Seung-Jae+V.+Lee
-
 A Genetic Change for Achieving a Long and Healthy Life
Researchers identified a single amino acid change in the tumor suppressor protein in PTEN that extends healthy periods while maintaining longevity
Living a long, healthy life is everyone’s wish, but it is not an easy one to achieve. Many aging studies are developing strategies to increase health spans, the period of life spent with good health, without chronic diseases and disabilities. Researchers at KAIST presented new insights for improving the health span by just regulating the activity of a protein.
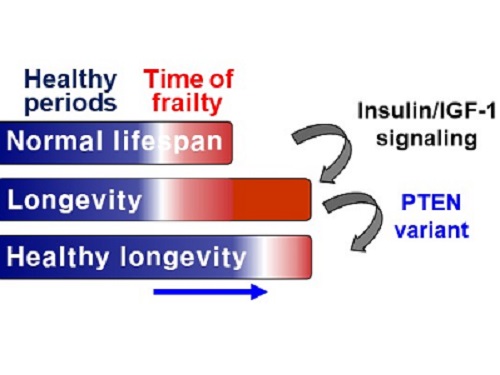
A research group under Professor Seung-Jae V. Lee from the Department of Biological Sciences identified a single amino acid change in the tumor suppressor protein phosphatase and tensin homolog (PTEN) that dramatically extends healthy periods while maintaining longevity. This study highlights the importance of the well-conserved tumor suppressor protein PTEN in health span regulation, which can be targeted to develop therapies for promoting healthy longevity in humans. The research was published in Nature Communications on September 24, 2021.
Insulin and insulin-like growth factor-1 (IGF-1) signaling (IIS) is one of the evolutionarily conserved aging-modulatory pathways present in life forms ranging from tiny roundworms to humans. The proper reduction of IIS leads to longevity in animals but often causes defects in multiple health parameters including impaired motility, reproduction, and growth.
The research team found that a specific amino acid change in the PTEN protein improves health status while retaining the longevity conferred by reduced IIS. They used the roundworm C. elegans, an excellent model animal that has been widely used for aging research, mainly because of its very short normal lifespan of about two to three weeks. The PTEN protein is a phosphatase that removes phosphate from lipids as well as proteins. Interestingly, the newly identified amino acid change delicately recalibrated the IIS by partially maintaining protein phosphatase activity while reducing lipid phosphatase activity.
As a result, the amino acid change in the PTEN protein maintained the activity of the longevity-promoting transcription factor Forkhead Box O (FOXO) protein while restricting the detrimental upregulation of another transcription factor, NRF2, leading to long and healthy life in animals with reduced IIS.
Professor Lee said, “Our study raises the exciting possibility of simultaneously promoting longevity and health in humans by slightly tweaking the activity of one protein, PTEN.”
This work was supported by the MInistry of Science and ICT through the National Research Foundation of Korea.
-Publication:Hae-Eun H. Park, Wooseon Hwang, Seokjin Ham, Eunah Kim, Ozlem Altintas, Sangsoon Park, Heehwa G. Son, Yujin Lee, Dongyeop Lee, Won Do Heo, and Seung-Jae V. Lee. 2021. “A PTEN variant uncouples longevity from impaired fitness in Caenorhabditis elegans with reduced insulin/IGF-1 signaling,” Nature Communications, 12(1), 5631. (https://doi.org/10.1038/s41467-021-25920-w)
-ProfileProfessor Seung-Jae V. LeeMolecular Genetics of Aging LaboratoryDepartment of Biological Sciences KAIST
2021.11.19 View 9726
A Genetic Change for Achieving a Long and Healthy Life
Researchers identified a single amino acid change in the tumor suppressor protein in PTEN that extends healthy periods while maintaining longevity
Living a long, healthy life is everyone’s wish, but it is not an easy one to achieve. Many aging studies are developing strategies to increase health spans, the period of life spent with good health, without chronic diseases and disabilities. Researchers at KAIST presented new insights for improving the health span by just regulating the activity of a protein.
A research group under Professor Seung-Jae V. Lee from the Department of Biological Sciences identified a single amino acid change in the tumor suppressor protein phosphatase and tensin homolog (PTEN) that dramatically extends healthy periods while maintaining longevity. This study highlights the importance of the well-conserved tumor suppressor protein PTEN in health span regulation, which can be targeted to develop therapies for promoting healthy longevity in humans. The research was published in Nature Communications on September 24, 2021.
Insulin and insulin-like growth factor-1 (IGF-1) signaling (IIS) is one of the evolutionarily conserved aging-modulatory pathways present in life forms ranging from tiny roundworms to humans. The proper reduction of IIS leads to longevity in animals but often causes defects in multiple health parameters including impaired motility, reproduction, and growth.
The research team found that a specific amino acid change in the PTEN protein improves health status while retaining the longevity conferred by reduced IIS. They used the roundworm C. elegans, an excellent model animal that has been widely used for aging research, mainly because of its very short normal lifespan of about two to three weeks. The PTEN protein is a phosphatase that removes phosphate from lipids as well as proteins. Interestingly, the newly identified amino acid change delicately recalibrated the IIS by partially maintaining protein phosphatase activity while reducing lipid phosphatase activity.
As a result, the amino acid change in the PTEN protein maintained the activity of the longevity-promoting transcription factor Forkhead Box O (FOXO) protein while restricting the detrimental upregulation of another transcription factor, NRF2, leading to long and healthy life in animals with reduced IIS.
Professor Lee said, “Our study raises the exciting possibility of simultaneously promoting longevity and health in humans by slightly tweaking the activity of one protein, PTEN.”
This work was supported by the MInistry of Science and ICT through the National Research Foundation of Korea.
-Publication:Hae-Eun H. Park, Wooseon Hwang, Seokjin Ham, Eunah Kim, Ozlem Altintas, Sangsoon Park, Heehwa G. Son, Yujin Lee, Dongyeop Lee, Won Do Heo, and Seung-Jae V. Lee. 2021. “A PTEN variant uncouples longevity from impaired fitness in Caenorhabditis elegans with reduced insulin/IGF-1 signaling,” Nature Communications, 12(1), 5631. (https://doi.org/10.1038/s41467-021-25920-w)
-ProfileProfessor Seung-Jae V. LeeMolecular Genetics of Aging LaboratoryDepartment of Biological Sciences KAIST
2021.11.19 View 9726 -
 Tinkering with Roundworm Proteins Offers Hope for Anti-aging Drugs
- The somatic nuclear protein kinase VRK-1 increases the worm’s lifespan through AMPK activation, and this mechanism can be applied to promoting human longevity, the study reveals. -
KAIST researchers have been able to dial up and down creatures’ lifespans by altering the activity of proteins found in roundworm cells that tell them to convert sugar into energy when their cellular energy is running low. Humans also have these proteins, offering up the intriguing possibilities for developing longevity-promoting drugs. These new findings were published on July 1 in Science Advances.
The roundworm Caenorhabditis elegans (C. elegans), a millimeter-long nematode commonly used in lab testing, enjoyed a boost in its lifespan when researchers tinkered with a couple of proteins involved in monitoring the energy use by its cells.
The proteins VRK-1 and AMPK work in tandem in roundworm cells, with the former telling the latter to get to work by sticking a phosphate molecule, composed of one phosphorus and four oxygen atoms, on it. In turn, AMPK’s role is to monitor energy levels in cells, when cellular energy is running low. In essence, VRK-1 regulates AMPK, and AMPK regulates the cellular energy status.
Using a range of different biological research tools, including introducing foreign genes into the worm, a group of researchers led by Professor Seung-Jae V. Lee from the Department of Biological Sciences at KAIST were able to dial up and down the activity of the gene that tells cells to produce the VRK-1 protein. This gene has remained pretty much unchanged throughout evolution. Most complex organisms have this same gene, including humans.
Lead author of the study Sangsoon Park and his colleagues confirmed that the overexpression, or increased production, of the VRK-1 protein boosted the lifespan of the C. elegans, which normally lives just two to three weeks, and the inhibition of VRK-1 production reduced its lifespan.
The research team found that the activity of the VRK-1-to-AMPK cellular-energy monitoring process is increased in low cellular energy status by reduced mitochondrial respiration, the set of metabolic chemical reactions that make use of the oxygen the worm breathes to convert macronutrients from food into the energy “currency” that cells spend to do everything they need to do.
It is already known that mitochondria, the energy-producing engine rooms in cells, play a crucial role in aging, and declines in the functioning of mitochondria are associated with age-related diseases. At the same time, the mild inhibition of mitochondrial respiration has been shown to promote longevity in a range of species, including flies and mammals.
When the research team performed similar tinkering with cultured human cells, they found they could also replicate this ramping up and down of the VRK-1-to-AMPK process that occurs in roundworms.
“This raises the intriguing possibility that VRK-1 also functions as a factor in governing human longevity, and so perhaps we can start developing longevity-promoting drugs that alter the activity of VRK-1,” explained Professor Lee.
At the very least, the research points us in an interesting direction for investigating new therapeutic strategies to combat metabolic disorders by targeting the modulation of VRK-1. Metabolic disorders involve the disruption of chemical reactions in the body, including diseases of the mitochondria.
But before metabolic disorder therapeutics or longevity drugs can be contemplated by scientists, further research still needs to be carried out to better understand how VRK-1 works to activate AMPK, as well as figure out the precise mechanics of how AMPK controls cellular energy.
This work was supported by the National Research Foundation (NRF), and the Ministry of Science and ICT (MSIT) of Korea.
Image credit: Seung-Jae V. LEE, KAIST.
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Park, S., et al. (2020) ‘VRK-1 extends life span by activation of AMPK via phosphorylation’. Science Advances, Volume 6. No. 27, eaaw7824. Available online at https://doi.org/10.1126/sciadv.aaw7824
Profile: Seung-Jae V. Lee, Ph.D.
Professor
seungjaevlee@kaist.ac.kr
https://sites.google.com/view/mgakaist
Molecular Genetics of Aging Laboratory
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.krDaejeon 34141, Korea
(END)
2020.07.31 View 13417
Tinkering with Roundworm Proteins Offers Hope for Anti-aging Drugs
- The somatic nuclear protein kinase VRK-1 increases the worm’s lifespan through AMPK activation, and this mechanism can be applied to promoting human longevity, the study reveals. -
KAIST researchers have been able to dial up and down creatures’ lifespans by altering the activity of proteins found in roundworm cells that tell them to convert sugar into energy when their cellular energy is running low. Humans also have these proteins, offering up the intriguing possibilities for developing longevity-promoting drugs. These new findings were published on July 1 in Science Advances.
The roundworm Caenorhabditis elegans (C. elegans), a millimeter-long nematode commonly used in lab testing, enjoyed a boost in its lifespan when researchers tinkered with a couple of proteins involved in monitoring the energy use by its cells.
The proteins VRK-1 and AMPK work in tandem in roundworm cells, with the former telling the latter to get to work by sticking a phosphate molecule, composed of one phosphorus and four oxygen atoms, on it. In turn, AMPK’s role is to monitor energy levels in cells, when cellular energy is running low. In essence, VRK-1 regulates AMPK, and AMPK regulates the cellular energy status.
Using a range of different biological research tools, including introducing foreign genes into the worm, a group of researchers led by Professor Seung-Jae V. Lee from the Department of Biological Sciences at KAIST were able to dial up and down the activity of the gene that tells cells to produce the VRK-1 protein. This gene has remained pretty much unchanged throughout evolution. Most complex organisms have this same gene, including humans.
Lead author of the study Sangsoon Park and his colleagues confirmed that the overexpression, or increased production, of the VRK-1 protein boosted the lifespan of the C. elegans, which normally lives just two to three weeks, and the inhibition of VRK-1 production reduced its lifespan.
The research team found that the activity of the VRK-1-to-AMPK cellular-energy monitoring process is increased in low cellular energy status by reduced mitochondrial respiration, the set of metabolic chemical reactions that make use of the oxygen the worm breathes to convert macronutrients from food into the energy “currency” that cells spend to do everything they need to do.
It is already known that mitochondria, the energy-producing engine rooms in cells, play a crucial role in aging, and declines in the functioning of mitochondria are associated with age-related diseases. At the same time, the mild inhibition of mitochondrial respiration has been shown to promote longevity in a range of species, including flies and mammals.
When the research team performed similar tinkering with cultured human cells, they found they could also replicate this ramping up and down of the VRK-1-to-AMPK process that occurs in roundworms.
“This raises the intriguing possibility that VRK-1 also functions as a factor in governing human longevity, and so perhaps we can start developing longevity-promoting drugs that alter the activity of VRK-1,” explained Professor Lee.
At the very least, the research points us in an interesting direction for investigating new therapeutic strategies to combat metabolic disorders by targeting the modulation of VRK-1. Metabolic disorders involve the disruption of chemical reactions in the body, including diseases of the mitochondria.
But before metabolic disorder therapeutics or longevity drugs can be contemplated by scientists, further research still needs to be carried out to better understand how VRK-1 works to activate AMPK, as well as figure out the precise mechanics of how AMPK controls cellular energy.
This work was supported by the National Research Foundation (NRF), and the Ministry of Science and ICT (MSIT) of Korea.
Image credit: Seung-Jae V. LEE, KAIST.
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Park, S., et al. (2020) ‘VRK-1 extends life span by activation of AMPK via phosphorylation’. Science Advances, Volume 6. No. 27, eaaw7824. Available online at https://doi.org/10.1126/sciadv.aaw7824
Profile: Seung-Jae V. Lee, Ph.D.
Professor
seungjaevlee@kaist.ac.kr
https://sites.google.com/view/mgakaist
Molecular Genetics of Aging Laboratory
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.krDaejeon 34141, Korea
(END)
2020.07.31 View 13417