzheimer’s disease is one of the most prevalent neurodegenerative disorders, affecting one in ten people over the age of 65. Early-onset dementia also increasingly affects younger people.
A number of pathogenic elements such as reactive oxygen species, amyloid-beta, and metal ions have been suggested as potential causes of Alzheimer’s disease. Each element itself can lead to Alzheimer’s disease, but interactions between them may also aggravate the patient’s condition or interfere with the appropriate clinical care.
For example, when interacting with amyloid-beta, metal ions foster the aggregation and accumulation of amyloid-beta peptides that can induce oxidative stress and toxicity in the brain and lead to neurodegeneration.
Because these pathogenic factors of Alzheimer’s disease are intertwined, developing therapeutic agents that are capable of simultaneously regulating metal ion dyshomeostasis, amyloid-beta agglutination, and oxidative stress responses remains a key to halting the progression of the disease.
A research team led by Professor Mi Hee Lim from the Department of Chemistry at KAIST demonstrated the feasibility of structure-mechanism-based molecular design for controlling a molecule’s chemical reactivity toward the various pathological factors of Alzheimer’s disease by tuning the redox properties of the molecule.
This study, featured as the ‘ACS Editors’ Choice’ in the May 6th issue of the Journal of the American Chemical Society (JACS), was conducted in conjunction with KAIST Professor Mu-Hyun Baik’s group and Professor Joo-Young Lee’s group at the Asan Medical Center.
Professor Lim and her collaborators rationally designed and generated 10 compact aromatic molecules presenting a range of redox potentials by adjusting the electronic distribution of the phenyl, phenylene, or pyridyl moiety to impart redox-dependent reactivities against the multiple pathogenic factors in Alzheimer’s disease.
During the team’s biochemical and biophysical studies, these designed molecular reagents displayed redox-dependent reactivities against numerous desirable targets that are associated with Alzheimer’s disease such as free radicals, metal-free amyloid-beta, and metal-bound amyloid-beta.
Further mechanistic results revealed that the redox properties of these designed molecular reagents were essential for their function. The team demonstrated that these reagents engaged in oxidative reactions with metal-free and metal-bound amyloid-beta and led to chemical modifications. The products of such oxidative transformations were observed to form covalent adducts with amyloid-beta and alter its aggregation.
Moreover, the administration of the most promising candidate molecule significantly attenuated the amyloid pathology in the brains of Alzheimer’s disease transgenic mice and improved their cognitive defects.
Professor Lim said, “This strategy is straightforward, time-saving, and cost-effective, and its effect is significant. We are excited to help enable the advancement of new therapeutic agents for neurodegenerative disorders, which can improve the lives of so many patients.”
This work was supported by the National Research Foundation (NRF) of Korea, the Institute for Basic Science (IBS), and the Asan Institute for Life Sciences.
Image credit: Professor Mi Hee Lim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of the news coverage of this paper only.
Publication:
Kim, M. et al. (2020) ‘Minimalistic Principles for Designing Small Molecules with Multiple Reactivities against Pathological Factors in Dementia.’ Journal of the American Chemical Society (JACS), Volume 142, Issue 18, pp.8183-8193. Available online at https://doi.org/10.1021/jacs.9b13100
Profile:
Mi Hee Lim
Professor
miheelim@kaist.ac.kr
http://sites.google.com/site/miheelimlab
Lim Laboratory
Department of Chemistry
KAIST
Profile:
Mu-Hyun Baik
Professor
mbaik2805@kaist.ac.kr
https://baik-laboratory.com/
Baik Laboratory
Department of Chemistry
KAIST
Profile:
Joo-Yong Lee
Professor
jlee@amc.seoul.kr
Asan Institute for Life Sciences
Asan Medical Center
(END)
2020.05.11 View 19107 -
 A Global Campaign of ‘Facts before Rumors’ on COVID-19 Launched
- A KAIST data scientist group responds to facts and rumors on COVID-19 for global awareness of the pandemic. -
Like the novel coronavirus, rumors have no borders. The world is fighting to contain the pandemic, but we also have to deal with the appalling spread of an infodemic that is as contagious as the virus. This infodemic, a pandemic of false information, is bringing chaos and extreme fear to the general public.
Professor Meeyoung Cha’s group at the School of Computing started a global campaign called ‘Facts before Rumors,’ to prevent the spread of false information from crossing borders. She explained, “We saw many rumors that had already been fact-checked long before in China and South Korea now begin to circulate in other countries, sometimes leading to detrimental results. We launched an official campaign, Facts before Rumors, to deliver COVID-19-related facts to countries where the number of cases is now increasing.” She released the first set of facts on March 26 via her Twitter account @nekozzang.
Professor Cha, a data scientist who has focused on detecting global fake news, is now part of the COVID-19 AI Task Force at the Global Strategy Institute at KAIST. She is also leading the Data Science Group at the Institute for Basic Science (IBS) as Chief Investigator.
Her research group worked in collaboration with the College of Nursing at Ewha Woman’s University to identify 15 claims about COVID-19 that circulated on social networks (SNS) and among the general public. The team fact-checked these claims based on information from the WHO and CDCs of Korea and the US. The research group is now working on translating the list of claims into Portuguese, Spanish, Persian, Chinese, Amharic, Hindi, and Vietnamese. Delivering facts before rumors, the team says, will help contain the disease and prevent any harm caused by misinformation.
The pandemic, which spread in China and South Korea before arriving in Europe and the US, is now moving into South America, Africa, and Southeast Asia. “We would like to play a part in preventing the further spread of the disease with the provision of only scientifically vetted, truthful facts,” said the team.
For this campaign, Professor Cha’s team investigated more than 200 rumored claims on COVID-19 in China during the early days of the pandemic. These claims spread in different levels: while some were only relevant locally or in larger regions of China, others propagated in Asia and are now spreading to countries that are currently most affected by the disease.
For example, the false claim which publicized that ‘Fireworks can help tame the virus in the air’ only spread in China. Other claims such as ‘Eating garlic helps people overcome the disease’ or ‘Gargling with salt water prevents the contraction of the disease,’ spread around the world even after being proved groundless.
The team noted, however, that the times at which these claims propagate are different from one country to another. “This opens up an opportunity to debunk rumors in some countries, even before they start to emerge,” said Professor Cha.
Kun-Woo Kim, a master’s candidate in the Department of Industrial Design who joined this campaign and designed the Facts before Rumors chart also expressed his hope that this campaign will help reduce the number of victims. He added, “I am very grateful to our scientists who quickly responded to the Fact Check in these challenging times.”
2020.03.27 View 15348
A Global Campaign of ‘Facts before Rumors’ on COVID-19 Launched
- A KAIST data scientist group responds to facts and rumors on COVID-19 for global awareness of the pandemic. -
Like the novel coronavirus, rumors have no borders. The world is fighting to contain the pandemic, but we also have to deal with the appalling spread of an infodemic that is as contagious as the virus. This infodemic, a pandemic of false information, is bringing chaos and extreme fear to the general public.
Professor Meeyoung Cha’s group at the School of Computing started a global campaign called ‘Facts before Rumors,’ to prevent the spread of false information from crossing borders. She explained, “We saw many rumors that had already been fact-checked long before in China and South Korea now begin to circulate in other countries, sometimes leading to detrimental results. We launched an official campaign, Facts before Rumors, to deliver COVID-19-related facts to countries where the number of cases is now increasing.” She released the first set of facts on March 26 via her Twitter account @nekozzang.
Professor Cha, a data scientist who has focused on detecting global fake news, is now part of the COVID-19 AI Task Force at the Global Strategy Institute at KAIST. She is also leading the Data Science Group at the Institute for Basic Science (IBS) as Chief Investigator.
Her research group worked in collaboration with the College of Nursing at Ewha Woman’s University to identify 15 claims about COVID-19 that circulated on social networks (SNS) and among the general public. The team fact-checked these claims based on information from the WHO and CDCs of Korea and the US. The research group is now working on translating the list of claims into Portuguese, Spanish, Persian, Chinese, Amharic, Hindi, and Vietnamese. Delivering facts before rumors, the team says, will help contain the disease and prevent any harm caused by misinformation.
The pandemic, which spread in China and South Korea before arriving in Europe and the US, is now moving into South America, Africa, and Southeast Asia. “We would like to play a part in preventing the further spread of the disease with the provision of only scientifically vetted, truthful facts,” said the team.
For this campaign, Professor Cha’s team investigated more than 200 rumored claims on COVID-19 in China during the early days of the pandemic. These claims spread in different levels: while some were only relevant locally or in larger regions of China, others propagated in Asia and are now spreading to countries that are currently most affected by the disease.
For example, the false claim which publicized that ‘Fireworks can help tame the virus in the air’ only spread in China. Other claims such as ‘Eating garlic helps people overcome the disease’ or ‘Gargling with salt water prevents the contraction of the disease,’ spread around the world even after being proved groundless.
The team noted, however, that the times at which these claims propagate are different from one country to another. “This opens up an opportunity to debunk rumors in some countries, even before they start to emerge,” said Professor Cha.
Kun-Woo Kim, a master’s candidate in the Department of Industrial Design who joined this campaign and designed the Facts before Rumors chart also expressed his hope that this campaign will help reduce the number of victims. He added, “I am very grateful to our scientists who quickly responded to the Fact Check in these challenging times.”
2020.03.27 View 15348 -
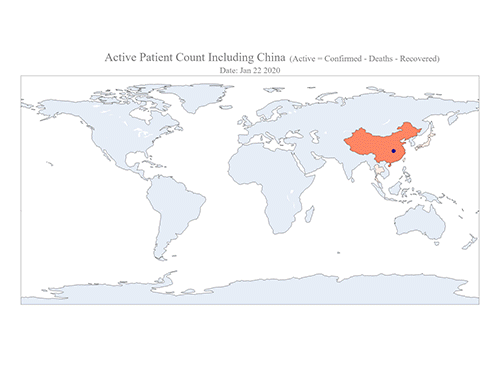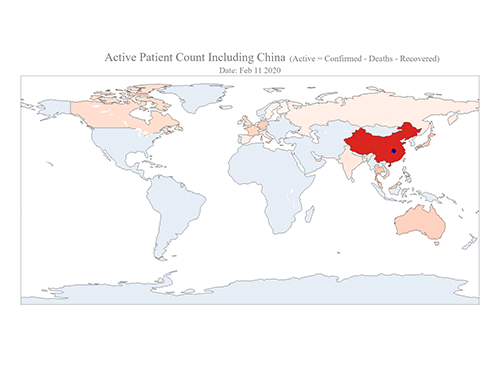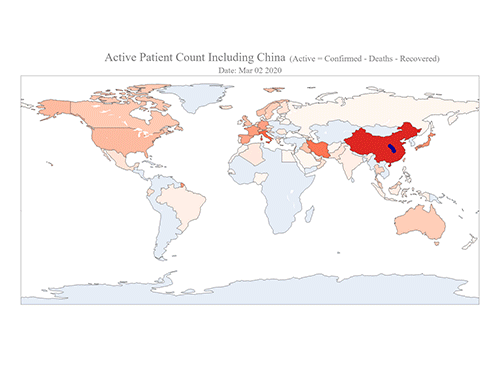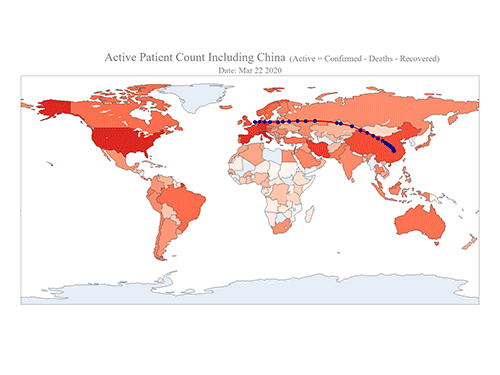 COVID-19 Map Shows How the Global Pandemic Moves
- A School of Computing team facilitated the data from COVID-19 to show the global spread of the virus. -
The COVID-19 map made by KAIST data scientists shows where and how the virus is spreading from China, reportedly the epicenter of the disease.
Professor Meeyoung Cha from the School of Computing and her group facilitated data based on the number of confirmed cases from January 22 to March 22 to analyze the trends of this global epidemic. The statistics include the number of confirmed cases, recoveries, and deaths across major continents based on the number of confirmed case data during that period.
The moving dot on the map strikingly shows how the confirmed cases are moving across the globe. According to their statistics, the centroid of the disease starts from near Wuhan in China and moved to Korea, then through the European region via Italy and Iran.
The data is collected by a graduate student from the School of Computing, Geng Sun, who started the process during the time he was quarantined since coming back from his home in China. An undergraduate colleague of Geng's, Gabriel Camilo Lima who made the map, is now working remotely from his home in Brazil since all undergraduate students were required to move out of the dormitory last week. The university closed all undergraduate housing and advised the undergraduate students to go back home in a preventive measure to stop the virus from spreading across the campus.
Gabriel said he calculated the centroid of all confirmed cases up to a given day. He explained, “I weighed each coordinate by the number of cases in that region and country and calculated an approximate center of gravity.”
“The Earth is round, so the shortest path from Asia to Europe is often through Russia. In early March, the center of gravity of new cases was moving from Asia to Europe. Therefore, the centroid is moving to the west and goes through Russia, even though Russia has not reported many cases,” he added.
Professor Cha, who is also responsible for the Data Science Group at the Institute for Basic Science (IBS) as the Chief Investigator, said their group will continue to update the map using public data at https://ds.ibs.re.kr/index.php/covid-19/.
(END)
2020.03.27 View 14769
COVID-19 Map Shows How the Global Pandemic Moves
- A School of Computing team facilitated the data from COVID-19 to show the global spread of the virus. -
The COVID-19 map made by KAIST data scientists shows where and how the virus is spreading from China, reportedly the epicenter of the disease.
Professor Meeyoung Cha from the School of Computing and her group facilitated data based on the number of confirmed cases from January 22 to March 22 to analyze the trends of this global epidemic. The statistics include the number of confirmed cases, recoveries, and deaths across major continents based on the number of confirmed case data during that period.
The moving dot on the map strikingly shows how the confirmed cases are moving across the globe. According to their statistics, the centroid of the disease starts from near Wuhan in China and moved to Korea, then through the European region via Italy and Iran.
The data is collected by a graduate student from the School of Computing, Geng Sun, who started the process during the time he was quarantined since coming back from his home in China. An undergraduate colleague of Geng's, Gabriel Camilo Lima who made the map, is now working remotely from his home in Brazil since all undergraduate students were required to move out of the dormitory last week. The university closed all undergraduate housing and advised the undergraduate students to go back home in a preventive measure to stop the virus from spreading across the campus.
Gabriel said he calculated the centroid of all confirmed cases up to a given day. He explained, “I weighed each coordinate by the number of cases in that region and country and calculated an approximate center of gravity.”
“The Earth is round, so the shortest path from Asia to Europe is often through Russia. In early March, the center of gravity of new cases was moving from Asia to Europe. Therefore, the centroid is moving to the west and goes through Russia, even though Russia has not reported many cases,” he added.
Professor Cha, who is also responsible for the Data Science Group at the Institute for Basic Science (IBS) as the Chief Investigator, said their group will continue to update the map using public data at https://ds.ibs.re.kr/index.php/covid-19/.
(END)
2020.03.27 View 14769 -
 2016 KAIST EEWS Workshop
The Energy, Environment, Water and Sustainability (EEWS) Graduate School of KAIST hosted a workshop entitled “Progress and Perspectives of Energy Science and Technology” on October 20, 2016. The workshop took place at the Fusion Hall of the KAIST Institute on campus.
About 400 experts in energy science and engineering participated in the event. Eight globally recognized scientists introduced the latest research trends in nanomaterials, energy theory, catalysts, and photocatalysts and led discussions on the current status and prospects of EEWS.
Professors Yi Cui of Stanford University, an expert in nanomaterials, and William A. Goddard of California Institute of Technology presented their research experiments on materials design and recent results on the direction of theory under the topics of energy and environment.
Dr. Miquel Salmeron, a former head of the Material Science Division of Lawrence Berkeley National Laboratory, and Professor Yuichi Ikuhara of Tokyo University introduced their analysis of catalysts and energy matters at an atomic scale.
Professor Sukbok Chang of the Chemistry Department at KAIST, a deputy editor of ACS Catalysis and the head of the Center for Catalytic Hydrocarbon Functionalizations at the Institute of Basic Science, and Professor Yang-Kook Sun of Energy Engineering at Hanyang University, who is also a deputy editor of ACS Energy Letters, presented their latest research results on new catalytic reaction development and energy storage.
The workshop consisted of three sections which addressed the design of energy and environment materials; analysis of energy and catalytic materials; and energy conversion and catalysts.
The EEWS Graduate School was established in 2008 with the sponsorship of the Korean government’s World Class University (WCU) project to support science education in Korea. Professor J. Fraser Stoddart, the winner of the 2016 Nobel Prize in Chemistry, was previously worked at the KAIST EEWS Graduate School as a WCU visiting professor for two years, from 2011 to 2013. Professor Ali Coskun, who was a postdoctoral researcher in the laboratory of Professor Stoddart, now teaches and conducts research as a full-time professor at the graduate school.
Dean Yousung Jung of the EEWS Graduate School said:
“This workshop has provided us with a meaningful opportunity to engage in discussions on energy science and technology with world-class scholars from all around the world. It is also a good venue for our graduate school to share with them what we have been doing in research and education.”
2016.10.20 View 15495
2016 KAIST EEWS Workshop
The Energy, Environment, Water and Sustainability (EEWS) Graduate School of KAIST hosted a workshop entitled “Progress and Perspectives of Energy Science and Technology” on October 20, 2016. The workshop took place at the Fusion Hall of the KAIST Institute on campus.
About 400 experts in energy science and engineering participated in the event. Eight globally recognized scientists introduced the latest research trends in nanomaterials, energy theory, catalysts, and photocatalysts and led discussions on the current status and prospects of EEWS.
Professors Yi Cui of Stanford University, an expert in nanomaterials, and William A. Goddard of California Institute of Technology presented their research experiments on materials design and recent results on the direction of theory under the topics of energy and environment.
Dr. Miquel Salmeron, a former head of the Material Science Division of Lawrence Berkeley National Laboratory, and Professor Yuichi Ikuhara of Tokyo University introduced their analysis of catalysts and energy matters at an atomic scale.
Professor Sukbok Chang of the Chemistry Department at KAIST, a deputy editor of ACS Catalysis and the head of the Center for Catalytic Hydrocarbon Functionalizations at the Institute of Basic Science, and Professor Yang-Kook Sun of Energy Engineering at Hanyang University, who is also a deputy editor of ACS Energy Letters, presented their latest research results on new catalytic reaction development and energy storage.
The workshop consisted of three sections which addressed the design of energy and environment materials; analysis of energy and catalytic materials; and energy conversion and catalysts.
The EEWS Graduate School was established in 2008 with the sponsorship of the Korean government’s World Class University (WCU) project to support science education in Korea. Professor J. Fraser Stoddart, the winner of the 2016 Nobel Prize in Chemistry, was previously worked at the KAIST EEWS Graduate School as a WCU visiting professor for two years, from 2011 to 2013. Professor Ali Coskun, who was a postdoctoral researcher in the laboratory of Professor Stoddart, now teaches and conducts research as a full-time professor at the graduate school.
Dean Yousung Jung of the EEWS Graduate School said:
“This workshop has provided us with a meaningful opportunity to engage in discussions on energy science and technology with world-class scholars from all around the world. It is also a good venue for our graduate school to share with them what we have been doing in research and education.”
2016.10.20 View 15495 -
 The Real Time Observation of the Birth of a Molecule
From right to left: Dr. Kyung-Hwan Kim, Professor Hyotcherl Lhee, and Jong-Gu Kim, a Ph.D. candidate
Professor Hyotcherl Lhee of the Department of Chemistry at KAIST and Japanese research teams jointly published their research results showing that they have succeeded in the direct observation of how atoms form a molecule in the online issue of Nature on February 19, 2015.
The researchers used water in which gold atoms ([Au(CN) 2- ]) are dissolved and fired X-ray pulses over the specimen in femtosecond timescales to study chemical reactions taking place among the gold atoms. They were able to examine in real time the instant process of how gold atoms bond together to become a molecule, to a trimer or tetramer state.
This direct viewing of the formation of a gold trimer complex ([Au(CN) 2- ] 3 ) will provide an opportunity to understand complex chemical and biological systems.
For details, please see the following press release that was distributed by the High Energy Accelerator Research Organization, KEK, in Japan:
Direct Observation of Bond Formations
February 18, 2015
A collaboration between researchers from KEK, the Institute for Basic Science (IBS), the Korea Advanced Institute of Science and Technology (KAIST), RIKEN, and the Japan Synchrotron Radiation Research Institute (JASRI) used the SACLA X-ray free electron laser (XFEL) facility for a real time visualization of the birth of a molecular that occurs via photoinduced formation of a chemical bonds. This achievement was published in the online version of the scientific journal “Nature” (published on 19 February 2015).
Direct “observation” of the bond making, through a chemical reaction, has been longstanding dream for chemists. However, the distance between atoms is very small, at about 100 picometer, and the bonding is completed very quickly, taking less than one picosecond (ps). Hence, previously, one could only imagine the bond formation between atoms while looking at the chemical reaction progressing in the test-tube.
In this study, the research group focused on the process of photoinduced bond formation between gold (Au) ions dissolved in water. In the ground state (S 0 state in Fig. 1) Au ions that are weakly bound to each other by an electron affinity and aligned in a bent geometry. Upon a photoexcitation, the S 0 state rapidly converts into an excited (S 1 state in Fig. 1) state where Au-Au covalent bonds are formed among Au ions aligned in a linear geometry. Subsequently, the S 1 state transforms to a triplet state (T 1 state in Fig. 1) in 1.6 ps while accompanying further contraction of Au-Au bonds by 0.1 Å. Later, the T 1 state of the trimer converts to a tetramer (tetramer state in Fig. 1) on nanosecond time scale. Finally, the Au ions returned to their original loosely interacting bent structure.
In this research, the direct observation of a very fast chemical reaction, induced by the photo-excitation, was succeeded (Fig. 2, 3). Therefore, this method is expected to be a fundamental technology for understanding the light energy conversion reaction. The research group is actively working to apply this method to the development of viable renewable energy resources, such as a photocatalysts for artificial photosynthesis using sunlight.
This research was supported by the X-ray Free Electron Laser Priority Strategy Program of the MEXT, PRESTO of the JST, and the the Innovative Areas "Artificial Photosynthesis (AnApple)" grant from the Japan Society for the Promotion of Science (JSPS).
Publication: Nature , 518 (19 February 2015)
Title: Direct observation of bond formation in solution with femtosecond X-ray scattering
Authors: K. H. Kim 1 , J. G. Kim 1 , S. Nozawa 1 , T. Sato 1 , K. Y. Oang, T. W. Kim, H. Ki, J. Jo, S. Park, C. Song, T. Sato, K. Ogawa, T. Togashi, K. Tono, M. Yabashi, T. Ishikawa, J. Kim, R. Ryoo, J. Kim, H. Ihee, S. Adachi. ※ 1: These authors contributed equally to the work.
DOI: 10.1038/nature14163
Figure 1. Structure of a gold cyano trimer complex (Au(CN) 2 - ) 3 .
Figure 2. Observed changes in the molecular structure of the gold complex
Figure 3. Schematic view of the research of photo-chemical reactions by the molecular movie
2015.02.27 View 14653
The Real Time Observation of the Birth of a Molecule
From right to left: Dr. Kyung-Hwan Kim, Professor Hyotcherl Lhee, and Jong-Gu Kim, a Ph.D. candidate
Professor Hyotcherl Lhee of the Department of Chemistry at KAIST and Japanese research teams jointly published their research results showing that they have succeeded in the direct observation of how atoms form a molecule in the online issue of Nature on February 19, 2015.
The researchers used water in which gold atoms ([Au(CN) 2- ]) are dissolved and fired X-ray pulses over the specimen in femtosecond timescales to study chemical reactions taking place among the gold atoms. They were able to examine in real time the instant process of how gold atoms bond together to become a molecule, to a trimer or tetramer state.
This direct viewing of the formation of a gold trimer complex ([Au(CN) 2- ] 3 ) will provide an opportunity to understand complex chemical and biological systems.
For details, please see the following press release that was distributed by the High Energy Accelerator Research Organization, KEK, in Japan:
Direct Observation of Bond Formations
February 18, 2015
A collaboration between researchers from KEK, the Institute for Basic Science (IBS), the Korea Advanced Institute of Science and Technology (KAIST), RIKEN, and the Japan Synchrotron Radiation Research Institute (JASRI) used the SACLA X-ray free electron laser (XFEL) facility for a real time visualization of the birth of a molecular that occurs via photoinduced formation of a chemical bonds. This achievement was published in the online version of the scientific journal “Nature” (published on 19 February 2015).
Direct “observation” of the bond making, through a chemical reaction, has been longstanding dream for chemists. However, the distance between atoms is very small, at about 100 picometer, and the bonding is completed very quickly, taking less than one picosecond (ps). Hence, previously, one could only imagine the bond formation between atoms while looking at the chemical reaction progressing in the test-tube.
In this study, the research group focused on the process of photoinduced bond formation between gold (Au) ions dissolved in water. In the ground state (S 0 state in Fig. 1) Au ions that are weakly bound to each other by an electron affinity and aligned in a bent geometry. Upon a photoexcitation, the S 0 state rapidly converts into an excited (S 1 state in Fig. 1) state where Au-Au covalent bonds are formed among Au ions aligned in a linear geometry. Subsequently, the S 1 state transforms to a triplet state (T 1 state in Fig. 1) in 1.6 ps while accompanying further contraction of Au-Au bonds by 0.1 Å. Later, the T 1 state of the trimer converts to a tetramer (tetramer state in Fig. 1) on nanosecond time scale. Finally, the Au ions returned to their original loosely interacting bent structure.
In this research, the direct observation of a very fast chemical reaction, induced by the photo-excitation, was succeeded (Fig. 2, 3). Therefore, this method is expected to be a fundamental technology for understanding the light energy conversion reaction. The research group is actively working to apply this method to the development of viable renewable energy resources, such as a photocatalysts for artificial photosynthesis using sunlight.
This research was supported by the X-ray Free Electron Laser Priority Strategy Program of the MEXT, PRESTO of the JST, and the the Innovative Areas "Artificial Photosynthesis (AnApple)" grant from the Japan Society for the Promotion of Science (JSPS).
Publication: Nature , 518 (19 February 2015)
Title: Direct observation of bond formation in solution with femtosecond X-ray scattering
Authors: K. H. Kim 1 , J. G. Kim 1 , S. Nozawa 1 , T. Sato 1 , K. Y. Oang, T. W. Kim, H. Ki, J. Jo, S. Park, C. Song, T. Sato, K. Ogawa, T. Togashi, K. Tono, M. Yabashi, T. Ishikawa, J. Kim, R. Ryoo, J. Kim, H. Ihee, S. Adachi. ※ 1: These authors contributed equally to the work.
DOI: 10.1038/nature14163
Figure 1. Structure of a gold cyano trimer complex (Au(CN) 2 - ) 3 .
Figure 2. Observed changes in the molecular structure of the gold complex
Figure 3. Schematic view of the research of photo-chemical reactions by the molecular movie
2015.02.27 View 14653