ES
-
 Professor Mikyoung Lim from Mathematical Sciences to Deliver Keynote at International Conference on Applied Inverse Problems
<Professor Mikyoung Lim from KAIST Department of Mathematical Sciences>
Professor Mikyoung Lim from KAIST Department of Mathematical Sciences gave a plenary talk on "Research on Inverse Problems based on Geometric Function Theory" at AIP 2025 (12th Applied Inverse Problems Conference). AIP is one of the leading international conferences in applied mathematics, organized biennially by the Inverse Problems International Association (IPIA). This year's conference was held from July 28 to August 1 in Rio de Janeiro, Brazil, and consisted of plenary talks, over 40 mini-symposia, and poster sessions. The IPIA began in 2007 and was re-established in 2022 as a non-profit international academic organization officially registered in Germany. At that time, Professor Lim served as an executive committee member for the re-establishment.
During the lecture, Professor Lim's research team introduced a new geometric solution and its applications to boundary value problems for electric/elastic equations, which they have been working on for the past 10 years. In particular, they presented a method for reconstructing partial differential equation boundary value problems into matrix equations and applying them to inverse problems using geometric function theory, a classical theory of complex analysis. A representative achievement was the formalization of the relationship between conformal mappings for simply connected domains in a plane and the measured values of solutions to equations of inhomogeneous conductors into a closed-form expression.
This research led to the plenary talk, as it was recognized for pioneering a new methodology for inverse problem research by connecting geometric function theory and layer potential theory.
2025.08.14 View 37
Professor Mikyoung Lim from Mathematical Sciences to Deliver Keynote at International Conference on Applied Inverse Problems
<Professor Mikyoung Lim from KAIST Department of Mathematical Sciences>
Professor Mikyoung Lim from KAIST Department of Mathematical Sciences gave a plenary talk on "Research on Inverse Problems based on Geometric Function Theory" at AIP 2025 (12th Applied Inverse Problems Conference). AIP is one of the leading international conferences in applied mathematics, organized biennially by the Inverse Problems International Association (IPIA). This year's conference was held from July 28 to August 1 in Rio de Janeiro, Brazil, and consisted of plenary talks, over 40 mini-symposia, and poster sessions. The IPIA began in 2007 and was re-established in 2022 as a non-profit international academic organization officially registered in Germany. At that time, Professor Lim served as an executive committee member for the re-establishment.
During the lecture, Professor Lim's research team introduced a new geometric solution and its applications to boundary value problems for electric/elastic equations, which they have been working on for the past 10 years. In particular, they presented a method for reconstructing partial differential equation boundary value problems into matrix equations and applying them to inverse problems using geometric function theory, a classical theory of complex analysis. A representative achievement was the formalization of the relationship between conformal mappings for simply connected domains in a plane and the measured values of solutions to equations of inhomogeneous conductors into a closed-form expression.
This research led to the plenary talk, as it was recognized for pioneering a new methodology for inverse problem research by connecting geometric function theory and layer potential theory.
2025.08.14 View 37 -
 KAIST Takes the Lead in Developing Core Technologies for Generative AI National R&D Project
KAIST (President Kwang Hyung Lee) is leading the transition to AI Transformation (AX) by advancing research topics based on the practical technological demands of industries, fostering AI talent, and demonstrating research outcomes in industrial settings. In this context, KAIST announced on the 13th of August that it is at the forefront of strengthening the nation's AI technology competitiveness by developing core AI technologies via national R&D projects for generative AI led by the Ministry of Science and ICT.
In the 'Generative AI Leading Talent Cultivation Project,' KAIST was selected as a joint research institution for all three projects—two led by industry partners and one by a research institution—and will thus be tasked with the dual challenge of developing core generative AI technologies and cultivating practical, core talent through industry-academia collaborations.
Moreover, in the 'Development of a Proprietary AI Foundation Model' project, KAIST faculty members are participating as key researchers in four out of five consortia, establishing the university as a central hub for domestic generative AI research.
Each project in the Generative AI Leading Talent Cultivation Project will receive 6.7 billion won, while each consortium in the proprietary AI foundation model development project will receive a total of 200 billion won in government support, including GPU infrastructure.
As part of the 'Generative AI Leading Talent Cultivation Project,' which runs until the end of 2028, KAIST is collaborating with LG AI Research. Professor Noseong Park from the School of Computing will participate as the principal investigator for KAIST, conducting research in the field of physics-based generative AI (Physical AI). This project focuses on developing image and video generation technologies based on physical laws and developing a 'World Model.'
<(From Left) Professor Noseong Park, Professor Jae-gil Lee, Professor Jiyoung Whang, Professor Sung-Eui Yoon, Professor Hyunwoo Kim>
In particular, research being conducted by Professor Noseong Park's team and Professor Sung-Eui Yoon's team proposes a model structure designed to help AI learn the real-world rules of the physical world more precisely. This is considered a core technology for Physical AI.
Professors Noseong Park, Jae-gil Lee, Jiyoung Hwang, Sung-Eui Yoon, and Hyun-Woo Kim from the School of Computing, who have been globally recognized for their achievements in the AI field, are jointly participating in this project. This year, they have presented work at top AI conferences such as ICLR, ICRA, ICCV, and ICML, including: ▲ Research on physics-based Ollivier Ricci-flow (ICLR 2025, Prof. Noseong Park) ▲ Technology to improve the navigation efficiency of quadruped robots (ICRA 2025, Prof. Sung-Eui Yoon) ▲ A multimodal large language model for text-video retrieval (ICCV 2025, Prof. Hyun-Woo Kim) ▲ Structured representation learning for knowledge generation (ICML 2025, Prof. Jiyoung Whang).
In the collaboration with NC AI, Professor Tae-Kyun Kim from the School of Computing is participating as the principal investigator to develop multimodal AI agent technology. The research will explore technologies applicable to the entire gaming industry, such as 3D modeling, animation, avatar expression generation, and character AI. It is expected to contribute to training practical AI talents by giving them hands-on experience in the industrial field and making the game production pipeline more efficient.
As the principal investigator, Professor Tae-Kyun Kim, a renowned scholar in 3D computer vision and generative AI, is developing key technologies for creating immersive avatars in the virtual and gaming industries. He will apply a first-person full-body motion diffusion model, which he developed through a joint research project with Meta, to VR and AR environments.
<Professor Tae-Kyun Kim, Minhyeok Seong, and Tae-Hyun Oh from the School of Computing, and Professor Sung-Hee Lee, Woon-Tack Woo, Jun-Yong Noh, and Kyung-Tae Lim from the Graduate School of Culture Technology, Professor Ki-min Lee, Seungryong Kim from the Kim Jae-chul Graduate School of AI>
Professor Tae-Kyun Kim, Minhyeok Seong, and Tae-Hyun Oh from the School of Computing, and Professors Sung-Hee Lee, Woon-Tack Woo, Jun-Yong Noh, and Kyung-Tae Lim from the Graduate School of Culture Technology, are participating in the NC AI project. They have presented globally recognized work at CVPR 2025 and ICLR 2025, including: ▲ A first-person full-body motion diffusion model (CVPR 2025, Prof. Tae-Kyun Kim) ▲ Stochastic diffusion synchronization technology for image generation (ICLR 2025, Prof. Minhyeok Seong) ▲ The creation of a large-scale 3D facial mesh video dataset (ICLR 2025, Prof. Tae-Hyun Oh) ▲ Object-adaptive agent motion generation technology, InterFaceRays (Eurographics 2025, Prof. Sung-Hee Lee) ▲ 3D neural face editing technology (CVPR 2025, Prof. Jun-Yong Noh) ▲ Research on selective search augmentation for multilingual vision-language models (COLING 2025, Prof. Kyung-Tae Lim).
In the project led by the Korea Electronics Technology Institute (KETI), Professor Seungryong Kim from the Kim Jae-chul Graduate School of AI is participating in generative AI technology development. His team recently developed new technology for extracting robust point-tracking information from video data in collaboration with Adobe Research and Google DeepMind, proposing a key technology for clearly understanding and generating videos.
Each industry partner will open joint courses with KAIST and provide their generative AI foundation models for education and research. Selected outstanding students will be dispatched to these companies to conduct practical research, and KAIST faculty will also serve as adjunct professors at the in-house AI graduate school established by LG AI Research.
<Egocentric Whole-Body Motion Diffusion (CVPR 2025, Prof. Taekyun Kim's Lab), Stochastic Diffusion Synchronization for Image Generation (ICLR 2025, Prof. Minhyuk Sung's Lab), A Large-Scale 3D Face Mesh Video Dataset (ICLR 2025, Prof. Taehyun Oh's Lab), InterFaceRays: Object-Adaptive Agent Action Generation (Eurographics 2025, Prof. Sunghee Lee's Lab), 3D Neural Face Editing (CVPR 2025, Prof. Junyong Noh's Lab), and Selective Retrieval Augmentation for Multilingual Vision-Language Models (COLING 2025, Prof. Kyeong-tae Lim's Lab)>
Meanwhile, KAIST showed an unrivaled presence by participating in four consortia for the Ministry of Science and ICT's 'Proprietary AI Foundation Model Development' project.
In the NC AI Consortium, Professors Tae-Kyun Kim, Sung-Eui Yoon, Noseong Park, Jiyoung Hwang, and Minhyeok Seong from the School of Computing are participating, focusing on the development of multimodal foundation models (LMMs) and robot-based models. They are particularly concentrating on developing LMMs that learn common sense about space, physics, and time. They have formed a research team optimized for developing next-generation, multimodal AI models that can understand and interact with the physical world, equipped with an 'all-purpose AI brain' capable of simultaneously understanding and processing diverse information such as text, images, video, and sound.
In the Upstage Consortium, Professors Jae-gil Lee and Hyeon-eon Oh from the School of Computing, both renowned scholars in data AI and NLP (natural language processing), along with Professor Kyung-Tae Lim from the Graduate School of Culture Technology, an LLM expert, are responsible for developing vertical models for industries such as finance, law, and manufacturing. The KAIST researchers will concentrate on developing practical AI models that are directly applicable to industrial settings and tailored to each specific industry.
The Naver Consortium includes Professor Tae-Hyun Oh from the School of Computing, who has developed key technology for multimodal learning and compositional language-vision models, Professor Hyun-Woo Kim, who has proposed video reasoning and generation methods using language models, and faculty from the Kim Jae-chul Graduate School of AI and the Department of Electrical Engineering.
In the SKT Consortium, Professor Ki-min Lee from the Kim Jae-chul Graduate School of AI, who has achieved outstanding results in text-to-image generation, human preference modeling, and visual robotic manipulation technology development, is participating. This technology is expected to play a key role in developing personalized services and customized AI solutions for telecommunications companies.
This outcome is considered a successful culmination of KAIST's strategy for developing AI technology based on industry demand and centered on on-site demonstrations.
KAIST President Kwang Hyung Lee said, "For AI technology to go beyond academic achievements and be connected to and practical for industry, continuous government support, research, and education centered on industry-academia collaboration are essential. KAIST will continue to strive to solve problems in industrial settings and make a real contribution to enhancing the competitiveness of the AI ecosystem."
He added that while the project led by Professor Sung-Ju Hwang from the Kim Jae-chul Graduate School of AI, which had applied as a lead institution for the proprietary foundation model development project, was unfortunately not selected, it was a meaningful challenge that stood out for its original approach and bold attempts. President Lee further commented, "Regardless of whether it was selected or not, such attempts will accumulate and make the Korean AI ecosystem even richer."
2025.08.13 View 93
KAIST Takes the Lead in Developing Core Technologies for Generative AI National R&D Project
KAIST (President Kwang Hyung Lee) is leading the transition to AI Transformation (AX) by advancing research topics based on the practical technological demands of industries, fostering AI talent, and demonstrating research outcomes in industrial settings. In this context, KAIST announced on the 13th of August that it is at the forefront of strengthening the nation's AI technology competitiveness by developing core AI technologies via national R&D projects for generative AI led by the Ministry of Science and ICT.
In the 'Generative AI Leading Talent Cultivation Project,' KAIST was selected as a joint research institution for all three projects—two led by industry partners and one by a research institution—and will thus be tasked with the dual challenge of developing core generative AI technologies and cultivating practical, core talent through industry-academia collaborations.
Moreover, in the 'Development of a Proprietary AI Foundation Model' project, KAIST faculty members are participating as key researchers in four out of five consortia, establishing the university as a central hub for domestic generative AI research.
Each project in the Generative AI Leading Talent Cultivation Project will receive 6.7 billion won, while each consortium in the proprietary AI foundation model development project will receive a total of 200 billion won in government support, including GPU infrastructure.
As part of the 'Generative AI Leading Talent Cultivation Project,' which runs until the end of 2028, KAIST is collaborating with LG AI Research. Professor Noseong Park from the School of Computing will participate as the principal investigator for KAIST, conducting research in the field of physics-based generative AI (Physical AI). This project focuses on developing image and video generation technologies based on physical laws and developing a 'World Model.'
<(From Left) Professor Noseong Park, Professor Jae-gil Lee, Professor Jiyoung Whang, Professor Sung-Eui Yoon, Professor Hyunwoo Kim>
In particular, research being conducted by Professor Noseong Park's team and Professor Sung-Eui Yoon's team proposes a model structure designed to help AI learn the real-world rules of the physical world more precisely. This is considered a core technology for Physical AI.
Professors Noseong Park, Jae-gil Lee, Jiyoung Hwang, Sung-Eui Yoon, and Hyun-Woo Kim from the School of Computing, who have been globally recognized for their achievements in the AI field, are jointly participating in this project. This year, they have presented work at top AI conferences such as ICLR, ICRA, ICCV, and ICML, including: ▲ Research on physics-based Ollivier Ricci-flow (ICLR 2025, Prof. Noseong Park) ▲ Technology to improve the navigation efficiency of quadruped robots (ICRA 2025, Prof. Sung-Eui Yoon) ▲ A multimodal large language model for text-video retrieval (ICCV 2025, Prof. Hyun-Woo Kim) ▲ Structured representation learning for knowledge generation (ICML 2025, Prof. Jiyoung Whang).
In the collaboration with NC AI, Professor Tae-Kyun Kim from the School of Computing is participating as the principal investigator to develop multimodal AI agent technology. The research will explore technologies applicable to the entire gaming industry, such as 3D modeling, animation, avatar expression generation, and character AI. It is expected to contribute to training practical AI talents by giving them hands-on experience in the industrial field and making the game production pipeline more efficient.
As the principal investigator, Professor Tae-Kyun Kim, a renowned scholar in 3D computer vision and generative AI, is developing key technologies for creating immersive avatars in the virtual and gaming industries. He will apply a first-person full-body motion diffusion model, which he developed through a joint research project with Meta, to VR and AR environments.
<Professor Tae-Kyun Kim, Minhyeok Seong, and Tae-Hyun Oh from the School of Computing, and Professor Sung-Hee Lee, Woon-Tack Woo, Jun-Yong Noh, and Kyung-Tae Lim from the Graduate School of Culture Technology, Professor Ki-min Lee, Seungryong Kim from the Kim Jae-chul Graduate School of AI>
Professor Tae-Kyun Kim, Minhyeok Seong, and Tae-Hyun Oh from the School of Computing, and Professors Sung-Hee Lee, Woon-Tack Woo, Jun-Yong Noh, and Kyung-Tae Lim from the Graduate School of Culture Technology, are participating in the NC AI project. They have presented globally recognized work at CVPR 2025 and ICLR 2025, including: ▲ A first-person full-body motion diffusion model (CVPR 2025, Prof. Tae-Kyun Kim) ▲ Stochastic diffusion synchronization technology for image generation (ICLR 2025, Prof. Minhyeok Seong) ▲ The creation of a large-scale 3D facial mesh video dataset (ICLR 2025, Prof. Tae-Hyun Oh) ▲ Object-adaptive agent motion generation technology, InterFaceRays (Eurographics 2025, Prof. Sung-Hee Lee) ▲ 3D neural face editing technology (CVPR 2025, Prof. Jun-Yong Noh) ▲ Research on selective search augmentation for multilingual vision-language models (COLING 2025, Prof. Kyung-Tae Lim).
In the project led by the Korea Electronics Technology Institute (KETI), Professor Seungryong Kim from the Kim Jae-chul Graduate School of AI is participating in generative AI technology development. His team recently developed new technology for extracting robust point-tracking information from video data in collaboration with Adobe Research and Google DeepMind, proposing a key technology for clearly understanding and generating videos.
Each industry partner will open joint courses with KAIST and provide their generative AI foundation models for education and research. Selected outstanding students will be dispatched to these companies to conduct practical research, and KAIST faculty will also serve as adjunct professors at the in-house AI graduate school established by LG AI Research.
<Egocentric Whole-Body Motion Diffusion (CVPR 2025, Prof. Taekyun Kim's Lab), Stochastic Diffusion Synchronization for Image Generation (ICLR 2025, Prof. Minhyuk Sung's Lab), A Large-Scale 3D Face Mesh Video Dataset (ICLR 2025, Prof. Taehyun Oh's Lab), InterFaceRays: Object-Adaptive Agent Action Generation (Eurographics 2025, Prof. Sunghee Lee's Lab), 3D Neural Face Editing (CVPR 2025, Prof. Junyong Noh's Lab), and Selective Retrieval Augmentation for Multilingual Vision-Language Models (COLING 2025, Prof. Kyeong-tae Lim's Lab)>
Meanwhile, KAIST showed an unrivaled presence by participating in four consortia for the Ministry of Science and ICT's 'Proprietary AI Foundation Model Development' project.
In the NC AI Consortium, Professors Tae-Kyun Kim, Sung-Eui Yoon, Noseong Park, Jiyoung Hwang, and Minhyeok Seong from the School of Computing are participating, focusing on the development of multimodal foundation models (LMMs) and robot-based models. They are particularly concentrating on developing LMMs that learn common sense about space, physics, and time. They have formed a research team optimized for developing next-generation, multimodal AI models that can understand and interact with the physical world, equipped with an 'all-purpose AI brain' capable of simultaneously understanding and processing diverse information such as text, images, video, and sound.
In the Upstage Consortium, Professors Jae-gil Lee and Hyeon-eon Oh from the School of Computing, both renowned scholars in data AI and NLP (natural language processing), along with Professor Kyung-Tae Lim from the Graduate School of Culture Technology, an LLM expert, are responsible for developing vertical models for industries such as finance, law, and manufacturing. The KAIST researchers will concentrate on developing practical AI models that are directly applicable to industrial settings and tailored to each specific industry.
The Naver Consortium includes Professor Tae-Hyun Oh from the School of Computing, who has developed key technology for multimodal learning and compositional language-vision models, Professor Hyun-Woo Kim, who has proposed video reasoning and generation methods using language models, and faculty from the Kim Jae-chul Graduate School of AI and the Department of Electrical Engineering.
In the SKT Consortium, Professor Ki-min Lee from the Kim Jae-chul Graduate School of AI, who has achieved outstanding results in text-to-image generation, human preference modeling, and visual robotic manipulation technology development, is participating. This technology is expected to play a key role in developing personalized services and customized AI solutions for telecommunications companies.
This outcome is considered a successful culmination of KAIST's strategy for developing AI technology based on industry demand and centered on on-site demonstrations.
KAIST President Kwang Hyung Lee said, "For AI technology to go beyond academic achievements and be connected to and practical for industry, continuous government support, research, and education centered on industry-academia collaboration are essential. KAIST will continue to strive to solve problems in industrial settings and make a real contribution to enhancing the competitiveness of the AI ecosystem."
He added that while the project led by Professor Sung-Ju Hwang from the Kim Jae-chul Graduate School of AI, which had applied as a lead institution for the proprietary foundation model development project, was unfortunately not selected, it was a meaningful challenge that stood out for its original approach and bold attempts. President Lee further commented, "Regardless of whether it was selected or not, such attempts will accumulate and make the Korean AI ecosystem even richer."
2025.08.13 View 93 -
 KAIST Develops AI That Automatically Designs Optimal Drug Candidates for Cancer-Targeting Mutations
< (From left) Ph.D candidate Wonho Zhung, Ph.D cadidate Joongwon Lee , Prof. Woo Young Kim , Ph.D candidate Jisu Seo >
Traditional drug development methods involve identifying a target protin (e.g., a cancer cell receptor) that causes disease, and then searching through countless molecular candidates (potential drugs) that could bind to that protein and block its function. This process is costly, time-consuming, and has a low success rate. KAIST researchers have developed an AI model that, using only information about the target protein, can design optimal drug candidates without any prior molecular data—opening up new possibilities for drug discovery.
KAIST (President Kwang Hyung Lee) announced on the 10th that a research team led by Professor Woo Youn Kim in the Department of Chemistry has developed an AI model named BInD (Bond and Interaction-generating Diffusion model), which can design and optimize drug candidate molecules tailored to a protein’s structure alone—without needing prior information about binding molecules. The model also predicts the binding mechanism (non-covalent interactions) between the drug and the target protein.
The core innovation of this technology lies in its “simultaneous design” approach. Previous AI models either focused on generating molecules or separately evaluating whether the generated molecule could bind to the target protein. In contrast, this new model considers the binding mechanism between the molecule and the protein during the generation process, enabling comprehensive design in one step. Since it pre-accounts for critical factors in protein-ligand binding, it has a much higher likelihood of generating effective and stable molecules. The generation process visually demonstrates how types and positions of atoms, covalent bonds, and interactions are created simultaneously to fit the protein’s binding site.
<Figure 1. Schematic of the diffusion model developed by the research team, which generates molecular structures and non-covalent interactions based on protein structures. Starting from a noise distribution, the model gradually removes noise (via reverse diffusion) to restore the atom positions, types, covalent bond types, and interaction types, thereby generating molecules. Interacting patterns are extracted from prior knowledge of known binding molecules or proteins, and through an inpainting technique, these patterns are kept fixed during the reverse diffusion process to guide the molecular generation.>
Moreover, this model is designed to meet multiple essential drug design criteria simultaneously—such as target binding affinity, drug-like properties, and structural stability. Traditional models often optimized for only one or two goals at the expense of others, but this new model balances various objectives, significantly enhancing its practical applicability.
The research team explained that the AI operates based on a “diffusion model”—a generative approach where a structure becomes increasingly refined from a random state. This is the same type of model used in AlphaFold 3, the 2024 Nobel Chemistry Prize-winning tool for protein-ligand structure generation, which has already demonstrated high efficiency.
Unlike AlphaFold 3, which provides spatial coordinates for atom positions, this study introduced a knowledge-based guide grounded in actual chemical laws—such as bond lengths and protein-ligand distances—enabling more chemically realistic structure generation.
<Figure 2. (Left) Target protein and the original bound molecule; (Right) Examples of molecules designed using the model developed in this study. The values for protein binding affinity (Vina), drug-likeness (QED), and synthetic accessibility (SA) are shown at the bottom.>
Additionally, the team applied an optimization strategy where outstanding binding patterns from prior results are reused. This allowed the model to generate even better drug candidates without additional training. Notably, the AI successfully produced molecules that selectively bind to the mutated residues of EGFR, a cancer-related target protein.
This study is also meaningful because it advances beyond the team’s previous research, which required prior input about the molecular conditions for the interaction pattern of protein binding.
Professor Woo Youn Kim commented that “the newly developed AI can learn and understand the key features required for strong binding to a target protein, and design optimal drug candidate molecules—even without any prior input. This could significantly shift the paradigm of drug development.” He added, “Since this technology generates molecular structures based on principles of chemical interactions, it is expected to enable faster and more reliable drug development.”
Joongwon Lee and Wonho Zhung, PhD students in the Department of Chemistry, participated as co-first authors of this study. The research results were published in the international journal Advanced Science (IF = 14.1) on July 11.
● Paper Title: BInD: Bond and Interaction-Generating Diffusion Model for Multi-Objective Structure-Based Drug Design
● DOI: 10.1002/advs.202502702
This research was supported by the National Research Foundation of Korea and the Ministry of Health and Welfare.
2025.08.12 View 205
KAIST Develops AI That Automatically Designs Optimal Drug Candidates for Cancer-Targeting Mutations
< (From left) Ph.D candidate Wonho Zhung, Ph.D cadidate Joongwon Lee , Prof. Woo Young Kim , Ph.D candidate Jisu Seo >
Traditional drug development methods involve identifying a target protin (e.g., a cancer cell receptor) that causes disease, and then searching through countless molecular candidates (potential drugs) that could bind to that protein and block its function. This process is costly, time-consuming, and has a low success rate. KAIST researchers have developed an AI model that, using only information about the target protein, can design optimal drug candidates without any prior molecular data—opening up new possibilities for drug discovery.
KAIST (President Kwang Hyung Lee) announced on the 10th that a research team led by Professor Woo Youn Kim in the Department of Chemistry has developed an AI model named BInD (Bond and Interaction-generating Diffusion model), which can design and optimize drug candidate molecules tailored to a protein’s structure alone—without needing prior information about binding molecules. The model also predicts the binding mechanism (non-covalent interactions) between the drug and the target protein.
The core innovation of this technology lies in its “simultaneous design” approach. Previous AI models either focused on generating molecules or separately evaluating whether the generated molecule could bind to the target protein. In contrast, this new model considers the binding mechanism between the molecule and the protein during the generation process, enabling comprehensive design in one step. Since it pre-accounts for critical factors in protein-ligand binding, it has a much higher likelihood of generating effective and stable molecules. The generation process visually demonstrates how types and positions of atoms, covalent bonds, and interactions are created simultaneously to fit the protein’s binding site.
<Figure 1. Schematic of the diffusion model developed by the research team, which generates molecular structures and non-covalent interactions based on protein structures. Starting from a noise distribution, the model gradually removes noise (via reverse diffusion) to restore the atom positions, types, covalent bond types, and interaction types, thereby generating molecules. Interacting patterns are extracted from prior knowledge of known binding molecules or proteins, and through an inpainting technique, these patterns are kept fixed during the reverse diffusion process to guide the molecular generation.>
Moreover, this model is designed to meet multiple essential drug design criteria simultaneously—such as target binding affinity, drug-like properties, and structural stability. Traditional models often optimized for only one or two goals at the expense of others, but this new model balances various objectives, significantly enhancing its practical applicability.
The research team explained that the AI operates based on a “diffusion model”—a generative approach where a structure becomes increasingly refined from a random state. This is the same type of model used in AlphaFold 3, the 2024 Nobel Chemistry Prize-winning tool for protein-ligand structure generation, which has already demonstrated high efficiency.
Unlike AlphaFold 3, which provides spatial coordinates for atom positions, this study introduced a knowledge-based guide grounded in actual chemical laws—such as bond lengths and protein-ligand distances—enabling more chemically realistic structure generation.
<Figure 2. (Left) Target protein and the original bound molecule; (Right) Examples of molecules designed using the model developed in this study. The values for protein binding affinity (Vina), drug-likeness (QED), and synthetic accessibility (SA) are shown at the bottom.>
Additionally, the team applied an optimization strategy where outstanding binding patterns from prior results are reused. This allowed the model to generate even better drug candidates without additional training. Notably, the AI successfully produced molecules that selectively bind to the mutated residues of EGFR, a cancer-related target protein.
This study is also meaningful because it advances beyond the team’s previous research, which required prior input about the molecular conditions for the interaction pattern of protein binding.
Professor Woo Youn Kim commented that “the newly developed AI can learn and understand the key features required for strong binding to a target protein, and design optimal drug candidate molecules—even without any prior input. This could significantly shift the paradigm of drug development.” He added, “Since this technology generates molecular structures based on principles of chemical interactions, it is expected to enable faster and more reliable drug development.”
Joongwon Lee and Wonho Zhung, PhD students in the Department of Chemistry, participated as co-first authors of this study. The research results were published in the international journal Advanced Science (IF = 14.1) on July 11.
● Paper Title: BInD: Bond and Interaction-Generating Diffusion Model for Multi-Objective Structure-Based Drug Design
● DOI: 10.1002/advs.202502702
This research was supported by the National Research Foundation of Korea and the Ministry of Health and Welfare.
2025.08.12 View 205 -
 KAIST Develops Bioelectrosynthesis Platform for Switch-Like Precision Control of Cell Signaling
<(From left)Professor Jimin Park, Ph.D candidate Myeongeun Lee, Ph.D cadidate Jaewoong Lee,Professor Jihan Kim>
Cells use various signaling molecules to regulate the nervous, immune, and vascular systems. Among these, nitric oxide (NO) and ammonia (NH₃) play important roles, but their chemical instability and gaseous nature make them difficult to generate or control externally. A KAIST research team has developed a platform that generates specific signaling molecules in situ from a single precursor under an applied electrical signal, enabling switch-like, precise spatiotemporal control of cellular responses. This approach could provide a foundation for future medical technologies such as electroceuticals, electrogenetics, and personalized cell therapies.
KAIST (President Kwang Hyung Lee) announced on August 11 that a research team led by Professor Jimin Park from the Department of Chemical and Biomolecular Engineering, in collaboration with Professor Jihan Kim's group, has developed a 'Bioelectrosynthesis Platform' capable of producing either nitric oxide or ammonia on demand using only an electrical signal. The platform allows control over the timing, spatial range, and duration of cell responses.
Inspired by enzymes involved in nitrite reduction, the researchers implemented an electrochemical strategy that selectively produces nitric oxide or ammonia from a single precursor, nitrite (NO₂⁻). By changing the catalyst, the team generated ammonia or nitric oxide from nitrite using a copper-molybdenum-sulfur catalyst (Cu2MoS4) and an iron-incorporated catalyst (FeCuMS4), respectively.
Through electrochemical measurements and computer simulations, the team revealed that Fe sites in the FeCuMoS4 catalyst bind nitric oxide intermediates more strongly, shifting product selectivity toward nitric oxide. Under the same electrical conditions, the Fe-containing catalyst preferentially produces nitric oxide, whereas the Cu2MoS4 catalyst favors ammonia production.
<Figure 1. Schematic diagram of a bio-electrosynthesis platform that synthesizes a desired signaling substance with an electrical signal (left) and the results of precise cell control using it (right)>
The research team demonstrated biological functionality by using the platform to activate ion channels in human cells. Specifically, electrochemically produced nitric oxide activated TRPV1 channels (responsive to heat and chemical stimuli), while electrochemically produced ammonia induced intracellular alkalinization and activated OTOP1 proton channels. By tuning the applied voltage and electrolysis duration, the team modulated the onset time, spatial extent, and termination of cellular responses, which effectively turned cellular signaling on and off like a switch.
<Figure 2. Experimental results showing the change in the production ratio of nitric oxide and ammonia signaling substances according to the type of catalyst (left) and computational simulation results showing the strong bond between iron and nitric oxide (right)>
Professor Jimin Park said, "This work is significant because it enables precise cellular control by selectively producing signaling molecules with electricity. We believe it has strong potential for applications in electroceutical technologies targeting the nervous system or metabolic disorders."
Myeongeun Lee and Jaewoong Lee, Ph.D. students in the Department of Chemical and Biomolecular Engineering at KAIST, served as the co-first authors. Professor Jihan Kim is a co-author. The paper was published online in 'Angewandte Chemie International Edition' on July 8, 2025 (DOI: 10.1002/ange.202508192).
Reference: https://doi.org/10.1002/ange.202508192
Authors: Myeongeun Lee†, Jaewoong Lee†, Yongha Kim, Changho Lee, Sang Yeon Oh, Prof. Jihan Kim, Prof. Jimin Park*
†These authors contributed equally. *Corresponding author.
2025.08.12 View 118
KAIST Develops Bioelectrosynthesis Platform for Switch-Like Precision Control of Cell Signaling
<(From left)Professor Jimin Park, Ph.D candidate Myeongeun Lee, Ph.D cadidate Jaewoong Lee,Professor Jihan Kim>
Cells use various signaling molecules to regulate the nervous, immune, and vascular systems. Among these, nitric oxide (NO) and ammonia (NH₃) play important roles, but their chemical instability and gaseous nature make them difficult to generate or control externally. A KAIST research team has developed a platform that generates specific signaling molecules in situ from a single precursor under an applied electrical signal, enabling switch-like, precise spatiotemporal control of cellular responses. This approach could provide a foundation for future medical technologies such as electroceuticals, electrogenetics, and personalized cell therapies.
KAIST (President Kwang Hyung Lee) announced on August 11 that a research team led by Professor Jimin Park from the Department of Chemical and Biomolecular Engineering, in collaboration with Professor Jihan Kim's group, has developed a 'Bioelectrosynthesis Platform' capable of producing either nitric oxide or ammonia on demand using only an electrical signal. The platform allows control over the timing, spatial range, and duration of cell responses.
Inspired by enzymes involved in nitrite reduction, the researchers implemented an electrochemical strategy that selectively produces nitric oxide or ammonia from a single precursor, nitrite (NO₂⁻). By changing the catalyst, the team generated ammonia or nitric oxide from nitrite using a copper-molybdenum-sulfur catalyst (Cu2MoS4) and an iron-incorporated catalyst (FeCuMS4), respectively.
Through electrochemical measurements and computer simulations, the team revealed that Fe sites in the FeCuMoS4 catalyst bind nitric oxide intermediates more strongly, shifting product selectivity toward nitric oxide. Under the same electrical conditions, the Fe-containing catalyst preferentially produces nitric oxide, whereas the Cu2MoS4 catalyst favors ammonia production.
<Figure 1. Schematic diagram of a bio-electrosynthesis platform that synthesizes a desired signaling substance with an electrical signal (left) and the results of precise cell control using it (right)>
The research team demonstrated biological functionality by using the platform to activate ion channels in human cells. Specifically, electrochemically produced nitric oxide activated TRPV1 channels (responsive to heat and chemical stimuli), while electrochemically produced ammonia induced intracellular alkalinization and activated OTOP1 proton channels. By tuning the applied voltage and electrolysis duration, the team modulated the onset time, spatial extent, and termination of cellular responses, which effectively turned cellular signaling on and off like a switch.
<Figure 2. Experimental results showing the change in the production ratio of nitric oxide and ammonia signaling substances according to the type of catalyst (left) and computational simulation results showing the strong bond between iron and nitric oxide (right)>
Professor Jimin Park said, "This work is significant because it enables precise cellular control by selectively producing signaling molecules with electricity. We believe it has strong potential for applications in electroceutical technologies targeting the nervous system or metabolic disorders."
Myeongeun Lee and Jaewoong Lee, Ph.D. students in the Department of Chemical and Biomolecular Engineering at KAIST, served as the co-first authors. Professor Jihan Kim is a co-author. The paper was published online in 'Angewandte Chemie International Edition' on July 8, 2025 (DOI: 10.1002/ange.202508192).
Reference: https://doi.org/10.1002/ange.202508192
Authors: Myeongeun Lee†, Jaewoong Lee†, Yongha Kim, Changho Lee, Sang Yeon Oh, Prof. Jihan Kim, Prof. Jimin Park*
†These authors contributed equally. *Corresponding author.
2025.08.12 View 118 -
 KAIST’s Wearable Robot Design Wins ‘2025 Red Dot Award Best of the Best’
<Professor Hyunjoon Park, M.S candidate Eun-ju Kang, Prospective M.S candidate Jae-seong Kim, undergraduate student Min-su Kim>
A team led by Professor Hyunjoon Park from the Department of Industrial Design won the ‘Best of the Best’ award at the 2025 Red Dot Design Awards, one of the world's top three design awards, for their 'Angel Robotics WSF1 VISION Concept.'
The design for the next-generation wearable robot for people with paraplegia successfully implements functionality, aesthetics, and social inclusion. This latest achievement follows the team's iF Design Award win for the WalkON Suit F1 prototype, which also won a gold medal at the Cybathlon last year. This marks consecutive wins at top-tier international design awards.
KAIST (President Kwang-hyung Lee) announced on the 8th of August that Move Lab, a research team led by Professor Hyunjoon Park from the Department of Industrial Design, won the 'Best of the Best' award in the Design Concept-Professional category at the prestigious '2025 Red Dot Design Awards' for their next-generation wearable robot design, the ‘Angel Robotics WSF1 VISION Concept.’
The German 'Red Dot Design Awards' is one of the world's most well-known design competitions. It is considered one of the world's top three design awards along with Germany’s iF Design Awards and America’s IDEA. The ‘Best of the Best’ award is given to the best design in a category and is awarded only to a very select few of the top designs (within the top 1%) among all Red Dot Award winners.
Professor Hyunjoon Park’s team was honored with the ‘Best of the Best’ award for a user-friendly follow-up development of the ‘WalkON Suit F1 prototype,’ which won a gold medal at the 2024 Cybathlon and an iF Design Award in 2025.
<Figure 1. WSF1 Vision Concept Main Image>
This award-winning design is the result of industry-academic cooperation with Angel Robotics Inc., founded by Professor Kyoungchul Kong from the KAIST Department of Mechanical Engineering. It is a concept design that proposes a next-generation wearable robot (an ultra-personal mobility device) that can be used by people with paraplegia in their daily lives.
The research team focused on transforming Angel Robotics Inc.'s advanced engineering platform into an intuitive and emotional, user-centric experience, implementing a design solution that simultaneously possesses functionality, aesthetics, and social inclusion.
<Figure 2. WSF1 Vision Concept Full Exterior (Front View)>
The WSF1 VISION Concept includes innovative features implemented in Professor Kyoungchul Kong’s Exo Lab, such as:
An autonomous access function where the robot finds the user on its own.
A front-loading mechanism designed for the user to put it on alone while seated.
Multi-directional walking functionality realized through 12 powerful torque actuators and the latest control algorithms.
AI vision technology, along with a multi-visual display system that provides navigation and omnidirectional vision.
This provides users with a safer and more convenient mobility experience.
The strong yet elegant silhouette was achieved through a design process that pursued perfection in proportion, surfaces, and details not seen in existing wearable robots. In particular, the fabric cover that wraps around the entire thigh from the robot's hip joint is a stylish element that respects the wearer's self-esteem and individuality, like fashionable athletic wear. It also acts as a device for the wearer to psychologically feel safe in interacting with the robot and blending in with the general public. This presents a new aesthetic for wearable robots where function and form are harmonized.
<Figure 3. WSF1 Vision Concept's Operating Principle. It walks autonomously and is worn from the front while the user is seated.>
KAIST Professor Hyunjoon Park said of the award, "We are focusing on using technology, aesthetics, and human-centered innovation to present advanced technical solutions as easy, enjoyable, and cool experiences for users. Based on Angel Robotics Inc.'s vision of 'recreating human ability with technology,' the WSF1 VISION Concept aimed to break away from the traditional framework of wearable robots and deliver a design experience that adds dignity, independence, and new style to the user's life."
<Figure 4. WSF1 Vision Concept Detail Image>
A physical model of the WSF1 VISION Concept is scheduled to be unveiled in the Future Hall of the 2025 Gwangju Design Biennale from August 30 to November 2. The theme is 'Po-yong-ji-deok' (the virtue of inclusion), and it will showcase the role of design language in creating an inclusive future society.
<Figure 5. WSF1 Vision Concept: Image of a Person Wearing and Walking>
2025.08.09 View 121
KAIST’s Wearable Robot Design Wins ‘2025 Red Dot Award Best of the Best’
<Professor Hyunjoon Park, M.S candidate Eun-ju Kang, Prospective M.S candidate Jae-seong Kim, undergraduate student Min-su Kim>
A team led by Professor Hyunjoon Park from the Department of Industrial Design won the ‘Best of the Best’ award at the 2025 Red Dot Design Awards, one of the world's top three design awards, for their 'Angel Robotics WSF1 VISION Concept.'
The design for the next-generation wearable robot for people with paraplegia successfully implements functionality, aesthetics, and social inclusion. This latest achievement follows the team's iF Design Award win for the WalkON Suit F1 prototype, which also won a gold medal at the Cybathlon last year. This marks consecutive wins at top-tier international design awards.
KAIST (President Kwang-hyung Lee) announced on the 8th of August that Move Lab, a research team led by Professor Hyunjoon Park from the Department of Industrial Design, won the 'Best of the Best' award in the Design Concept-Professional category at the prestigious '2025 Red Dot Design Awards' for their next-generation wearable robot design, the ‘Angel Robotics WSF1 VISION Concept.’
The German 'Red Dot Design Awards' is one of the world's most well-known design competitions. It is considered one of the world's top three design awards along with Germany’s iF Design Awards and America’s IDEA. The ‘Best of the Best’ award is given to the best design in a category and is awarded only to a very select few of the top designs (within the top 1%) among all Red Dot Award winners.
Professor Hyunjoon Park’s team was honored with the ‘Best of the Best’ award for a user-friendly follow-up development of the ‘WalkON Suit F1 prototype,’ which won a gold medal at the 2024 Cybathlon and an iF Design Award in 2025.
<Figure 1. WSF1 Vision Concept Main Image>
This award-winning design is the result of industry-academic cooperation with Angel Robotics Inc., founded by Professor Kyoungchul Kong from the KAIST Department of Mechanical Engineering. It is a concept design that proposes a next-generation wearable robot (an ultra-personal mobility device) that can be used by people with paraplegia in their daily lives.
The research team focused on transforming Angel Robotics Inc.'s advanced engineering platform into an intuitive and emotional, user-centric experience, implementing a design solution that simultaneously possesses functionality, aesthetics, and social inclusion.
<Figure 2. WSF1 Vision Concept Full Exterior (Front View)>
The WSF1 VISION Concept includes innovative features implemented in Professor Kyoungchul Kong’s Exo Lab, such as:
An autonomous access function where the robot finds the user on its own.
A front-loading mechanism designed for the user to put it on alone while seated.
Multi-directional walking functionality realized through 12 powerful torque actuators and the latest control algorithms.
AI vision technology, along with a multi-visual display system that provides navigation and omnidirectional vision.
This provides users with a safer and more convenient mobility experience.
The strong yet elegant silhouette was achieved through a design process that pursued perfection in proportion, surfaces, and details not seen in existing wearable robots. In particular, the fabric cover that wraps around the entire thigh from the robot's hip joint is a stylish element that respects the wearer's self-esteem and individuality, like fashionable athletic wear. It also acts as a device for the wearer to psychologically feel safe in interacting with the robot and blending in with the general public. This presents a new aesthetic for wearable robots where function and form are harmonized.
<Figure 3. WSF1 Vision Concept's Operating Principle. It walks autonomously and is worn from the front while the user is seated.>
KAIST Professor Hyunjoon Park said of the award, "We are focusing on using technology, aesthetics, and human-centered innovation to present advanced technical solutions as easy, enjoyable, and cool experiences for users. Based on Angel Robotics Inc.'s vision of 'recreating human ability with technology,' the WSF1 VISION Concept aimed to break away from the traditional framework of wearable robots and deliver a design experience that adds dignity, independence, and new style to the user's life."
<Figure 4. WSF1 Vision Concept Detail Image>
A physical model of the WSF1 VISION Concept is scheduled to be unveiled in the Future Hall of the 2025 Gwangju Design Biennale from August 30 to November 2. The theme is 'Po-yong-ji-deok' (the virtue of inclusion), and it will showcase the role of design language in creating an inclusive future society.
<Figure 5. WSF1 Vision Concept: Image of a Person Wearing and Walking>
2025.08.09 View 121 -
 Key Figures in the Establishment of KAIST, Specially Invited to the Presidential Office’s National Appointment Ceremony
KAIST announced on August 6 that Professor Emeritus Jung-Woong Ra from the Department of Electrical Engineering and Won-ki Kwon, former Vice Minister of the Ministry of Science and Technology, who played pivotal roles in the establishment of KAIST, were selected as special guests for the 'National Appointment Ceremony' hosted by the Presidential Office on August 15th.
The Presidential Office selected special invitees across eight categories for the ceremony. These include individuals born in 1945 (referred to as 'Liberation Babies'), those involved in the founding of KAIST in 1971, independence activists and national patriots, overseas workers in Germany and the Middle East, AI industry professionals, residents from regions facing depopulation, leading figures in K-culture, military personnel, firefighters, police officers, families of fallen public servants and victims of social disasters, as well as promising talents in economics, science, culture, and the arts.
Considering the historical significance of its establishment and its symbolic meaning for the development of national science and technology, KAIST Professor Emeritus Jung-Woong Ra, who was a key figure in the establishment of the Department of Electrical Engineering after being appointed as a professor in 1971, and former Vice Minister Kwon Won-ki, who was the first practical leader of the establishment project. Both were officially included on the special invitation list.
Briefing from the Presidential Office regarding the 'National Appointment Ceremony' (2025.07.28) https://www.president.go.kr/newsroom/briefing/grehGMuP
2025.08.06 View 229
Key Figures in the Establishment of KAIST, Specially Invited to the Presidential Office’s National Appointment Ceremony
KAIST announced on August 6 that Professor Emeritus Jung-Woong Ra from the Department of Electrical Engineering and Won-ki Kwon, former Vice Minister of the Ministry of Science and Technology, who played pivotal roles in the establishment of KAIST, were selected as special guests for the 'National Appointment Ceremony' hosted by the Presidential Office on August 15th.
The Presidential Office selected special invitees across eight categories for the ceremony. These include individuals born in 1945 (referred to as 'Liberation Babies'), those involved in the founding of KAIST in 1971, independence activists and national patriots, overseas workers in Germany and the Middle East, AI industry professionals, residents from regions facing depopulation, leading figures in K-culture, military personnel, firefighters, police officers, families of fallen public servants and victims of social disasters, as well as promising talents in economics, science, culture, and the arts.
Considering the historical significance of its establishment and its symbolic meaning for the development of national science and technology, KAIST Professor Emeritus Jung-Woong Ra, who was a key figure in the establishment of the Department of Electrical Engineering after being appointed as a professor in 1971, and former Vice Minister Kwon Won-ki, who was the first practical leader of the establishment project. Both were officially included on the special invitation list.
Briefing from the Presidential Office regarding the 'National Appointment Ceremony' (2025.07.28) https://www.president.go.kr/newsroom/briefing/grehGMuP
2025.08.06 View 229 -
 Immune Signals Directly Modulate Brain's Emotional Circuits: Unraveling the Mechanism Behind Anxiety-Inducing Behaviors
KAIST's Department of Brain and Cognitive Sciences, led by Professor Jeong-Tae Kwon, has collaborated with MIT and Harvard Medical School to make a groundbreaking discovery. For the first time globally, their joint research has revealed that cytokines, released during immune responses, directly influence the brain's emotional circuits to regulate anxiety behavior.
The study provided experimental evidence for a bidirectional regulatory mechanism: inflammatory cytokines IL-17A and IL-17C act on specific neurons in the amygdala, a region known for emotional regulation, increasing their excitability and consequently inducing anxiety. Conversely, the anti-inflammatory cytokine IL-10 was found to suppress excitability in these very same neurons, thereby contributing to anxiety alleviation.
In a mouse model, the research team observed that while skin inflammation was mitigated by immunotherapy (IL-17RA antibody), anxiety levels paradoxically rose. This was attributed to elevated circulating IL-17 family cytokines leading to the overactivation of amygdala neurons.
Key finding: Inflammatory cytokines IL-17A/17C promote anxiety by acting on excitable amygdala neurons (via IL-17RA/RE receptors), whereas anti-inflammatory cytokine IL-10 alleviates anxiety by suppressing excitability through IL-10RA receptors on the same neurons.
The researchers further elucidated that the anti-inflammatory cytokine IL-10 works to reduce the excitability of these amygdala neurons, thereby mitigating anxiety responses.
This research marks the first instance of demonstrating that immune responses, such as infections or inflammation, directly impact emotional regulation at the level of brain circuits, extending beyond simple physical reactions. This is a profoundly significant achievement, as it proposes a crucial biological mechanism that interlinks immunity, emotion, and behavior through identical neurons within the brain.
The findings of this research were published in the esteemed international journal Cell on April 17th of this year.
Paper Information:
Title: Inflammatory and anti-inflammatory cytokines bidirectionally modulate amygdala circuits regulating anxiety
Journal: Cell (Vol. 188, 2190–2220), April 17, 2025
DOI: https://doi.org/10.1016/j.cell.2025.03.005
Corresponding Authors: Professor Gloria Choi (MIT), Professor Jun R. Huh (Harvard Medical School)
2025.07.24 View 492
Immune Signals Directly Modulate Brain's Emotional Circuits: Unraveling the Mechanism Behind Anxiety-Inducing Behaviors
KAIST's Department of Brain and Cognitive Sciences, led by Professor Jeong-Tae Kwon, has collaborated with MIT and Harvard Medical School to make a groundbreaking discovery. For the first time globally, their joint research has revealed that cytokines, released during immune responses, directly influence the brain's emotional circuits to regulate anxiety behavior.
The study provided experimental evidence for a bidirectional regulatory mechanism: inflammatory cytokines IL-17A and IL-17C act on specific neurons in the amygdala, a region known for emotional regulation, increasing their excitability and consequently inducing anxiety. Conversely, the anti-inflammatory cytokine IL-10 was found to suppress excitability in these very same neurons, thereby contributing to anxiety alleviation.
In a mouse model, the research team observed that while skin inflammation was mitigated by immunotherapy (IL-17RA antibody), anxiety levels paradoxically rose. This was attributed to elevated circulating IL-17 family cytokines leading to the overactivation of amygdala neurons.
Key finding: Inflammatory cytokines IL-17A/17C promote anxiety by acting on excitable amygdala neurons (via IL-17RA/RE receptors), whereas anti-inflammatory cytokine IL-10 alleviates anxiety by suppressing excitability through IL-10RA receptors on the same neurons.
The researchers further elucidated that the anti-inflammatory cytokine IL-10 works to reduce the excitability of these amygdala neurons, thereby mitigating anxiety responses.
This research marks the first instance of demonstrating that immune responses, such as infections or inflammation, directly impact emotional regulation at the level of brain circuits, extending beyond simple physical reactions. This is a profoundly significant achievement, as it proposes a crucial biological mechanism that interlinks immunity, emotion, and behavior through identical neurons within the brain.
The findings of this research were published in the esteemed international journal Cell on April 17th of this year.
Paper Information:
Title: Inflammatory and anti-inflammatory cytokines bidirectionally modulate amygdala circuits regulating anxiety
Journal: Cell (Vol. 188, 2190–2220), April 17, 2025
DOI: https://doi.org/10.1016/j.cell.2025.03.005
Corresponding Authors: Professor Gloria Choi (MIT), Professor Jun R. Huh (Harvard Medical School)
2025.07.24 View 492 -
 KAIST School of Transdisciplinary Studies Is Driving Innovation in Korean Education
<(From Left) Professor Jaeseung Jeong, haed of the School of Transdiciplinary Studies, Dr, Albert Chau, Vice President of Hong Kong Baptist University>
KAIST (President Kwang Hyung Lee) announced on the 24th of July that its School of Transdisciplinary Studies has been consistently showcasing the results of its experiments and practices for educational innovation both domestically and abroad.
On June 27, Professor Jaeseung Jeong, head of the School of Transdisciplinary Studies, was invited to speak at the “Pacific Asia Summit on Transdisciplinary Education 2025 (PASTE 2025)” held at Hong Kong Baptist University. He presented the Korean model of transdisciplinary education under the title “The Philosophy and Achievements of the KAIST School of Transdisciplinary Studies.”
In his talk, Professor Jeong pointed out the limitations of conventional education systems that rely on answer-centered evaluation, perfectionism, and competitiveness, claiming that they hinder creativity and integrative thinking. He then introduced the philosophy and operational practices of the School of Transdisciplinary Studies, which was established in 2019 to overcome these issues.
Professor Jeong outlined five key principles that define the school's educational philosophy: ①a broad and integrative academic foundation, ②student-driven and customized education, ③creativity and execution, ④a sense of social responsibility and global citizenship, and ⑤learning driven by intrinsic motivation and curiosity. He explained that students are admitted without a declared major, allowed to design their own learning plans, and evaluated under a P/NR system* that focuses on growth rather than competition.
*P/NR system: A non-competitive grading system led by KAIST’s School of Transdisciplinary Studies. Instead of traditional letter grades (A/B/C/Fail), students receive Pass (P) or No Record (NR), with the latter not appearing as a failure and not affecting GPA.
Professor Jeong emphasized, “This experiment at KAIST represents a new educational paradigm that values questions over knowledge, culture over structure, and inquiry over competition. Students are bridging academic learning and real-world practice by addressing societal challenges through technology, which could lead to a fundamental shift in global higher education.”
His presentation provided an opportunity to spotlight how KAIST’s experimental approach to nurturing transdisciplinary talent is pointing to new directions for the global education community beyond Korea.
< Hyungjoon Jang, a student at the School of Transdisciplinary Studies>
The achievements of KAIST’s transdisciplinary education model are also reflected in students’ academic accomplishments. Hyungjoon Jang, a student at the School of Transdisciplinary Studies, participated in a collaborative study led by his mentor, Professor Jaekyung Kim in the Department of Mathematical Sciences, along with researchers from Chungnam National University and the Institute for Basic Science (IBS). Their groundbreaking analytical method enables the accurate estimation of inhibition constants using only a single inhibitor concentration. The paper was published in the prestigious journal Nature Communications in June, with Jang listed as co–first author.
Jang played a leading role throughout the research process by developing the experimental methodology, creating a software package to support the method, drafting the manuscript, and engaging in peer review. He also effectively communicated mathematical and statistical models to pharmaceutical experts by mastering presentation techniques and visual explanation strategies, thereby setting a strong example of interdisciplinary collaboration.
He emphasized that “the School of Transdisciplinary Studies’ mentor system allowed regular research feedback and the systematic acquisition of essential knowledge and analytical skills through courses in biochemistry and computational neuroscience.”
This example demonstrates how undergraduate students at the School of Transdisciplinary Studies can take leading roles in cutting-edge interdisciplinary research.
The school’s educational philosophy is also reflected in students’ practical actions. Inseo Jeong, a current student and founder of the startup MPAge Inc., made a meaningful donation to help establish a creative makerspace in the school.
<Inseo Jeong, founder of MPAG>
Inseo Jeong explained that the decision was made to express gratitude for the knowledge gained and the mentorship received from professors, saying that at the School of Transdisciplinary Studies, she learned not only how to solve problems with technology but also how to view society, and that learning has helped her grow. She added, “The deep understanding of humanity and the world emphasized by Professor Jaeseung Jeong will be a great asset not only to entrepreneurs but to all students pursuing diverse paths,” expressing support for her fellow students.
Inseo Jeong collaborated for over two years with Professor Hyunwook Ka of the School of Transdisciplinary Studies on software research for individuals with hearing impairments. After numerous algorithm designs and experimental iterations, their work, which considered the social scalability of technology, was presented at the world-renowned CSUN Assistive Technology Conference held at California State University, Northridge. The project has filed for a patent under KAIST’s name.
※ Presentation title: Evidence-Based Adaptive Transcription for Sign Language Users
KAIST is now working to complete the makerspace on the third floor of the Administrative Annex (N2) in Room 314 with a size of approximately 33 m2 during the summer. The makerspace is expected to serve as a hands-on, integrative learning environment where various ideas can be realized and implemented, playing a key role in fostering students’ creative problem-solving and integrative thinking skills.
KAIST President Kwang Hyung Lee stated, “The School of Transdisciplinary Studies is both an experimental ground and a practical field for overcoming the limitations of traditional education and nurturing global talents with creative problem-solving skills and integrative thinking, which are essential for the future.” He added, “KAIST will continue to lead efforts to cultivate question-asking, inquiry-driven, transdisciplinary talents and propose new paradigms for education and research.”
2025.07.24 View 395
KAIST School of Transdisciplinary Studies Is Driving Innovation in Korean Education
<(From Left) Professor Jaeseung Jeong, haed of the School of Transdiciplinary Studies, Dr, Albert Chau, Vice President of Hong Kong Baptist University>
KAIST (President Kwang Hyung Lee) announced on the 24th of July that its School of Transdisciplinary Studies has been consistently showcasing the results of its experiments and practices for educational innovation both domestically and abroad.
On June 27, Professor Jaeseung Jeong, head of the School of Transdisciplinary Studies, was invited to speak at the “Pacific Asia Summit on Transdisciplinary Education 2025 (PASTE 2025)” held at Hong Kong Baptist University. He presented the Korean model of transdisciplinary education under the title “The Philosophy and Achievements of the KAIST School of Transdisciplinary Studies.”
In his talk, Professor Jeong pointed out the limitations of conventional education systems that rely on answer-centered evaluation, perfectionism, and competitiveness, claiming that they hinder creativity and integrative thinking. He then introduced the philosophy and operational practices of the School of Transdisciplinary Studies, which was established in 2019 to overcome these issues.
Professor Jeong outlined five key principles that define the school's educational philosophy: ①a broad and integrative academic foundation, ②student-driven and customized education, ③creativity and execution, ④a sense of social responsibility and global citizenship, and ⑤learning driven by intrinsic motivation and curiosity. He explained that students are admitted without a declared major, allowed to design their own learning plans, and evaluated under a P/NR system* that focuses on growth rather than competition.
*P/NR system: A non-competitive grading system led by KAIST’s School of Transdisciplinary Studies. Instead of traditional letter grades (A/B/C/Fail), students receive Pass (P) or No Record (NR), with the latter not appearing as a failure and not affecting GPA.
Professor Jeong emphasized, “This experiment at KAIST represents a new educational paradigm that values questions over knowledge, culture over structure, and inquiry over competition. Students are bridging academic learning and real-world practice by addressing societal challenges through technology, which could lead to a fundamental shift in global higher education.”
His presentation provided an opportunity to spotlight how KAIST’s experimental approach to nurturing transdisciplinary talent is pointing to new directions for the global education community beyond Korea.
< Hyungjoon Jang, a student at the School of Transdisciplinary Studies>
The achievements of KAIST’s transdisciplinary education model are also reflected in students’ academic accomplishments. Hyungjoon Jang, a student at the School of Transdisciplinary Studies, participated in a collaborative study led by his mentor, Professor Jaekyung Kim in the Department of Mathematical Sciences, along with researchers from Chungnam National University and the Institute for Basic Science (IBS). Their groundbreaking analytical method enables the accurate estimation of inhibition constants using only a single inhibitor concentration. The paper was published in the prestigious journal Nature Communications in June, with Jang listed as co–first author.
Jang played a leading role throughout the research process by developing the experimental methodology, creating a software package to support the method, drafting the manuscript, and engaging in peer review. He also effectively communicated mathematical and statistical models to pharmaceutical experts by mastering presentation techniques and visual explanation strategies, thereby setting a strong example of interdisciplinary collaboration.
He emphasized that “the School of Transdisciplinary Studies’ mentor system allowed regular research feedback and the systematic acquisition of essential knowledge and analytical skills through courses in biochemistry and computational neuroscience.”
This example demonstrates how undergraduate students at the School of Transdisciplinary Studies can take leading roles in cutting-edge interdisciplinary research.
The school’s educational philosophy is also reflected in students’ practical actions. Inseo Jeong, a current student and founder of the startup MPAge Inc., made a meaningful donation to help establish a creative makerspace in the school.
<Inseo Jeong, founder of MPAG>
Inseo Jeong explained that the decision was made to express gratitude for the knowledge gained and the mentorship received from professors, saying that at the School of Transdisciplinary Studies, she learned not only how to solve problems with technology but also how to view society, and that learning has helped her grow. She added, “The deep understanding of humanity and the world emphasized by Professor Jaeseung Jeong will be a great asset not only to entrepreneurs but to all students pursuing diverse paths,” expressing support for her fellow students.
Inseo Jeong collaborated for over two years with Professor Hyunwook Ka of the School of Transdisciplinary Studies on software research for individuals with hearing impairments. After numerous algorithm designs and experimental iterations, their work, which considered the social scalability of technology, was presented at the world-renowned CSUN Assistive Technology Conference held at California State University, Northridge. The project has filed for a patent under KAIST’s name.
※ Presentation title: Evidence-Based Adaptive Transcription for Sign Language Users
KAIST is now working to complete the makerspace on the third floor of the Administrative Annex (N2) in Room 314 with a size of approximately 33 m2 during the summer. The makerspace is expected to serve as a hands-on, integrative learning environment where various ideas can be realized and implemented, playing a key role in fostering students’ creative problem-solving and integrative thinking skills.
KAIST President Kwang Hyung Lee stated, “The School of Transdisciplinary Studies is both an experimental ground and a practical field for overcoming the limitations of traditional education and nurturing global talents with creative problem-solving skills and integrative thinking, which are essential for the future.” He added, “KAIST will continue to lead efforts to cultivate question-asking, inquiry-driven, transdisciplinary talents and propose new paradigms for education and research.”
2025.07.24 View 395 -
 Why Do Plants Attack Themselves? The Secret of Genetic Conflict Revealed
<Professor Ji-Joon Song of the KAIST Department of Biological Sciences>
Plants, with their unique immune systems, sometimes launch 'autoimmune responses' by mistakenly identifying their own protein structures as pathogens. In particular, 'hybrid necrosis,' a phenomenon where descendant plants fail to grow healthily and perish after cross-breeding different varieties, has long been a difficult challenge for botanists and agricultural researchers. In response, an international research team has successfully elucidated the mechanism inducing plant autoimmune responses and proposed a novel strategy for cultivar improvement that can predict and avoid these reactions.
Professor Ji-Joon Song's research team at KAIST, in collaboration with teams from the National University of Singapore (NUS) and the University of Oxford, announced on the 21st of July that they have elucidated the structure and function of the 'DM3' protein complex, which triggers plant autoimmune responses, using cryo-electron microscopy (Cryo-EM) technology.
This research is drawing attention because it identifies defects in protein structure as the cause of hybrid necrosis, which occurs due to an abnormal reaction of immune receptors during cross-breeding between plant hybrids.
This protein (DM3) is originally an enzyme involved in the plant's immune response, but problems arise when the structure of the DM3 protein is damaged in a specific protein combination called 'DANGEROUS MIX (DM)'.
Notably, one variant of DM3, the 'DM3Col-0' variant, forms a stable complex with six proteins and is recognized as normal, thus not triggering an immune response. In contrast, another 'DM3Hh-0' variant has improper binding between its six proteins, causing the plant to recognize it as an 'abnormal state' and trigger an immune alarm, leading to autoimmunity.
The research team visualized this structure using atomic-resolution cryo-electron microscopy (Cryo-EM) and revealed that the immune-inducing ability is not due to the enzymatic function of the DM3 protein, but rather to 'differences in protein binding affinity.'
<Figure 1. Mechanism of Plant Autoimmunity Triggered by the Collapse of the DM3 Protein Complex>
This demonstrates that plants can initiate an immune response by recognizing not only 'external pathogens' but also 'internal protein structures' when they undergo abnormal changes, treating them as if they were pathogens.
The study shows how sensitively the plant immune system changes and triggers autoimmune responses when genes are mixed and protein structures change during the cross-breeding of different plant varieties. It significantly advanced the understanding of genetic incompatibility that can occur during natural cross-breeding and cultivar improvement processes.
Dr. Gijeong Kim, the co-first author, stated, "Through international research collaboration, we presented a new perspective on understanding the plant immune system by leveraging the autoimmune phenomenon, completing a high-quality study that encompasses structural biochemistry, genetics, and cell biological experiments."
Professor Ji-Joon Song of the KAIST Department of Biological Sciences, who led the research, said, "The fact that the immune system can detect not only external pathogens but also structural abnormalities in its own proteins will set a new standard for plant biotechnology and crop breeding strategies. Cryo-electron microscopy-based structural analysis will be an important tool for understanding the essence of gene interactions."
This research, with Professor Ji-Joon Song and Professor Eunyoung Chae of the University of Oxford as co-corresponding authors, Dr. Gijeong Kim (currently a postdoctoral researcher at the University of Zurich) and Dr. Wei-Lin Wan of the National University of Singapore as co-first authors, and Ph.D candidate Nayun Kim, as the second author, was published on July 17th in Molecular Cell, a sister journal of the international academic journal Cell.
This research was supported by the KAIST Grand Challenge 30 project.
Article Title: Structural determinants of DANGEROUS MIX 3, an alpha/beta hydrolase that triggers NLR-mediated genetic incompatibility in plants DOI: https://doi.org/10.1016/j.molcel.2025.06.021
2025.07.21 View 537
Why Do Plants Attack Themselves? The Secret of Genetic Conflict Revealed
<Professor Ji-Joon Song of the KAIST Department of Biological Sciences>
Plants, with their unique immune systems, sometimes launch 'autoimmune responses' by mistakenly identifying their own protein structures as pathogens. In particular, 'hybrid necrosis,' a phenomenon where descendant plants fail to grow healthily and perish after cross-breeding different varieties, has long been a difficult challenge for botanists and agricultural researchers. In response, an international research team has successfully elucidated the mechanism inducing plant autoimmune responses and proposed a novel strategy for cultivar improvement that can predict and avoid these reactions.
Professor Ji-Joon Song's research team at KAIST, in collaboration with teams from the National University of Singapore (NUS) and the University of Oxford, announced on the 21st of July that they have elucidated the structure and function of the 'DM3' protein complex, which triggers plant autoimmune responses, using cryo-electron microscopy (Cryo-EM) technology.
This research is drawing attention because it identifies defects in protein structure as the cause of hybrid necrosis, which occurs due to an abnormal reaction of immune receptors during cross-breeding between plant hybrids.
This protein (DM3) is originally an enzyme involved in the plant's immune response, but problems arise when the structure of the DM3 protein is damaged in a specific protein combination called 'DANGEROUS MIX (DM)'.
Notably, one variant of DM3, the 'DM3Col-0' variant, forms a stable complex with six proteins and is recognized as normal, thus not triggering an immune response. In contrast, another 'DM3Hh-0' variant has improper binding between its six proteins, causing the plant to recognize it as an 'abnormal state' and trigger an immune alarm, leading to autoimmunity.
The research team visualized this structure using atomic-resolution cryo-electron microscopy (Cryo-EM) and revealed that the immune-inducing ability is not due to the enzymatic function of the DM3 protein, but rather to 'differences in protein binding affinity.'
<Figure 1. Mechanism of Plant Autoimmunity Triggered by the Collapse of the DM3 Protein Complex>
This demonstrates that plants can initiate an immune response by recognizing not only 'external pathogens' but also 'internal protein structures' when they undergo abnormal changes, treating them as if they were pathogens.
The study shows how sensitively the plant immune system changes and triggers autoimmune responses when genes are mixed and protein structures change during the cross-breeding of different plant varieties. It significantly advanced the understanding of genetic incompatibility that can occur during natural cross-breeding and cultivar improvement processes.
Dr. Gijeong Kim, the co-first author, stated, "Through international research collaboration, we presented a new perspective on understanding the plant immune system by leveraging the autoimmune phenomenon, completing a high-quality study that encompasses structural biochemistry, genetics, and cell biological experiments."
Professor Ji-Joon Song of the KAIST Department of Biological Sciences, who led the research, said, "The fact that the immune system can detect not only external pathogens but also structural abnormalities in its own proteins will set a new standard for plant biotechnology and crop breeding strategies. Cryo-electron microscopy-based structural analysis will be an important tool for understanding the essence of gene interactions."
This research, with Professor Ji-Joon Song and Professor Eunyoung Chae of the University of Oxford as co-corresponding authors, Dr. Gijeong Kim (currently a postdoctoral researcher at the University of Zurich) and Dr. Wei-Lin Wan of the National University of Singapore as co-first authors, and Ph.D candidate Nayun Kim, as the second author, was published on July 17th in Molecular Cell, a sister journal of the international academic journal Cell.
This research was supported by the KAIST Grand Challenge 30 project.
Article Title: Structural determinants of DANGEROUS MIX 3, an alpha/beta hydrolase that triggers NLR-mediated genetic incompatibility in plants DOI: https://doi.org/10.1016/j.molcel.2025.06.021
2025.07.21 View 537 -
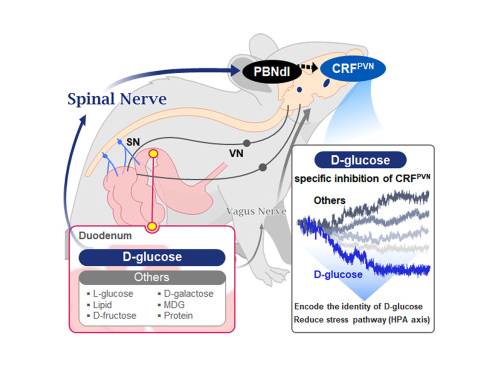 KAIST Shows That the Brain Can Distinguish Glucose: Clues to Treat Obesity and Diabetes
<(From left)Prof. Greg S.B Suh, Dr. Jieun Kim, Dr. Shinhye Kim, Researcher Wongyo Jeong)
“How does our brain distinguish glucose from the many nutrients absorbed in the gut?” Starting with this question, a KAIST research team has demonstrated that the brain can selectively recognize specific nutrients—particularly glucose—beyond simply detecting total calorie content. This study is expected to offer a new paradigm for appetite control and the treatment of metabolic diseases.
On the 9th, KAIST (President Kwang Hyung Lee) announced that Professor Greg S.B. Suh’s team in the Department of Biological Sciences, in collaboration with Professor Young-Gyun Park’s team (BarNeuro), Professor Seung-Hee Lee’s team (Department of Biological Sciences), and the Albert Einstein College of Medicine in New York, had identified the existence of a gut-brain circuit that allows animals in a hungry state to selectively detect and prefer glucose in the gut.
Organisms derive energy from various nutrients including sugars, proteins, and fats. Previous studies have shown that total caloric information in the gut suppresses hunger neurons in the hypothalamus to regulate appetite. However, the existence of a gut-brain circuit that specifically responds to glucose and corresponding brain cells had not been demonstrated until now.
In this study, the team successfully identified a “gut-brain circuit” that senses glucose—essential for brain function—and regulates food intake behavior for required nutrients.
They further proved, for the first time, that this circuit responds within seconds to not only hunger or external stimuli but also to specific caloric nutrients directly introduced into the small intestine, particularly D-glucose, through the activity of “CRF neurons*” in the brain’s hypothalamus.
*CRF neurons: These neurons secrete corticotropin-releasing factor (CRF) in the hypothalamus and are central to the hypothalamic-pituitary-adrenal (HPA) axis, the body’s core physiological system for responding to stress. CRF neurons are known to regulate neuroendocrine balance in response to stress stimuli.
Using optogenetics to precisely track neural activity in real time, the researchers injected various nutrients—D-glucose, L-glucose, amino acids, and fats—directly into the small intestines of mice and observed the results.
They discovered that among the CRF neurons located in the paraventricular nucleus (PVN)* of the hypothalamus, only those specific to D-glucose showed selective responses. These neurons did not respond—or showed inverse reactions—to other sugars or to proteins and fats. This is the first demonstration that single neurons in the brain can guide nutrient-specific responses depending on gut nutrient influx.
*PVN (Paraventricular Nucleus): A key nucleus within the hypothalamus responsible for maintaining bodily homeostasis.
The team also revealed that glucose-sensing signals in the small intestine are transmitted via the spinal cord to the dorsolateral parabrachial nucleus (PBNdl) of the brain, and from there to CRF neurons in the PVN. In contrast, signals for amino acids and fats are transmitted to the brain through the vagus nerve, a different pathway.
In optogenetic inhibition experiments, suppressing CRF neurons in fasting mice eliminated their preference for glucose, proving that this circuit is essential for glucose-specific nutrient preference.
This study was inspired by Professor Suh’s earlier research at NYU using fruit flies, where he identified “DH44 neurons” that selectively detect glucose and sugar in the gut. Based on the hypothesis that hypothalamic neurons in mammals would show similar functional responses to glucose, the current study was launched.
To test this hypothesis, Dr. Jineun Kim (KAIST Ph.D. graduate, now at Caltech) demonstrated during her doctoral research that hungry mice preferred glucose among various intragastrically infused nutrients and that CRF neurons exhibited rapid and specific responses.
Along with Wongyo Jung (KAIST B.S. graduate, now Ph.D. student at Caltech), they modeled and experimentally confirmed the critical role of CRF neurons. Dr. Shinhye Kim, through collaboration, revealed that specific spinal neurons play a key role in conveying intestinal nutrient information to the brain.
Dr. Jineun Kim and Dr. Shinhye Kim said, “This study started from a simple but fundamental question—‘How does the brain distinguish glucose from various nutrients absorbed in the gut?’ We have shown that spinal-based gut-brain circuits play a central role in energy metabolism and homeostasis by transmitting specific gut nutrient signals to the brain.”
Professor Suh added, “By identifying a gut-brain pathway specialized for glucose, this research offers a new therapeutic target for metabolic diseases such as obesity and diabetes. Our future research will explore similar circuits for sensing other essential nutrients like amino acids and fats and their interaction mechanisms.”
Ph.D. student Jineun Kim, Dr. Shinhye Kim, and student Wongyo Jung (co-first authors) contributed to this study, which was published online in the international journal Neuron on June 20, 2025.
※ Paper Title: Encoding the glucose identity by discrete hypothalamic neurons via the gut-brain axis ※ DOI: https://doi.org/10.1016/j.neuron.2025.05.024
This study was supported by the Samsung Science & Technology Foundation, the National Research Foundation of Korea (NRF) Leader Research Program, the POSCO Cheongam Science Fellowship, the Asan Foundation Biomedical Science Scholarship, the Institute for Basic Science (IBS), and the KAIST KAIX program.
2025.07.09 View 773
KAIST Shows That the Brain Can Distinguish Glucose: Clues to Treat Obesity and Diabetes
<(From left)Prof. Greg S.B Suh, Dr. Jieun Kim, Dr. Shinhye Kim, Researcher Wongyo Jeong)
“How does our brain distinguish glucose from the many nutrients absorbed in the gut?” Starting with this question, a KAIST research team has demonstrated that the brain can selectively recognize specific nutrients—particularly glucose—beyond simply detecting total calorie content. This study is expected to offer a new paradigm for appetite control and the treatment of metabolic diseases.
On the 9th, KAIST (President Kwang Hyung Lee) announced that Professor Greg S.B. Suh’s team in the Department of Biological Sciences, in collaboration with Professor Young-Gyun Park’s team (BarNeuro), Professor Seung-Hee Lee’s team (Department of Biological Sciences), and the Albert Einstein College of Medicine in New York, had identified the existence of a gut-brain circuit that allows animals in a hungry state to selectively detect and prefer glucose in the gut.
Organisms derive energy from various nutrients including sugars, proteins, and fats. Previous studies have shown that total caloric information in the gut suppresses hunger neurons in the hypothalamus to regulate appetite. However, the existence of a gut-brain circuit that specifically responds to glucose and corresponding brain cells had not been demonstrated until now.
In this study, the team successfully identified a “gut-brain circuit” that senses glucose—essential for brain function—and regulates food intake behavior for required nutrients.
They further proved, for the first time, that this circuit responds within seconds to not only hunger or external stimuli but also to specific caloric nutrients directly introduced into the small intestine, particularly D-glucose, through the activity of “CRF neurons*” in the brain’s hypothalamus.
*CRF neurons: These neurons secrete corticotropin-releasing factor (CRF) in the hypothalamus and are central to the hypothalamic-pituitary-adrenal (HPA) axis, the body’s core physiological system for responding to stress. CRF neurons are known to regulate neuroendocrine balance in response to stress stimuli.
Using optogenetics to precisely track neural activity in real time, the researchers injected various nutrients—D-glucose, L-glucose, amino acids, and fats—directly into the small intestines of mice and observed the results.
They discovered that among the CRF neurons located in the paraventricular nucleus (PVN)* of the hypothalamus, only those specific to D-glucose showed selective responses. These neurons did not respond—or showed inverse reactions—to other sugars or to proteins and fats. This is the first demonstration that single neurons in the brain can guide nutrient-specific responses depending on gut nutrient influx.
*PVN (Paraventricular Nucleus): A key nucleus within the hypothalamus responsible for maintaining bodily homeostasis.
The team also revealed that glucose-sensing signals in the small intestine are transmitted via the spinal cord to the dorsolateral parabrachial nucleus (PBNdl) of the brain, and from there to CRF neurons in the PVN. In contrast, signals for amino acids and fats are transmitted to the brain through the vagus nerve, a different pathway.
In optogenetic inhibition experiments, suppressing CRF neurons in fasting mice eliminated their preference for glucose, proving that this circuit is essential for glucose-specific nutrient preference.
This study was inspired by Professor Suh’s earlier research at NYU using fruit flies, where he identified “DH44 neurons” that selectively detect glucose and sugar in the gut. Based on the hypothesis that hypothalamic neurons in mammals would show similar functional responses to glucose, the current study was launched.
To test this hypothesis, Dr. Jineun Kim (KAIST Ph.D. graduate, now at Caltech) demonstrated during her doctoral research that hungry mice preferred glucose among various intragastrically infused nutrients and that CRF neurons exhibited rapid and specific responses.
Along with Wongyo Jung (KAIST B.S. graduate, now Ph.D. student at Caltech), they modeled and experimentally confirmed the critical role of CRF neurons. Dr. Shinhye Kim, through collaboration, revealed that specific spinal neurons play a key role in conveying intestinal nutrient information to the brain.
Dr. Jineun Kim and Dr. Shinhye Kim said, “This study started from a simple but fundamental question—‘How does the brain distinguish glucose from various nutrients absorbed in the gut?’ We have shown that spinal-based gut-brain circuits play a central role in energy metabolism and homeostasis by transmitting specific gut nutrient signals to the brain.”
Professor Suh added, “By identifying a gut-brain pathway specialized for glucose, this research offers a new therapeutic target for metabolic diseases such as obesity and diabetes. Our future research will explore similar circuits for sensing other essential nutrients like amino acids and fats and their interaction mechanisms.”
Ph.D. student Jineun Kim, Dr. Shinhye Kim, and student Wongyo Jung (co-first authors) contributed to this study, which was published online in the international journal Neuron on June 20, 2025.
※ Paper Title: Encoding the glucose identity by discrete hypothalamic neurons via the gut-brain axis ※ DOI: https://doi.org/10.1016/j.neuron.2025.05.024
This study was supported by the Samsung Science & Technology Foundation, the National Research Foundation of Korea (NRF) Leader Research Program, the POSCO Cheongam Science Fellowship, the Asan Foundation Biomedical Science Scholarship, the Institute for Basic Science (IBS), and the KAIST KAIX program.
2025.07.09 View 773 -
 KAIST Develops Novel Candidiasis Treatment Overcoming Side Effects and Resistance
<(From left) Ph. D Candidate Ju Yeon Chung, Prof.Hyun Jung Chung, Ph.D candidate Seungju Yang, Ph.D candidate Ayoung Park, Dr. Yoon-Kyoung Hong from Asan Medical Center, Prof. Yong Pil Chong, Dr. Eunhee Jeon>
Candida, a type of fungus, which can spread throughout the body via the bloodstream, leading to organ damage and sepsis. Recently, the incidence of candidiasis has surged due to the increase in immunosuppressive therapies, medical implants, and transplantation. Korean researchers have successfully developed a next-generation treatment that, unlike existing antifungals, selectively acts only on Candida, achieving both high therapeutic efficacy and low side effects simultaneously.
KAIST (President Kwang Hyung Lee) announced on the 8th that a research team led by Professor Hyun-Jung Chung of the Department of Biological Sciences, in collaboration with Professor Yong Pil Jeong's team at Asan Medical Center, developed a gene-based nanotherapy (FTNx) that simultaneously inhibits two key enzymes in the Candida cell wall.
Current antifungal drugs for Candida have low target selectivity, which can affect human cells. Furthermore, their therapeutic efficacy is gradually decreasing due to the emergence of new resistant strains. Especially for immunocompromised patients, the infection progresses rapidly and has a poor prognosis, making the development of new treatments to overcome the limitations of existing therapies urgent.
The developed treatment can be administered systemically, and by combining gene suppression technology with nanomaterial technology, it effectively overcomes the structural limitations of existing compound-based drugs and successfully achieves selective treatment against only Candida.
The research team created a gold nanoparticle-based complex loaded with short DNA fragments called antisense oligonucleotides (ASO), which simultaneously target two crucial enzymes—β-1,3-glucan synthase (FKS1) and chitin synthase (CHS3)—important for forming the cell wall of the Candida fungus.
By applying a surface coating technology that binds to a specific glycolipid structure (a structure combining sugar and fat) on the Candida cell wall, a targeted delivery device was implemented. This successfully achieved a precise targeting effect, ensuring the complex is not delivered to human cells at all but acts selectively only on Candida.
<Figure 1: Overview of antifungal therapy design and experimental approach>
This complex, after entering Candida cells, cleaves the mRNA produced by the FKS1 and CHS3 genes, thereby inhibiting translation and simultaneously blocking the synthesis of cell wall components β-1,3-glucan and chitin. As a result, the
Candida cell wall loses its structural stability and collapses, suppressing bacterial survival and proliferation.
In fact, experiments using a systemic candidiasis model in mice confirmed the therapeutic effect: a significant reduction in
Candida count in the organs, normalization of immune responses, and a notable increase in survival rates were observed in the treated group.
Professor Hyun-Jung Chung, who led the research, stated, "This study presents a method to overcome the issues of human toxicity and drug resistance spread with existing treatments, marking an important turning point by demonstrating the applicability of gene therapy for systemic infections". She added, "We plan to continue research on optimizing administration methods and verifying toxicity for future clinical application."
This research involved Ju Yeon Chung and Yoon-Kyoung Hong as co-first authors , and was published in the international journal 'Nature Communications' on July 1st.
Paper Title: Effective treatment of systemic candidiasis by synergistic targeting of cell wall synthesis
DOI: 10.1038/s41467-025-60684-7
This research was supported by the Ministry of Health and Welfare and the National Research Foundation of Korea.
2025.07.08 View 722
KAIST Develops Novel Candidiasis Treatment Overcoming Side Effects and Resistance
<(From left) Ph. D Candidate Ju Yeon Chung, Prof.Hyun Jung Chung, Ph.D candidate Seungju Yang, Ph.D candidate Ayoung Park, Dr. Yoon-Kyoung Hong from Asan Medical Center, Prof. Yong Pil Chong, Dr. Eunhee Jeon>
Candida, a type of fungus, which can spread throughout the body via the bloodstream, leading to organ damage and sepsis. Recently, the incidence of candidiasis has surged due to the increase in immunosuppressive therapies, medical implants, and transplantation. Korean researchers have successfully developed a next-generation treatment that, unlike existing antifungals, selectively acts only on Candida, achieving both high therapeutic efficacy and low side effects simultaneously.
KAIST (President Kwang Hyung Lee) announced on the 8th that a research team led by Professor Hyun-Jung Chung of the Department of Biological Sciences, in collaboration with Professor Yong Pil Jeong's team at Asan Medical Center, developed a gene-based nanotherapy (FTNx) that simultaneously inhibits two key enzymes in the Candida cell wall.
Current antifungal drugs for Candida have low target selectivity, which can affect human cells. Furthermore, their therapeutic efficacy is gradually decreasing due to the emergence of new resistant strains. Especially for immunocompromised patients, the infection progresses rapidly and has a poor prognosis, making the development of new treatments to overcome the limitations of existing therapies urgent.
The developed treatment can be administered systemically, and by combining gene suppression technology with nanomaterial technology, it effectively overcomes the structural limitations of existing compound-based drugs and successfully achieves selective treatment against only Candida.
The research team created a gold nanoparticle-based complex loaded with short DNA fragments called antisense oligonucleotides (ASO), which simultaneously target two crucial enzymes—β-1,3-glucan synthase (FKS1) and chitin synthase (CHS3)—important for forming the cell wall of the Candida fungus.
By applying a surface coating technology that binds to a specific glycolipid structure (a structure combining sugar and fat) on the Candida cell wall, a targeted delivery device was implemented. This successfully achieved a precise targeting effect, ensuring the complex is not delivered to human cells at all but acts selectively only on Candida.
<Figure 1: Overview of antifungal therapy design and experimental approach>
This complex, after entering Candida cells, cleaves the mRNA produced by the FKS1 and CHS3 genes, thereby inhibiting translation and simultaneously blocking the synthesis of cell wall components β-1,3-glucan and chitin. As a result, the
Candida cell wall loses its structural stability and collapses, suppressing bacterial survival and proliferation.
In fact, experiments using a systemic candidiasis model in mice confirmed the therapeutic effect: a significant reduction in
Candida count in the organs, normalization of immune responses, and a notable increase in survival rates were observed in the treated group.
Professor Hyun-Jung Chung, who led the research, stated, "This study presents a method to overcome the issues of human toxicity and drug resistance spread with existing treatments, marking an important turning point by demonstrating the applicability of gene therapy for systemic infections". She added, "We plan to continue research on optimizing administration methods and verifying toxicity for future clinical application."
This research involved Ju Yeon Chung and Yoon-Kyoung Hong as co-first authors , and was published in the international journal 'Nature Communications' on July 1st.
Paper Title: Effective treatment of systemic candidiasis by synergistic targeting of cell wall synthesis
DOI: 10.1038/s41467-025-60684-7
This research was supported by the Ministry of Health and Welfare and the National Research Foundation of Korea.
2025.07.08 View 722 -
 KAIST Presents a Breakthrough in Overcoming Drug Resistance in Cancer – Hope for Treating Intractable Diseases like Diabetes
<(From the left) Prof. Hyun Uk Kim, Ph.D candiate Hae Deok Jung, Ph.D candidate Jina Lim, Prof.Yoosik Kim from the Department of Chemical and Biomolecular Engineering>
One of the biggest obstacles in cancer treatment is drug resistance in cancer cells. Conventional efforts have focused on identifying new drug targets to eliminate these resistant cells, but such approaches can often lead to even stronger resistance. Now, researchers at KAIST have developed a computational framework to predict key metabolic genes that can re-sensitize resistant cancer cells to treatment. This technique holds promise not only for a variety of cancer therapies but also for treating metabolic diseases such as diabetes.
On the 7th of July, KAIST (President Kwang Hyung Lee) announced that a research team led by Professors Hyun Uk Kim and Yoosik Kim from the Department of Chemical and Biomolecular Engineering had developed a computational framework that predicts metabolic gene targets to re-sensitize drug-resistant breast cancer cells. This was achieved using a metabolic network model capable of simulating human metabolism.
Focusing on metabolic alterations—key characteristics in the formation of drug resistance—the researchers developed a metabolism-based approach to identify gene targets that could enhance drug responsiveness by regulating the metabolism of drug-resistant breast cancer cells.
< Computational framework that can identify metabolic gene targets to revert the metabolic state of the drug-resistant cells to that of the drug-sensitive parental cells>
The team first constructed cell-specific metabolic network models by integrating proteomic data obtained from two different types of drug-resistant MCF7 breast cancer cell lines: one resistant to doxorubicin and the other to paclitaxel. They then performed gene knockout simulations* on all of the metabolic genes and analyzed the results.
*Gene knockout simulation: A computational method to predict changes in a biological network by virtually removing specific genes.
As a result, they discovered that suppressing certain genes could make previously resistant cancer cells responsive to anticancer drugs again. Specifically, they identified GOT1 as a target in doxorubicin-resistant cells, GPI in paclitaxel-resistant cells, and SLC1A5 as a common target for both drugs.
The predictions were experimentally validated by suppressing proteins encoded by these genes, which led to the re-sensitization of the drug-resistant cancer cells.
Furthermore, consistent re-sensitization effects were also observed when the same proteins were inhibited in other types of breast cancer cells that had developed resistance to the same drugs.
Professor Yoosik Kim remarked, “Cellular metabolism plays a crucial role in various intractable diseases including infectious and degenerative conditions. This new technology, which predicts metabolic regulation switches, can serve as a foundational tool not only for treating drug-resistant breast cancer but also for a wide range of diseases that currently lack effective therapies.”
Professor Hyun Uk Kim, who led the study, emphasized, “The significance of this research lies in our ability to accurately predict key metabolic genes that can make resistant cancer cells responsive to treatment again—using only computer simulations and minimal experimental data. This framework can be widely applied to discover new therapeutic targets in various cancers and metabolic diseases.”
The study, in which Ph.D. candidates JinA Lim and Hae Deok Jung from KAIST participated as co-first authors, was published online on June 25 in Proceedings of the National Academy of Sciences (PNAS), a leading multidisciplinary journal that covers top-tier research in life sciences, physics, engineering, and social sciences.
※ Title: Genome-scale knockout simulation and clustering analysis of drug-resistant breast cancer cells reveal drug sensitization targets ※ DOI: https://doi.org/10.1073/pnas.2425384122 ※ Authors: JinA Lim (KAIST, co-first author), Hae Deok Jung (KAIST, co-first author), Han Suk Ryu (Seoul National University Hospital, corresponding author), Yoosik Kim (KAIST, corresponding author), Hyun Uk Kim (KAIST, corresponding author), and five others.
This research was supported by the Ministry of Science and ICT through the National Research Foundation of Korea, and the Electronics and Telecommunications Research Institute (ETRI).
2025.07.08 View 1188
KAIST Presents a Breakthrough in Overcoming Drug Resistance in Cancer – Hope for Treating Intractable Diseases like Diabetes
<(From the left) Prof. Hyun Uk Kim, Ph.D candiate Hae Deok Jung, Ph.D candidate Jina Lim, Prof.Yoosik Kim from the Department of Chemical and Biomolecular Engineering>
One of the biggest obstacles in cancer treatment is drug resistance in cancer cells. Conventional efforts have focused on identifying new drug targets to eliminate these resistant cells, but such approaches can often lead to even stronger resistance. Now, researchers at KAIST have developed a computational framework to predict key metabolic genes that can re-sensitize resistant cancer cells to treatment. This technique holds promise not only for a variety of cancer therapies but also for treating metabolic diseases such as diabetes.
On the 7th of July, KAIST (President Kwang Hyung Lee) announced that a research team led by Professors Hyun Uk Kim and Yoosik Kim from the Department of Chemical and Biomolecular Engineering had developed a computational framework that predicts metabolic gene targets to re-sensitize drug-resistant breast cancer cells. This was achieved using a metabolic network model capable of simulating human metabolism.
Focusing on metabolic alterations—key characteristics in the formation of drug resistance—the researchers developed a metabolism-based approach to identify gene targets that could enhance drug responsiveness by regulating the metabolism of drug-resistant breast cancer cells.
< Computational framework that can identify metabolic gene targets to revert the metabolic state of the drug-resistant cells to that of the drug-sensitive parental cells>
The team first constructed cell-specific metabolic network models by integrating proteomic data obtained from two different types of drug-resistant MCF7 breast cancer cell lines: one resistant to doxorubicin and the other to paclitaxel. They then performed gene knockout simulations* on all of the metabolic genes and analyzed the results.
*Gene knockout simulation: A computational method to predict changes in a biological network by virtually removing specific genes.
As a result, they discovered that suppressing certain genes could make previously resistant cancer cells responsive to anticancer drugs again. Specifically, they identified GOT1 as a target in doxorubicin-resistant cells, GPI in paclitaxel-resistant cells, and SLC1A5 as a common target for both drugs.
The predictions were experimentally validated by suppressing proteins encoded by these genes, which led to the re-sensitization of the drug-resistant cancer cells.
Furthermore, consistent re-sensitization effects were also observed when the same proteins were inhibited in other types of breast cancer cells that had developed resistance to the same drugs.
Professor Yoosik Kim remarked, “Cellular metabolism plays a crucial role in various intractable diseases including infectious and degenerative conditions. This new technology, which predicts metabolic regulation switches, can serve as a foundational tool not only for treating drug-resistant breast cancer but also for a wide range of diseases that currently lack effective therapies.”
Professor Hyun Uk Kim, who led the study, emphasized, “The significance of this research lies in our ability to accurately predict key metabolic genes that can make resistant cancer cells responsive to treatment again—using only computer simulations and minimal experimental data. This framework can be widely applied to discover new therapeutic targets in various cancers and metabolic diseases.”
The study, in which Ph.D. candidates JinA Lim and Hae Deok Jung from KAIST participated as co-first authors, was published online on June 25 in Proceedings of the National Academy of Sciences (PNAS), a leading multidisciplinary journal that covers top-tier research in life sciences, physics, engineering, and social sciences.
※ Title: Genome-scale knockout simulation and clustering analysis of drug-resistant breast cancer cells reveal drug sensitization targets ※ DOI: https://doi.org/10.1073/pnas.2425384122 ※ Authors: JinA Lim (KAIST, co-first author), Hae Deok Jung (KAIST, co-first author), Han Suk Ryu (Seoul National University Hospital, corresponding author), Yoosik Kim (KAIST, corresponding author), Hyun Uk Kim (KAIST, corresponding author), and five others.
This research was supported by the Ministry of Science and ICT through the National Research Foundation of Korea, and the Electronics and Telecommunications Research Institute (ETRI).
2025.07.08 View 1188