healthcare
-
 KAIST Develops Healthcare Device Tracking Chronic Diabetic Wounds
A KAIST research team has developed an effective wireless system that monitors the wound healing process by tracking the spatiotemporal temperature changes and heat transfer characteristics of damaged areas such as diabetic wounds.
On the 5th of March, KAIST (represented by President Kwang Hyung Lee) announced that the research team led by Professor Kyeongha Kwon from KAIST’s School of Electrical Engineering, in association with Chung-Ang University professor Hanjun Ryu, developed digital healthcare technology that tracks the wound healing process in real time, which allows appropriate treatments to be administered.
< Figure 1. Schematic illustrations and diagrams of real-time wound monitoring systems. >
The skin serves as a barrier protecting the body from harmful substances, therefore damage to the skin may cause severe health risks to patients in need of intensive care. Especially in the case of diabetic patients, chronic wounds are easily formed due to complications in normal blood circulation and the wound healing process. In the United States alone, hundreds of billions of dollars of medical costs stem from regenerating the skin from such wounds. While various methods exist to promote wound healing, personalized management is essential depending on the condition of each patient's wounds.
Accordingly, the research team tracked the heating response within the wound by utilizing the differences in temperature between the damaged area and the surrounding healthy skin. They then measured heat transfer characteristics to observe moisture changes near the skin surface, ultimately establishing a basis for understanding the formation process of scar tissue. The team conducted experiments using diabetic mice models regarding the delay in wound healing under pathological conditions, and it was demonstrated that the collected data accurately tracks the wound healing process and the formation of scar tissue.
To minimize the tissue damage that may occur in the process of removing the tracking device after healing, the system integrates biodegradable sensor modules capable of natural decomposition within the body. These biodegradable modules disintegrate within the body after use, thus reducing the risk of additional discomfort or tissue damage upon device removal. Furthermore, the device could one day be used for monitoring inside the wound area as there is no need for removal.
Professor Kyeongha Kwon, who led the research, anticipates that continuous monitoring of wound temperature and heat transfer characteristics will enable medical professionals to more accurately assess the status of diabetic patients' wounds and provide appropriate treatment. He further predicted that the implementation of biodegradable sensors allows for the safe decomposition of the device after wound healing without the need for removal, making live monitoring possible not only in hospitals but also at home.
The research team plans to integrate antimicrobial materials into this device, aiming to expand its technological capabilities to enable the observation and prevention of inflammatory responses, bacterial infections, and other complications. The goal is to provide a multi-purpose wound monitoring platform capable of real-time antimicrobial monitoring in hospitals or homes by detecting changes in temperature and heat transfer characteristics indicative of infection levels.
< Image 1. Image of the bioresorbable temperature sensor >
The results of this study were published on February 19th in the international journal Advanced Healthcare Materials and selected as the inside back cover article, titled "Materials and Device Designs for Wireless Monitoring of Temperature and Thermal Transport Properties of Wound Beds during Healing."
This research was conducted with support from the Basic Research Program, the Regional Innovation Center Program, and the BK21 Program.
2024.03.11 View 6263
KAIST Develops Healthcare Device Tracking Chronic Diabetic Wounds
A KAIST research team has developed an effective wireless system that monitors the wound healing process by tracking the spatiotemporal temperature changes and heat transfer characteristics of damaged areas such as diabetic wounds.
On the 5th of March, KAIST (represented by President Kwang Hyung Lee) announced that the research team led by Professor Kyeongha Kwon from KAIST’s School of Electrical Engineering, in association with Chung-Ang University professor Hanjun Ryu, developed digital healthcare technology that tracks the wound healing process in real time, which allows appropriate treatments to be administered.
< Figure 1. Schematic illustrations and diagrams of real-time wound monitoring systems. >
The skin serves as a barrier protecting the body from harmful substances, therefore damage to the skin may cause severe health risks to patients in need of intensive care. Especially in the case of diabetic patients, chronic wounds are easily formed due to complications in normal blood circulation and the wound healing process. In the United States alone, hundreds of billions of dollars of medical costs stem from regenerating the skin from such wounds. While various methods exist to promote wound healing, personalized management is essential depending on the condition of each patient's wounds.
Accordingly, the research team tracked the heating response within the wound by utilizing the differences in temperature between the damaged area and the surrounding healthy skin. They then measured heat transfer characteristics to observe moisture changes near the skin surface, ultimately establishing a basis for understanding the formation process of scar tissue. The team conducted experiments using diabetic mice models regarding the delay in wound healing under pathological conditions, and it was demonstrated that the collected data accurately tracks the wound healing process and the formation of scar tissue.
To minimize the tissue damage that may occur in the process of removing the tracking device after healing, the system integrates biodegradable sensor modules capable of natural decomposition within the body. These biodegradable modules disintegrate within the body after use, thus reducing the risk of additional discomfort or tissue damage upon device removal. Furthermore, the device could one day be used for monitoring inside the wound area as there is no need for removal.
Professor Kyeongha Kwon, who led the research, anticipates that continuous monitoring of wound temperature and heat transfer characteristics will enable medical professionals to more accurately assess the status of diabetic patients' wounds and provide appropriate treatment. He further predicted that the implementation of biodegradable sensors allows for the safe decomposition of the device after wound healing without the need for removal, making live monitoring possible not only in hospitals but also at home.
The research team plans to integrate antimicrobial materials into this device, aiming to expand its technological capabilities to enable the observation and prevention of inflammatory responses, bacterial infections, and other complications. The goal is to provide a multi-purpose wound monitoring platform capable of real-time antimicrobial monitoring in hospitals or homes by detecting changes in temperature and heat transfer characteristics indicative of infection levels.
< Image 1. Image of the bioresorbable temperature sensor >
The results of this study were published on February 19th in the international journal Advanced Healthcare Materials and selected as the inside back cover article, titled "Materials and Device Designs for Wireless Monitoring of Temperature and Thermal Transport Properties of Wound Beds during Healing."
This research was conducted with support from the Basic Research Program, the Regional Innovation Center Program, and the BK21 Program.
2024.03.11 View 6263 -
 KAIST to showcase a pack of KAIST Start-ups at CES 2023
- KAIST is to run an Exclusive Booth at the Venetian Expo (Hall G) in Eureka Park, at CES 2023, to be held in Las Vegas from Thursday, January 5th through Sunday, the 8th.
- Twelve businesses recently put together by KAIST faculty, alumni, and the start-ups given legal usage of KAIST technologies will be showcased.
- Out of the participating start-ups, the products by Fluiz and Hills Robotics were selected as the “CES Innovation Award 2023 Honoree”, scoring top in their respective categories.
On January 3, KAIST announced that there will be a KAIST booth at Consumer Electronics Show (CES) 2023, the most influential tech event in the world, to be held in Las Vegas from January 3 to 8.
At this exclusive corner, KAIST will introduce the technologies of KAIST start-ups over the exhibition period.
KAIST first started holding its exclusive booth in CES 2019 with five start-up businesses, following up at CES 2020 with 12 start-ups and at CES 2022 with 10 start-ups. At CES 2023, which would be KAIST’s fourth conference, KAIST will be accompanying 12 businesses including start-ups by the faculty members, alumni, and technology transfer companies that just began their businesses with technologies from their research findings that stands a head above others.
To maximize the publicity opportunity, KAIST will support each company’s marketing strategies through cooperation with the Korea International Trade Association (KITA), and provide an opportunity for the school and each startup to create global identity and exhibit the excellence of their technologies at the convention.
The following companies will be at the KAIST Booth in Eureka Park:
The twelve startups mentioned above aim to achieve global technology commecialization in their respective fields of expertise spanning from eXtended Reality (XR) and gaming, to AI and robotics, vehicle and transport, mobile platform, smart city, autonomous driving, healthcare, internet of thing (IoT), through joint research and development, technology transfer and investment attraction from world’s leading institutions and enterprises.
In particular, Fluiz and Hills Robotics won the CES Innovation Award as 2023 Honorees and is expected to attain greater achievements in the future.
A staff member from the KAIST Institute of Technology Value Creation said, “The KAIST Showcase for CES 2023 has prepared a new pitching space for each of the companies for their own IR efforts, and we hope that KAIST startups will actively and effectively market their products and technologies while they are at the convention. We hope it will help them utilize their time here to establish their name in presence here which will eventually serve as a good foothold for them and their predecessors to further global commercialization goals.”
2023.01.04 View 14743
KAIST to showcase a pack of KAIST Start-ups at CES 2023
- KAIST is to run an Exclusive Booth at the Venetian Expo (Hall G) in Eureka Park, at CES 2023, to be held in Las Vegas from Thursday, January 5th through Sunday, the 8th.
- Twelve businesses recently put together by KAIST faculty, alumni, and the start-ups given legal usage of KAIST technologies will be showcased.
- Out of the participating start-ups, the products by Fluiz and Hills Robotics were selected as the “CES Innovation Award 2023 Honoree”, scoring top in their respective categories.
On January 3, KAIST announced that there will be a KAIST booth at Consumer Electronics Show (CES) 2023, the most influential tech event in the world, to be held in Las Vegas from January 3 to 8.
At this exclusive corner, KAIST will introduce the technologies of KAIST start-ups over the exhibition period.
KAIST first started holding its exclusive booth in CES 2019 with five start-up businesses, following up at CES 2020 with 12 start-ups and at CES 2022 with 10 start-ups. At CES 2023, which would be KAIST’s fourth conference, KAIST will be accompanying 12 businesses including start-ups by the faculty members, alumni, and technology transfer companies that just began their businesses with technologies from their research findings that stands a head above others.
To maximize the publicity opportunity, KAIST will support each company’s marketing strategies through cooperation with the Korea International Trade Association (KITA), and provide an opportunity for the school and each startup to create global identity and exhibit the excellence of their technologies at the convention.
The following companies will be at the KAIST Booth in Eureka Park:
The twelve startups mentioned above aim to achieve global technology commecialization in their respective fields of expertise spanning from eXtended Reality (XR) and gaming, to AI and robotics, vehicle and transport, mobile platform, smart city, autonomous driving, healthcare, internet of thing (IoT), through joint research and development, technology transfer and investment attraction from world’s leading institutions and enterprises.
In particular, Fluiz and Hills Robotics won the CES Innovation Award as 2023 Honorees and is expected to attain greater achievements in the future.
A staff member from the KAIST Institute of Technology Value Creation said, “The KAIST Showcase for CES 2023 has prepared a new pitching space for each of the companies for their own IR efforts, and we hope that KAIST startups will actively and effectively market their products and technologies while they are at the convention. We hope it will help them utilize their time here to establish their name in presence here which will eventually serve as a good foothold for them and their predecessors to further global commercialization goals.”
2023.01.04 View 14743 -
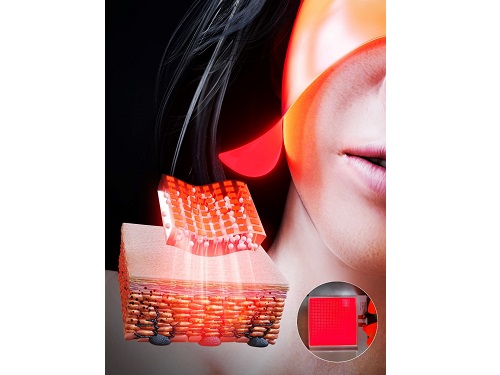 KAIST Team Develops Surface-Lighting MicroLED Patch with Significant Melanogenesis Inhibition Effect
A KAIST research team led by Ph.d candidate Jae Hee Lee and Professor Keon Jae Lee from the Department of Materials Science and Engineering has developed a surface-lighting microLED patch for UV-induced melanogenesis inhibition.
Melanin is brown or dark pigments existing in the skin, which can be abnormally synthesized by external UV or stress. Since the excessive melanin leads to skin diseases such as spots and freckles, proper treatment is required to return normal skin condition.
Recently, LED-based photo-stimulators have been released for skin care, however, their therapeutic effect is still controversial. Since conventional LED stimulators cannot conformally attach to the human skin, distance-induced side effects are caused by light loss and high heat transfer. To achieve effective phototreatment, the LED stimulator needs to be irradiated in contact with the human skin surface, enabling proper and uniform light deliver to the dermis with minimal optical loss.
In this work, the research team fabricated skin-attachable surface-lighting microLED (SµLED, 4 × 4 cm2) patch by utilizing a thousand of microLED chips and silica-embedded light diffusion layer. 100 µm-sized LED chips are vertically-interconnected for high flexibility and low heat generation, allowing its long-term operation on the human skin.
< Image 1. The overall concept of SµLED patch. a) SµLED patch operated on the human skin. b) Schematic illustration of SµLED patch structure. c) 4 × 4 cm2-sized SµLED patch. d) Schematic illustration of the advantages of SµLED patch such as efficient light delivery, low heat generation, and surface-lighting irradiation. >
The research team confirmed melanogenesis inhibition by irradiating the SµLED patch and the conventional LED (CLED) on the artificial human skin and mice dorsal skin. The SµLED-treated groups of human cells and mouse tissues showed minimal epidermal photo-toxicity and consistently effective reduction in synthesized melanin, compared to CLED-treated groups. In addition, significant suppression of proteins/catalysts expression involved in melanin synthesis such as MITF (microphthalmia-associated transcription factor), Melan-A and tyrosinase was verified.
< Image 2. The efficacy of melanogenesis inhibition on 3D human skin cells. a). Different irradiation conditions for a-MSH (major factor to stimulate melanin synthesis) treated cells. b) The ratio of pigmented area to total epidermis area. c) Relative variance of melanin level in 1 cm2-sized skin cells. A low variance means that melanin is evenly distributed, and a high variance means that the melanin is irregularly distributed. d) Optical images after in vitro experiments for 12 days. Scale bar, 1cm. e) Histological analysis of 3D skin, showing the greatest reduction in melanin after SµLED irradiation. Scale bar, 20 µm. >
< Image 3. The efficacy of melanogenesis inhibition on mouse dorsal skin. a) Optical images of mice dorsal skin after photo-treatment for 20 days. b) Histological analysis of mice dorsal skin. Less brown color means less expression of protein/catalysis involved in melanin synthesis. Scale bar, 50 µm. >
Prof. Keon Jae Lee said, “Our inorganic-based SµLED patch has outstanding characteristics in light efficiency, reliability, and durability. The SµLED patch is expected to give a great impact on the cosmetic field by reducing side effects and maximizing phototherapeutic effects.” The core technology of cosmetic SµLED has been transferred to Fronics co., Ltd, founded by Prof. Lee. Fronics is building foundry and equipment for mass production of SµLED masks for whole face cover and plans to release the products in March next year.
This paper entitled “Wearable Surface-Lighting Micro-Light-Emitting Diode Patch for Melanogenesis Inhibition” was published in the November 2022 issue of Advanced Healthcare Materials.
2022.11.22 View 11404
KAIST Team Develops Surface-Lighting MicroLED Patch with Significant Melanogenesis Inhibition Effect
A KAIST research team led by Ph.d candidate Jae Hee Lee and Professor Keon Jae Lee from the Department of Materials Science and Engineering has developed a surface-lighting microLED patch for UV-induced melanogenesis inhibition.
Melanin is brown or dark pigments existing in the skin, which can be abnormally synthesized by external UV or stress. Since the excessive melanin leads to skin diseases such as spots and freckles, proper treatment is required to return normal skin condition.
Recently, LED-based photo-stimulators have been released for skin care, however, their therapeutic effect is still controversial. Since conventional LED stimulators cannot conformally attach to the human skin, distance-induced side effects are caused by light loss and high heat transfer. To achieve effective phototreatment, the LED stimulator needs to be irradiated in contact with the human skin surface, enabling proper and uniform light deliver to the dermis with minimal optical loss.
In this work, the research team fabricated skin-attachable surface-lighting microLED (SµLED, 4 × 4 cm2) patch by utilizing a thousand of microLED chips and silica-embedded light diffusion layer. 100 µm-sized LED chips are vertically-interconnected for high flexibility and low heat generation, allowing its long-term operation on the human skin.
< Image 1. The overall concept of SµLED patch. a) SµLED patch operated on the human skin. b) Schematic illustration of SµLED patch structure. c) 4 × 4 cm2-sized SµLED patch. d) Schematic illustration of the advantages of SµLED patch such as efficient light delivery, low heat generation, and surface-lighting irradiation. >
The research team confirmed melanogenesis inhibition by irradiating the SµLED patch and the conventional LED (CLED) on the artificial human skin and mice dorsal skin. The SµLED-treated groups of human cells and mouse tissues showed minimal epidermal photo-toxicity and consistently effective reduction in synthesized melanin, compared to CLED-treated groups. In addition, significant suppression of proteins/catalysts expression involved in melanin synthesis such as MITF (microphthalmia-associated transcription factor), Melan-A and tyrosinase was verified.
< Image 2. The efficacy of melanogenesis inhibition on 3D human skin cells. a). Different irradiation conditions for a-MSH (major factor to stimulate melanin synthesis) treated cells. b) The ratio of pigmented area to total epidermis area. c) Relative variance of melanin level in 1 cm2-sized skin cells. A low variance means that melanin is evenly distributed, and a high variance means that the melanin is irregularly distributed. d) Optical images after in vitro experiments for 12 days. Scale bar, 1cm. e) Histological analysis of 3D skin, showing the greatest reduction in melanin after SµLED irradiation. Scale bar, 20 µm. >
< Image 3. The efficacy of melanogenesis inhibition on mouse dorsal skin. a) Optical images of mice dorsal skin after photo-treatment for 20 days. b) Histological analysis of mice dorsal skin. Less brown color means less expression of protein/catalysis involved in melanin synthesis. Scale bar, 50 µm. >
Prof. Keon Jae Lee said, “Our inorganic-based SµLED patch has outstanding characteristics in light efficiency, reliability, and durability. The SµLED patch is expected to give a great impact on the cosmetic field by reducing side effects and maximizing phototherapeutic effects.” The core technology of cosmetic SµLED has been transferred to Fronics co., Ltd, founded by Prof. Lee. Fronics is building foundry and equipment for mass production of SµLED masks for whole face cover and plans to release the products in March next year.
This paper entitled “Wearable Surface-Lighting Micro-Light-Emitting Diode Patch for Melanogenesis Inhibition” was published in the November 2022 issue of Advanced Healthcare Materials.
2022.11.22 View 11404 -
 KAIST KPC4IR Presents the AI Global Guide for Healthcare
The benchmark for the responsible usage of AI technology in the healthcare sector will promote clarity and high standards for technological applications
The KAIST Korea Policy Center for the Fourth Industrial Revolution (KPC4IR) published 'Using AI to Support Healthcare Decisions: A Guide for Society.' This global guide is designed to serve as a benchmark for the responsible usage of AI technologies, and will promote clarity and high standards for technological applications in the healthcare sector. The guide details what should be considered when making clinical decisions to help reduce the chances of the AI giving false or misleading results.
The KPC4IR presented the guide in collaboration with the Lloyd’s Register Foundation Institute for the Public Understanding of Risk at the National University of Singapore (NUS IPUR) and Sense about Science, a non-profit organization in the UK specialized in science communication, during the 2021 SIG-KDD (Special Interest Group on Knowledge Discovery and Data Mining) Conference on August 15.
AI technology is being widely used in the healthcare sector and has already proved its accuracy and efficiency in diagnosing and predicting diseases. Despite its huge impact on our daily lives in every sector of society, AI technology has some drawbacks and comes with risks, especially due to biased algorithms.
“We focused on the ‘reliability’ of AI applications in the healthcare sector to make all data well represented, in good quality. The technology will eventually innovate to better serve the people’s new demand, especially critical demands for safety and precision in healthcare services. This global guide will help both developers and people’s understanding of the appropriate technology applications,” says Director So Young Kim at the KPC4IR.
The guide, for instance, says that to scrutinize quality and reliability, the source of the data must be clearly known; the data must have been collected or selected for the purpose it’s being used for; the limitations and assumptions for that purpose have been clearly stated; the biases have been addressed; and it has been properly tested in the real world. It also reflects the importance of the representativeness of data that will affect the accuracy of the AI applications.
“By being transparent and demonstrating the steps taken to check that the AI is reliable, researchers and developers can help give people confidence about providing their data,” the guide states.
For this guide, the KPC4IR and its collaborators collected data after working with numerous experts from the Graduate School of AI at KAIST, the Science and Technology Policy Institute in Korea, Asan Medical Center in Seoul, Seoul National University Bundang Hospital, and AI solution companies.
2021.08.17 View 8493
KAIST KPC4IR Presents the AI Global Guide for Healthcare
The benchmark for the responsible usage of AI technology in the healthcare sector will promote clarity and high standards for technological applications
The KAIST Korea Policy Center for the Fourth Industrial Revolution (KPC4IR) published 'Using AI to Support Healthcare Decisions: A Guide for Society.' This global guide is designed to serve as a benchmark for the responsible usage of AI technologies, and will promote clarity and high standards for technological applications in the healthcare sector. The guide details what should be considered when making clinical decisions to help reduce the chances of the AI giving false or misleading results.
The KPC4IR presented the guide in collaboration with the Lloyd’s Register Foundation Institute for the Public Understanding of Risk at the National University of Singapore (NUS IPUR) and Sense about Science, a non-profit organization in the UK specialized in science communication, during the 2021 SIG-KDD (Special Interest Group on Knowledge Discovery and Data Mining) Conference on August 15.
AI technology is being widely used in the healthcare sector and has already proved its accuracy and efficiency in diagnosing and predicting diseases. Despite its huge impact on our daily lives in every sector of society, AI technology has some drawbacks and comes with risks, especially due to biased algorithms.
“We focused on the ‘reliability’ of AI applications in the healthcare sector to make all data well represented, in good quality. The technology will eventually innovate to better serve the people’s new demand, especially critical demands for safety and precision in healthcare services. This global guide will help both developers and people’s understanding of the appropriate technology applications,” says Director So Young Kim at the KPC4IR.
The guide, for instance, says that to scrutinize quality and reliability, the source of the data must be clearly known; the data must have been collected or selected for the purpose it’s being used for; the limitations and assumptions for that purpose have been clearly stated; the biases have been addressed; and it has been properly tested in the real world. It also reflects the importance of the representativeness of data that will affect the accuracy of the AI applications.
“By being transparent and demonstrating the steps taken to check that the AI is reliable, researchers and developers can help give people confidence about providing their data,” the guide states.
For this guide, the KPC4IR and its collaborators collected data after working with numerous experts from the Graduate School of AI at KAIST, the Science and Technology Policy Institute in Korea, Asan Medical Center in Seoul, Seoul National University Bundang Hospital, and AI solution companies.
2021.08.17 View 8493 -
 Wearable Strain Sensor Using Light Transmittance Helps Measure Physical Signals Better
KAIST researchers have developed a novel wearable strain sensor based on the modulation of optical transmittance of a carbon nanotube (CNT)-embedded elastomer. The sensor is capable of sensitive, stable, and continuous measurement of physical signals. This technology, featured in the March 4th issue of ACS Applied Materials & Interfaces as a front cover article, shows great potential for the detection of subtle human motions and the real-time monitoring of body postures for healthcare applications.
A wearable strain sensor must have high sensitivity, flexibility, and stretchability, as well as low cost. Those used especially for health monitoring should also be tied to long-term solid performance, and be environmentally stable. Various stretchable strain sensors based on piezo-resistive and capacitive principles have been developed to meet all these requirements.
Conventional piezo-resistive strain sensors using functional nanomaterials, including CNTs as the most common example, have shown high sensitivity and great sensing performance. However, they suffer from poor long-term stability and linearity, as well as considerable signal hysteresis. As an alternative, piezo-capacitive strain sensors with better stability, lower hysteresis, and higher stretchability have been suggested. But due to the fact that piezo-capacitive strain sensors exhibit limited sensitivity and strong electromagnetic interference caused by the conductive objects in the surrounding environment, these conventional stretchable strain sensors are still facing limitations that are yet to be resolved.
A KAIST research team led by Professor Inkyu Park from the Department of Mechanical Engineering suggested that an optical-type stretchable strain sensor can be a good alternative to resolve the limitations of conventional piezo-resistive and piezo-capacitive strain sensors, because they have high stability and are less affected by environmental disturbances. The team then introduced an optical wearable strain sensor based on the light transmittance changes of a CNT-embedded elastomer, which further addresses the low sensitivity problem of conventional optical stretchable strain sensors.
In order to achieve a large dynamic range for the sensor, Professor Park and his researchers chose Ecoflex as an elastomeric substrate with good mechanical durability, flexibility, and attachability on human skin, and the new optical wearable strain sensor developed by the research group actually shows a wide dynamic range of 0 to 400%.
In addition, the researchers propagated the microcracks under tensile strain within the film of multi-walled CNTs embedded in the Ecoflex substrate, changing the optical transmittance of the film. By doing so, it was possible for them to develop a wearable strain sensor having a sensitivity 10 times higher than conventional optical stretchable strain sensors.
The proposed sensor has also passed the durability test with excellent results. The sensor’s response after 13,000 sets of cyclic loading was stable without any noticeable drift. This suggests that the sensor response can be used without degradation, even if the sensor is repeatedly used for a long time and in various environmental conditions.
Using the developed sensor, the research team could measure the finger bending motion and used it for robot control. They also developed a three-axes sensor array for body posture monitoring. The sensor was able to monitor human motions with small strains such as a pulse near the carotid artery and muscle movement around the mouth during pronunciation.
Professor Park said, “In this study, our group developed a new wearable strain sensor platform that overcomes many limitations of previously developed resistive, capacitive, and optical-type stretchable strain sensors. Our sensor could be widely used in a variety of fields including soft robotics, wearable electronics, electronic skin, healthcare, and even entertainment.”
This work was supported by the National Research Foundation (NRF) of Korea.
Publication:
Jimin Gu, Donguk Kwon, Junseong Ahn, and Inkyu Park. (2020) “Wearable Strain sensors Using Light Transmittance Change of Carbon Nanotube-Embedded Elastomers with Microcracks” ACS Applied Materials & Interfaces. Volume 12. Issue 9. Available online at https://doi.org/10.1021/acsami.9b18069
Profile:
Inkyu Park
Professor
inkyu@kaist.ac.kr
http://mintlab1.kaist.ac.kr
Micro/Nano Transducers Laboratory (MINT Lab)
Department of Mechanical Engineering (ME)Korea Advanced Institute of Science and Technology (KAIST)
Profile:
Jimin Gu
Ph.D. Candidate
mint9411@kaist.ac.kr
http://mintlab1.kaist.ac.kr
MINT Lab
KAIST ME
(END)
2020.03.20 View 19473
Wearable Strain Sensor Using Light Transmittance Helps Measure Physical Signals Better
KAIST researchers have developed a novel wearable strain sensor based on the modulation of optical transmittance of a carbon nanotube (CNT)-embedded elastomer. The sensor is capable of sensitive, stable, and continuous measurement of physical signals. This technology, featured in the March 4th issue of ACS Applied Materials & Interfaces as a front cover article, shows great potential for the detection of subtle human motions and the real-time monitoring of body postures for healthcare applications.
A wearable strain sensor must have high sensitivity, flexibility, and stretchability, as well as low cost. Those used especially for health monitoring should also be tied to long-term solid performance, and be environmentally stable. Various stretchable strain sensors based on piezo-resistive and capacitive principles have been developed to meet all these requirements.
Conventional piezo-resistive strain sensors using functional nanomaterials, including CNTs as the most common example, have shown high sensitivity and great sensing performance. However, they suffer from poor long-term stability and linearity, as well as considerable signal hysteresis. As an alternative, piezo-capacitive strain sensors with better stability, lower hysteresis, and higher stretchability have been suggested. But due to the fact that piezo-capacitive strain sensors exhibit limited sensitivity and strong electromagnetic interference caused by the conductive objects in the surrounding environment, these conventional stretchable strain sensors are still facing limitations that are yet to be resolved.
A KAIST research team led by Professor Inkyu Park from the Department of Mechanical Engineering suggested that an optical-type stretchable strain sensor can be a good alternative to resolve the limitations of conventional piezo-resistive and piezo-capacitive strain sensors, because they have high stability and are less affected by environmental disturbances. The team then introduced an optical wearable strain sensor based on the light transmittance changes of a CNT-embedded elastomer, which further addresses the low sensitivity problem of conventional optical stretchable strain sensors.
In order to achieve a large dynamic range for the sensor, Professor Park and his researchers chose Ecoflex as an elastomeric substrate with good mechanical durability, flexibility, and attachability on human skin, and the new optical wearable strain sensor developed by the research group actually shows a wide dynamic range of 0 to 400%.
In addition, the researchers propagated the microcracks under tensile strain within the film of multi-walled CNTs embedded in the Ecoflex substrate, changing the optical transmittance of the film. By doing so, it was possible for them to develop a wearable strain sensor having a sensitivity 10 times higher than conventional optical stretchable strain sensors.
The proposed sensor has also passed the durability test with excellent results. The sensor’s response after 13,000 sets of cyclic loading was stable without any noticeable drift. This suggests that the sensor response can be used without degradation, even if the sensor is repeatedly used for a long time and in various environmental conditions.
Using the developed sensor, the research team could measure the finger bending motion and used it for robot control. They also developed a three-axes sensor array for body posture monitoring. The sensor was able to monitor human motions with small strains such as a pulse near the carotid artery and muscle movement around the mouth during pronunciation.
Professor Park said, “In this study, our group developed a new wearable strain sensor platform that overcomes many limitations of previously developed resistive, capacitive, and optical-type stretchable strain sensors. Our sensor could be widely used in a variety of fields including soft robotics, wearable electronics, electronic skin, healthcare, and even entertainment.”
This work was supported by the National Research Foundation (NRF) of Korea.
Publication:
Jimin Gu, Donguk Kwon, Junseong Ahn, and Inkyu Park. (2020) “Wearable Strain sensors Using Light Transmittance Change of Carbon Nanotube-Embedded Elastomers with Microcracks” ACS Applied Materials & Interfaces. Volume 12. Issue 9. Available online at https://doi.org/10.1021/acsami.9b18069
Profile:
Inkyu Park
Professor
inkyu@kaist.ac.kr
http://mintlab1.kaist.ac.kr
Micro/Nano Transducers Laboratory (MINT Lab)
Department of Mechanical Engineering (ME)Korea Advanced Institute of Science and Technology (KAIST)
Profile:
Jimin Gu
Ph.D. Candidate
mint9411@kaist.ac.kr
http://mintlab1.kaist.ac.kr
MINT Lab
KAIST ME
(END)
2020.03.20 View 19473 -
 What Fuels a “Domino Effect” in Cancer Drug Resistance?
KAIST researchers have identified mechanisms that relay prior acquired resistance to the first-line chemotherapy to the second-line targeted therapy, fueling a “domino effect” in cancer drug resistance. Their study featured in the February 7 edition of Science Advances suggests a new strategy for improving the second-line setting of cancer treatment for patients who showed resistance to anti-cancer drugs.
Resistance to cancer drugs is often managed in the clinic by chemotherapy and targeted therapy. Unlike chemotherapy that works by repressing fast-proliferating cells, targeted therapy blocks a single oncogenic pathway to halt tumor growth. In many cases, targeted therapy is engaged as a maintenance therapy or employed in the second-line after front-line chemotherapy.
A team of researchers led by Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering and the KAIST Institute for Health Science and Technology (KIHST) has discovered an unexpected resistance signature that occurs between chemotherapy and targeted therapy. The team further identified a set of integrated mechanisms that promotes this kind of sequential therapy resistance.
“There have been multiple clinical accounts reflecting that targeted therapies tend to be least successful in patients who have exhausted all standard treatments,” said the first author of the paper Mark Borris D. Aldonza. He continued, “These accounts ignited our hypothesis that failed responses to some chemotherapies might speed up the evolution of resistance to other drugs, particularly those with specific targets.”
Aldonza and his colleagues extracted large amounts of drug-resistance information from the open-source database the Genomics of Drug Sensitivity in Cancer (GDSC), which contains thousands of drug response data entries from various human cancer cell lines. Their big data analysis revealed that cancer cell lines resistant to chemotherapies classified as anti-mitotic drugs (AMDs), toxins that inhibit overacting cell division, are also resistant to a class of targeted therapies called epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs).
In all of the cancer types analyzed, more than 84 percent of those resistant to AMDs, representatively ‘paclitaxel’, were also resistant to at least nine EGFR-TKIs. In lung, pancreatic, and breast cancers where paclitaxel is often used as a first-line, standard-of-care regimen, greater than 92 percent showed resistance to EGFR-TKIs. Professor Kim said, “It is surprising to see that such collateral resistance can occur specifically between two chemically different classes of drugs.”
To figure out how failed responses to paclitaxel leads to resistance to EGFR-TKIs, the team validated co-resistance signatures that they found in the database by generating and analyzing a subset of slow-doubling, paclitaxel-resistant cancer models called ‘persisters’.
The results demonstrated that paclitaxel-resistant cancers remodel their stress response by first becoming more stem cell-like, evolving the ability to self-renew to adapt to more stressful conditions like drug exposures. More surprisingly, when the researchers characterized the metabolic state of the cells, EGFR-TKI persisters derived from paclitaxel-resistant cancer cells showed high dependencies to energy-producing processes such as glycolysis and glutaminolysis.
“We found that, without an energy stimulus like glucose, these cells transform to becoming more senescent, a characteristic of cells that have arrested cell division. However, this senescence is controlled by stem cell factors, which the paclitaxel-resistant cancers use to escape from this arrested state given a favorable condition to re-grow,” said Aldonza.
Professor Kim explained, “Before this research, there was no reason to expect that acquiring the cancer stem cell phenotype that dramatically leads to a cascade of changes in cellular states affecting metabolism and cell death is linked with drug-specific sequential resistance between two classes of therapies.”
He added, “The expansion of our work to other working models of drug resistance in a much more clinically-relevant setting, perhaps in clinical trials, will take on increasing importance, as sequential treatment strategies will continue to be adapted to various forms of anti-cancer therapy regimens.”
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF-2016R1C1B2009886), and the KAIST Future Systems Healthcare Project (KAISTHEALTHCARE42) funded by the Korean Ministry of Science and ICT (MSIT). Undergraduate student Aldonza participated in this research project and presented the findings as the lead author as part of the Undergraduate Research Participation (URP) Program at KAIST.
< Figure 1. Schematic overview of the study. >
< Figure 2. Big data analysis revealing co-resistance signatures between classes of anti-cancer drugs. >
Publication:
Aldonza et al. (2020) Prior acquired resistance to paclitaxel relays diverse EGFR-targeted therapy persistence mechanisms. Science Advances, Vol. 6, No. 6, eaav7416. Available online at http://dx.doi.org/10.1126/sciadv.aav7416
Profile: Prof. Yoosik Kim, MA, PhD
ysyoosik@kaist.ac.kr
https://qcbio.kaist.ac.kr/
Assistant Professor
Bio Network Analysis Laboratory
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Mark Borris D. Aldonza
borris@kaist.ac.kr
Undergraduate Student
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.10 View 15061
What Fuels a “Domino Effect” in Cancer Drug Resistance?
KAIST researchers have identified mechanisms that relay prior acquired resistance to the first-line chemotherapy to the second-line targeted therapy, fueling a “domino effect” in cancer drug resistance. Their study featured in the February 7 edition of Science Advances suggests a new strategy for improving the second-line setting of cancer treatment for patients who showed resistance to anti-cancer drugs.
Resistance to cancer drugs is often managed in the clinic by chemotherapy and targeted therapy. Unlike chemotherapy that works by repressing fast-proliferating cells, targeted therapy blocks a single oncogenic pathway to halt tumor growth. In many cases, targeted therapy is engaged as a maintenance therapy or employed in the second-line after front-line chemotherapy.
A team of researchers led by Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering and the KAIST Institute for Health Science and Technology (KIHST) has discovered an unexpected resistance signature that occurs between chemotherapy and targeted therapy. The team further identified a set of integrated mechanisms that promotes this kind of sequential therapy resistance.
“There have been multiple clinical accounts reflecting that targeted therapies tend to be least successful in patients who have exhausted all standard treatments,” said the first author of the paper Mark Borris D. Aldonza. He continued, “These accounts ignited our hypothesis that failed responses to some chemotherapies might speed up the evolution of resistance to other drugs, particularly those with specific targets.”
Aldonza and his colleagues extracted large amounts of drug-resistance information from the open-source database the Genomics of Drug Sensitivity in Cancer (GDSC), which contains thousands of drug response data entries from various human cancer cell lines. Their big data analysis revealed that cancer cell lines resistant to chemotherapies classified as anti-mitotic drugs (AMDs), toxins that inhibit overacting cell division, are also resistant to a class of targeted therapies called epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs).
In all of the cancer types analyzed, more than 84 percent of those resistant to AMDs, representatively ‘paclitaxel’, were also resistant to at least nine EGFR-TKIs. In lung, pancreatic, and breast cancers where paclitaxel is often used as a first-line, standard-of-care regimen, greater than 92 percent showed resistance to EGFR-TKIs. Professor Kim said, “It is surprising to see that such collateral resistance can occur specifically between two chemically different classes of drugs.”
To figure out how failed responses to paclitaxel leads to resistance to EGFR-TKIs, the team validated co-resistance signatures that they found in the database by generating and analyzing a subset of slow-doubling, paclitaxel-resistant cancer models called ‘persisters’.
The results demonstrated that paclitaxel-resistant cancers remodel their stress response by first becoming more stem cell-like, evolving the ability to self-renew to adapt to more stressful conditions like drug exposures. More surprisingly, when the researchers characterized the metabolic state of the cells, EGFR-TKI persisters derived from paclitaxel-resistant cancer cells showed high dependencies to energy-producing processes such as glycolysis and glutaminolysis.
“We found that, without an energy stimulus like glucose, these cells transform to becoming more senescent, a characteristic of cells that have arrested cell division. However, this senescence is controlled by stem cell factors, which the paclitaxel-resistant cancers use to escape from this arrested state given a favorable condition to re-grow,” said Aldonza.
Professor Kim explained, “Before this research, there was no reason to expect that acquiring the cancer stem cell phenotype that dramatically leads to a cascade of changes in cellular states affecting metabolism and cell death is linked with drug-specific sequential resistance between two classes of therapies.”
He added, “The expansion of our work to other working models of drug resistance in a much more clinically-relevant setting, perhaps in clinical trials, will take on increasing importance, as sequential treatment strategies will continue to be adapted to various forms of anti-cancer therapy regimens.”
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF-2016R1C1B2009886), and the KAIST Future Systems Healthcare Project (KAISTHEALTHCARE42) funded by the Korean Ministry of Science and ICT (MSIT). Undergraduate student Aldonza participated in this research project and presented the findings as the lead author as part of the Undergraduate Research Participation (URP) Program at KAIST.
< Figure 1. Schematic overview of the study. >
< Figure 2. Big data analysis revealing co-resistance signatures between classes of anti-cancer drugs. >
Publication:
Aldonza et al. (2020) Prior acquired resistance to paclitaxel relays diverse EGFR-targeted therapy persistence mechanisms. Science Advances, Vol. 6, No. 6, eaav7416. Available online at http://dx.doi.org/10.1126/sciadv.aav7416
Profile: Prof. Yoosik Kim, MA, PhD
ysyoosik@kaist.ac.kr
https://qcbio.kaist.ac.kr/
Assistant Professor
Bio Network Analysis Laboratory
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Mark Borris D. Aldonza
borris@kaist.ac.kr
Undergraduate Student
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.10 View 15061 -
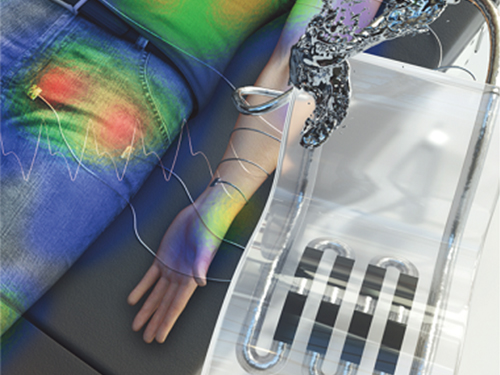 New Liquid Metal Wearable Pressure Sensor Created for Health Monitoring Applications
Soft pressure sensors have received significant research attention in a variety of fields, including soft robotics, electronic skin, and wearable electronics. Wearable soft pressure sensors have great potential for the real-time health monitoring and for the early diagnosis of diseases.
A KAIST research team led by Professor Inkyu Park from the Department of Mechanical Engineering developed a highly sensitive wearable pressure sensor for health monitoring applications. This work was reported in Advanced Healthcare Materials on November 21 as a front cover article.
This technology is capable of sensitive, precise, and continuous measurement of physiological and physical signals and shows great potential for health monitoring applications and the early diagnosis of diseases.
A soft pressure sensor is required to have high compliance, high sensitivity, low cost, long-term performance stability, and environmental stability in order to be employed for continuous health monitoring. Conventional solid-state soft pressure sensors using functional materials including carbon nanotubes and graphene have showed great sensing performance. However, these sensors suffer from limited stretchability, signal drifting, and long-term instability due to the distance between the stretchable substrate and the functional materials.
To overcome these issues, liquid-state electronics using liquid metal have been introduced for various wearable applications. Of these materials, Galinstan, a eutectic metal alloy of gallium, indium, and tin, has great mechanical and electrical properties that can be employed in wearable applications. But today’s liquid metal-based pressure sensors have low-pressure sensitivity, limiting their applicability for health monitoring devices.
The research team developed a 3D-printed rigid microbump array-integrated, liquid metal-based soft pressure sensor. With the help of 3D printing, the integration of a rigid microbump array and the master mold for a liquid metal microchannel could be achieved simultaneously, reducing the complexity of the manufacturing process. Through the integration of the rigid microbump and the microchannel, the new pressure sensor has an extremely low detection limit and enhanced pressure sensitivity compared to previously reported liquid metal-based pressure sensors. The proposed sensor also has a negligible signal drift over 10,000 cycles of pressure, bending, and stretching and exhibited excellent stability when subjected to various environmental conditions.
These performance outcomes make it an excellent sensor for various health monitoring devices. First, the research team demonstrated a wearable wristband device that can continuously monitor one’s pulse during exercise and be employed in a noninvasive cuffless BP monitoring system based on PTT calculations. Then, they introduced a wireless wearable heel pressure monitoring system that integrates three 3D-BLiPS with a wireless communication module.
Professor Park said, “It was possible to measure health indicators including pulse and blood pressure continuously as well as pressure of body parts using our proposed soft pressure sensor. We expect it to be used in health care applications, such as the prevention and the monitoring of the pressure-driven diseases such as pressure ulcers in the near future. There will be more opportunities for future research including a whole-body pressure monitoring system related to other physical parameters.”
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT.
< Figure 1. The front cover image of Advanced Healthcare Materials, Volume 8, Issue 22. >
< Figure 2. Highly sensitive liquid metal-based soft pressure sensor integrated with 3D-printed microbump array. >
< Figure 3. High pressure sensitivity and reliable sensing performances of the proposed sensor and wireless heel pressure monitoring application. >
-ProfileProfessor Inkyu ParkMicro/Nano Transducers Laboratoryhttp://mintlab1.kaist.ac.kr/
Department of Mechanical EngineeringKAIST
2019.12.20 View 14812
New Liquid Metal Wearable Pressure Sensor Created for Health Monitoring Applications
Soft pressure sensors have received significant research attention in a variety of fields, including soft robotics, electronic skin, and wearable electronics. Wearable soft pressure sensors have great potential for the real-time health monitoring and for the early diagnosis of diseases.
A KAIST research team led by Professor Inkyu Park from the Department of Mechanical Engineering developed a highly sensitive wearable pressure sensor for health monitoring applications. This work was reported in Advanced Healthcare Materials on November 21 as a front cover article.
This technology is capable of sensitive, precise, and continuous measurement of physiological and physical signals and shows great potential for health monitoring applications and the early diagnosis of diseases.
A soft pressure sensor is required to have high compliance, high sensitivity, low cost, long-term performance stability, and environmental stability in order to be employed for continuous health monitoring. Conventional solid-state soft pressure sensors using functional materials including carbon nanotubes and graphene have showed great sensing performance. However, these sensors suffer from limited stretchability, signal drifting, and long-term instability due to the distance between the stretchable substrate and the functional materials.
To overcome these issues, liquid-state electronics using liquid metal have been introduced for various wearable applications. Of these materials, Galinstan, a eutectic metal alloy of gallium, indium, and tin, has great mechanical and electrical properties that can be employed in wearable applications. But today’s liquid metal-based pressure sensors have low-pressure sensitivity, limiting their applicability for health monitoring devices.
The research team developed a 3D-printed rigid microbump array-integrated, liquid metal-based soft pressure sensor. With the help of 3D printing, the integration of a rigid microbump array and the master mold for a liquid metal microchannel could be achieved simultaneously, reducing the complexity of the manufacturing process. Through the integration of the rigid microbump and the microchannel, the new pressure sensor has an extremely low detection limit and enhanced pressure sensitivity compared to previously reported liquid metal-based pressure sensors. The proposed sensor also has a negligible signal drift over 10,000 cycles of pressure, bending, and stretching and exhibited excellent stability when subjected to various environmental conditions.
These performance outcomes make it an excellent sensor for various health monitoring devices. First, the research team demonstrated a wearable wristband device that can continuously monitor one’s pulse during exercise and be employed in a noninvasive cuffless BP monitoring system based on PTT calculations. Then, they introduced a wireless wearable heel pressure monitoring system that integrates three 3D-BLiPS with a wireless communication module.
Professor Park said, “It was possible to measure health indicators including pulse and blood pressure continuously as well as pressure of body parts using our proposed soft pressure sensor. We expect it to be used in health care applications, such as the prevention and the monitoring of the pressure-driven diseases such as pressure ulcers in the near future. There will be more opportunities for future research including a whole-body pressure monitoring system related to other physical parameters.”
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT.
< Figure 1. The front cover image of Advanced Healthcare Materials, Volume 8, Issue 22. >
< Figure 2. Highly sensitive liquid metal-based soft pressure sensor integrated with 3D-printed microbump array. >
< Figure 3. High pressure sensitivity and reliable sensing performances of the proposed sensor and wireless heel pressure monitoring application. >
-ProfileProfessor Inkyu ParkMicro/Nano Transducers Laboratoryhttp://mintlab1.kaist.ac.kr/
Department of Mechanical EngineeringKAIST
2019.12.20 View 14812 -
 KAIST-KU Joint Research Center for Smart Healthcare & Transportation
(President Shin shakes hands with KU acting Presidedent Arif Al Hammdi at the KAIST-KU Joint Research Center opening ceremony on April 8.)
KAIST opened the KAIST-Khalifa University Joint Research Center with Khalifa University on April 8. The opening ceremony was held at Khalifa University and was attended by President Sung-Chul Shin and Khalifa University Acting President Arif Al Hammadi.
The new research center reflects the evolution of the long-established partnership between the two institutions. The two universities have already made very close collaborations in research and education in the fields of nuclear and quantum engineering.
The launch of this center expanded their fields of collaboration to smart healthcare and smart transportation, key emerging sectors in the Fourth Industrial Revolution. President Shin signed an MOU with the UAE Minister of State for Advanced Science Sarah Amiri and Khalifa University to expand mutual collaboration in technology development and fostering human capital last year.
The center will conduct research and education on autonomous vehicles, infrastructure for autonomous vehicle operation, wireless charging for electric vehicles, and infrastructure for electric autonomous vehicles. As for smart healthcare, the center will focus on healthcare robotics as well as sensors and wearable devices for personal healthcare services.
President Shin, who accompanied a research team from the Graduate School of Green Transportation, said, “We are very delighted to enter into this expanded collaboration with KU. This partnership justifies our long-standing collaboration in the areas of emerging technologies in the Fourth Industrial Revolution while fostering human capital.”
KU Acting President Arif Al Hammadi added, “The outcome of these research projects will establish the status of both institutions as champions of the Fourth Industrial Revolution, bringing benefits to our communities. We believe the new research center will further consolidate our status as a globally active, research-intensive academic institution, developing international collaborations that benefit the community in general.”
2019.04.09 View 8589
KAIST-KU Joint Research Center for Smart Healthcare & Transportation
(President Shin shakes hands with KU acting Presidedent Arif Al Hammdi at the KAIST-KU Joint Research Center opening ceremony on April 8.)
KAIST opened the KAIST-Khalifa University Joint Research Center with Khalifa University on April 8. The opening ceremony was held at Khalifa University and was attended by President Sung-Chul Shin and Khalifa University Acting President Arif Al Hammadi.
The new research center reflects the evolution of the long-established partnership between the two institutions. The two universities have already made very close collaborations in research and education in the fields of nuclear and quantum engineering.
The launch of this center expanded their fields of collaboration to smart healthcare and smart transportation, key emerging sectors in the Fourth Industrial Revolution. President Shin signed an MOU with the UAE Minister of State for Advanced Science Sarah Amiri and Khalifa University to expand mutual collaboration in technology development and fostering human capital last year.
The center will conduct research and education on autonomous vehicles, infrastructure for autonomous vehicle operation, wireless charging for electric vehicles, and infrastructure for electric autonomous vehicles. As for smart healthcare, the center will focus on healthcare robotics as well as sensors and wearable devices for personal healthcare services.
President Shin, who accompanied a research team from the Graduate School of Green Transportation, said, “We are very delighted to enter into this expanded collaboration with KU. This partnership justifies our long-standing collaboration in the areas of emerging technologies in the Fourth Industrial Revolution while fostering human capital.”
KU Acting President Arif Al Hammadi added, “The outcome of these research projects will establish the status of both institutions as champions of the Fourth Industrial Revolution, bringing benefits to our communities. We believe the new research center will further consolidate our status as a globally active, research-intensive academic institution, developing international collaborations that benefit the community in general.”
2019.04.09 View 8589 -
 Cross-Generation Collaborative Labs Open
KAIST opened two cross-generation collaborative labs last month. This novel approach will pair up senior and junior faculty members for sustaining research and academic achievements even after the senior researcher retires.
This is one of the Vision 2031 innovation initiatives established to extend the spectrum of knowledge and research competitiveness. The selected labs will be funded for five years and the funding will be extended if necessary. KAIST will continue to select new labs every year.
A five-member selection committee including the Nobel Laureates Professor Klaus Von Klitzing at the Max-Planck Institute for Solid State Research and Dr. Kurt Wüthrich from ETH Zürich selected the first two labs with senior-junior pairs in March.
(Two renowned scholars' Cross-Generation Collaborative Labs which opened last month.
Distinguished Professor Lee's lab (above) andChair Professor Sung's lab)
Both labs are run by world-renowned scholars: the Systems Metabolic Engineering and Systems Healthcare Laboratory headed by Distinguished Professor Sang-Yup Lee in the Department of Chemical and Biomolecular Engineering and the Acousto-Microfluidics Research Center for Next-Generation Healthcare led by Chair Professor Hyung Jin Sung in the Department of Mechanical Engineering.
Distinguished Professor Lee will be teamed up with Professor Hyun Uk Kim, and their lab aims to mass produce new eco-friendly chemical materials as well as higher-value-added materials which will be used for medicine. The new platform technologies created in the lab are expected to provide information which will benefit human healthcare.
Meanwhile, the Acousto-Microfluidics Research Center for Next-Generation Healthcare will team up with Professors Hyoungsoo Kim and Yeunwoo Cho under Chair Professor Sung. The lab will conduct research on controlling fluids and objects exquisitely on a micro-nano scale by using high-frequency acoustic waves. The lab plans to develop a next-generation healthcare platform for customized diagnoses as well as disease treatment.
KAIST President Sung-Chul Shin, who introduced this novel idea in his research innovation initiative, said that he hopes the Cross-Generation Collaborative Labs will contribute to honoring senior scholars’ research legacies and passing knowledge down to junior researchers in order to further develop their academic achievements. He said, “I sincerely hope the labs will make numerous research breakthroughs in the very near future.”
2018.05.03 View 12381
Cross-Generation Collaborative Labs Open
KAIST opened two cross-generation collaborative labs last month. This novel approach will pair up senior and junior faculty members for sustaining research and academic achievements even after the senior researcher retires.
This is one of the Vision 2031 innovation initiatives established to extend the spectrum of knowledge and research competitiveness. The selected labs will be funded for five years and the funding will be extended if necessary. KAIST will continue to select new labs every year.
A five-member selection committee including the Nobel Laureates Professor Klaus Von Klitzing at the Max-Planck Institute for Solid State Research and Dr. Kurt Wüthrich from ETH Zürich selected the first two labs with senior-junior pairs in March.
(Two renowned scholars' Cross-Generation Collaborative Labs which opened last month.
Distinguished Professor Lee's lab (above) andChair Professor Sung's lab)
Both labs are run by world-renowned scholars: the Systems Metabolic Engineering and Systems Healthcare Laboratory headed by Distinguished Professor Sang-Yup Lee in the Department of Chemical and Biomolecular Engineering and the Acousto-Microfluidics Research Center for Next-Generation Healthcare led by Chair Professor Hyung Jin Sung in the Department of Mechanical Engineering.
Distinguished Professor Lee will be teamed up with Professor Hyun Uk Kim, and their lab aims to mass produce new eco-friendly chemical materials as well as higher-value-added materials which will be used for medicine. The new platform technologies created in the lab are expected to provide information which will benefit human healthcare.
Meanwhile, the Acousto-Microfluidics Research Center for Next-Generation Healthcare will team up with Professors Hyoungsoo Kim and Yeunwoo Cho under Chair Professor Sung. The lab will conduct research on controlling fluids and objects exquisitely on a micro-nano scale by using high-frequency acoustic waves. The lab plans to develop a next-generation healthcare platform for customized diagnoses as well as disease treatment.
KAIST President Sung-Chul Shin, who introduced this novel idea in his research innovation initiative, said that he hopes the Cross-Generation Collaborative Labs will contribute to honoring senior scholars’ research legacies and passing knowledge down to junior researchers in order to further develop their academic achievements. He said, “I sincerely hope the labs will make numerous research breakthroughs in the very near future.”
2018.05.03 View 12381 -
 KAIST & the Classic 500 Co Sign for Mobile Healthcare Research
KAIST and The Classic 500 Co., Ltd., an elder care provider based in Seoul, signed a memorandum of understanding to expand medical services by cooperating on the research of medical services and IT on March 24, 2015.
Twenty people from the two institutions, including President Steve Kang, Dong-Hyun Bak, CEO of The Classic 500 and Mun-Sul Jeong, a former KAIST Chairman of the Board, attended the signing ceremony.
Under the agreement, the two institutions will cooperate on mobile healthcare research and the development of a telemedicine system. They will also research and develop a system to better serve society with medical services.
The Classic 500, established by Konkuk University in Korea, provides nursing care services and assisted living facilities for senior citizens.
2015.03.26 View 9108
KAIST & the Classic 500 Co Sign for Mobile Healthcare Research
KAIST and The Classic 500 Co., Ltd., an elder care provider based in Seoul, signed a memorandum of understanding to expand medical services by cooperating on the research of medical services and IT on March 24, 2015.
Twenty people from the two institutions, including President Steve Kang, Dong-Hyun Bak, CEO of The Classic 500 and Mun-Sul Jeong, a former KAIST Chairman of the Board, attended the signing ceremony.
Under the agreement, the two institutions will cooperate on mobile healthcare research and the development of a telemedicine system. They will also research and develop a system to better serve society with medical services.
The Classic 500, established by Konkuk University in Korea, provides nursing care services and assisted living facilities for senior citizens.
2015.03.26 View 9108 -
 KAIST and Hancom Sign for Development of Mobile Healthcare
KAIST signed a memorandum of understanding with Hancom, Inc., an office suite developer in Korea, to foster mobile healthcare software programs. President Steve Kang and Chairman Sang-Chul Kim of Hancom held a signing ceremony on March 13, 2015 at the KAIST campus.
Based on the agreement, KAIST and Hancom will exchange research personnel to build Dr. M, a smart healthcare platform developed by the university, collaborate in research and development, and cooperate in the transfer of research developments from the university to the software industry including Hancom.
KAIST and Hancom also signed a memorandum of understanding on the development of software in April 2014. The Hancom-KAIST Research Center opened on campus last October.
2015.03.20 View 9802
KAIST and Hancom Sign for Development of Mobile Healthcare
KAIST signed a memorandum of understanding with Hancom, Inc., an office suite developer in Korea, to foster mobile healthcare software programs. President Steve Kang and Chairman Sang-Chul Kim of Hancom held a signing ceremony on March 13, 2015 at the KAIST campus.
Based on the agreement, KAIST and Hancom will exchange research personnel to build Dr. M, a smart healthcare platform developed by the university, collaborate in research and development, and cooperate in the transfer of research developments from the university to the software industry including Hancom.
KAIST and Hancom also signed a memorandum of understanding on the development of software in April 2014. The Hancom-KAIST Research Center opened on campus last October.
2015.03.20 View 9802 -
 'Dr. M,' Mobile Healthcare Showroom Opened at KI
Portable and wearable computers have made the way we manage our health easier and potentially more effective. Researchers from six departments and one graduate school at KAIST collaborated and conducted a one-year project called the “Mobile Healthcare Innovation” to develop a mobile healthcare system. Their research results are on exhibit on campus at the “Dr. M Showroom” which was open on March 13, 2015.
Located on the second floor of the College of Information and Electrical Engineering building, the showroom displays the entirety of mobile healthcare system developed during 2014, from the collection of biological data through smart sensors to analyzing big data to provide customized healthcare models for patients.
Standing in for a mobile doctor, Dr. M is a networked medical service system provided through the Internet of Things (IoC), wearable electronics, smart home, and smart car. Under this care, people can monitor their health on a daily basis at any-time and place, helping them to lower the risk of serious health problems. Patients who have chronic diseases such as diabetes or cardiovascular illness can inform doctors of their health status in real time. Moreover, people living in remote regions can receive quality medical services without traveling long distances.
At the showroom, about 40 convergence technologies are displayed, including biological sensors, low-power communication devices, IoC technology, big data, disease analysis, and prediction technology, presenting how these technologies are connected and worked systematically. For example, all the data earned from biological sensors are analyzed to produce relevant user information. Once abnormalities are discovered, the results would be sent immediately to medical staff for treatment.
As part of Dr. M, KAIST has been implementing the establishment of a “Mobile Healthcare Campus,” distributing small, wearable wristbands to 100 students. The wristbands read students’ biological signals and send them to researchers for monitoring. In addition, KAIST plans to collaborate with local hospitals, nursing care centers, communications, and mobile healthcare service providers for the commercialization of Dr. M system.
Professor Hoi-Jun Yoo of the Electrical Engineering Department, who has led the Mobile Healthcare Innovation project said, “One of the great advantages Dr. M can offer is the capability to customize healthcare service based on individuals and ages. For individuals in their twenties, for example, healthcare services such as skincare and diet programs will be more relevant whereas blood pressure monitoring for patients in their fifties and early diagnosis for the recurrence of diseases for those in their seventies. If we define human history in terms of major technology advancements, the first big one was computation, communication for the second, and I think ubiquitous healthcare will be the third one. We will continue to develop Dr. M in collaboration with medical and research organizations.”
A total of 32 professors from the Departments of Electrical Engineering, Computer Science, Industrial and Systems Engineering, Industrial Design, Web Science, Knowledge Service Engineering, and the Information Security Graduate School participated in the Mobile Healthcare Innovation project.
2015.03.17 View 10876
'Dr. M,' Mobile Healthcare Showroom Opened at KI
Portable and wearable computers have made the way we manage our health easier and potentially more effective. Researchers from six departments and one graduate school at KAIST collaborated and conducted a one-year project called the “Mobile Healthcare Innovation” to develop a mobile healthcare system. Their research results are on exhibit on campus at the “Dr. M Showroom” which was open on March 13, 2015.
Located on the second floor of the College of Information and Electrical Engineering building, the showroom displays the entirety of mobile healthcare system developed during 2014, from the collection of biological data through smart sensors to analyzing big data to provide customized healthcare models for patients.
Standing in for a mobile doctor, Dr. M is a networked medical service system provided through the Internet of Things (IoC), wearable electronics, smart home, and smart car. Under this care, people can monitor their health on a daily basis at any-time and place, helping them to lower the risk of serious health problems. Patients who have chronic diseases such as diabetes or cardiovascular illness can inform doctors of their health status in real time. Moreover, people living in remote regions can receive quality medical services without traveling long distances.
At the showroom, about 40 convergence technologies are displayed, including biological sensors, low-power communication devices, IoC technology, big data, disease analysis, and prediction technology, presenting how these technologies are connected and worked systematically. For example, all the data earned from biological sensors are analyzed to produce relevant user information. Once abnormalities are discovered, the results would be sent immediately to medical staff for treatment.
As part of Dr. M, KAIST has been implementing the establishment of a “Mobile Healthcare Campus,” distributing small, wearable wristbands to 100 students. The wristbands read students’ biological signals and send them to researchers for monitoring. In addition, KAIST plans to collaborate with local hospitals, nursing care centers, communications, and mobile healthcare service providers for the commercialization of Dr. M system.
Professor Hoi-Jun Yoo of the Electrical Engineering Department, who has led the Mobile Healthcare Innovation project said, “One of the great advantages Dr. M can offer is the capability to customize healthcare service based on individuals and ages. For individuals in their twenties, for example, healthcare services such as skincare and diet programs will be more relevant whereas blood pressure monitoring for patients in their fifties and early diagnosis for the recurrence of diseases for those in their seventies. If we define human history in terms of major technology advancements, the first big one was computation, communication for the second, and I think ubiquitous healthcare will be the third one. We will continue to develop Dr. M in collaboration with medical and research organizations.”
A total of 32 professors from the Departments of Electrical Engineering, Computer Science, Industrial and Systems Engineering, Industrial Design, Web Science, Knowledge Service Engineering, and the Information Security Graduate School participated in the Mobile Healthcare Innovation project.
2015.03.17 View 10876