electrode+material
-
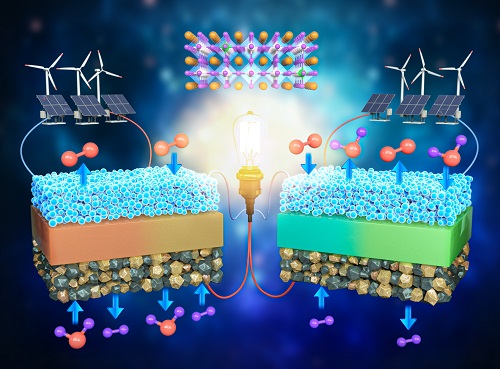 A KAIST Research Team Develops an Ultra-High Performing “Universal Electrode” for Next-Generation Fuel Cells
Fuel cells are devices that generate electricity with high efficiency using hydrogen, a clean energy source, and are expected to play an important part in the upcoming hydrogen society. The recent development of an excellent universal electrode material that is applicable to all next-generation fuel cells and can withstand 700 hours of operation has therefore garnered a great deal of attention.
On August 9, a joint research team led by Prof. WooChul Jung from the KAIST Department of Materials Science and Engineering, Prof. Kang Taek Lee from the KAIST Department of Mechanical Engineering, and Prof. Jun Hyuk Kim from the Department of Chemical Engineering at Hongik University announced the development of an electrode material that is applicable to both oxygen- and proton-conducting solid oxide cells.
Depending on the type of ion conducted by the electrolyte, ceramic fuel cells are categorized into either solid oxide fuel cells (SOFC) or protonic ceramic fuel cells (PCFC). As they can both convert between electricity and hydrogen production, fuel cells can be categorized into a total of four device types. These devices are applicable in hydrogen fuel cell vehicles, hydrogen charging stations, and power generation systems, and are henceforth emerging as core next-generation technologies for a carbon-neutral society.
However, these devices have a chronic problem where the speed of their slowest reaction would decrease with a drop of driving temperature, which greatly reduces device efficiency. Various studies have been conducted to solve this, but most reported that electrode materials have low catalytic activity and their applications are limited to specific devices, which limits them from being used as SOFCs that require reversible power conversion and hydrogen production.
< Figure 1. Schematic diagram of high-performance oxygen ion conductive solid oxide fuel cell (SOFC) and proton conductive ceramic fuel cell (PCFC) operates with the new universal electrodes >
To solve this issue, the research team doped a perovskite oxide material with Ta5+, a high valence ion that did not receive much attention in the field. Through this, the team successfully stabilized what is usually a highly unstable crystal structure, and confirmed that catalytic activity improved by 100 times.
The electrode material developed by the team was applied to all four of the mentioned device types. Furthermore, their efficiencies were greater than any of the devices reported thus far, and showed excellent performance by stably running for much longer (700 hours) compared to existing materials that deteriorated within the first 100 hours of operation.
< Figure 2. (a) Power conversion and hydrogen production performance chart for the protonic ceramic fuel cell (PCFC) with the new universal electrodes (b) and performance comparison with other reported devices >
This research, in which KAIST’s Ph.D. candidates Dongyeon Kim and Sejong Ahn, and Professor Jun Hyuk Kim from Hongik University contributed as co-first authors, was published in the internationally renowned Energy & Environmental Science under the title, "Oxygen-Electrode for Reversible Solid Oxide Electrochemical Cells at Reduced Temperatures".
Prof. WooChul Jung said, “We broke free from the idea that we must develop a completely new material to solve an existing problem, and instead suggested a way to control the crystal structure of a lesser-known material to develop a high-efficiency fuel cell, and that’s what makes these results more significant.”
Prof. Kang Taek Lee added, “Unlike previously reported materials that could only be applied to one device type at a time, our material has the flexibility of being applicable to all four. We therefore look forward to its contribution in the commercialization of eco-friendly energy technology including fuel cells and water-splitting equipment for hydrogen production.”
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Ministry of Science and ICT.
2023.08.22 View 8740
A KAIST Research Team Develops an Ultra-High Performing “Universal Electrode” for Next-Generation Fuel Cells
Fuel cells are devices that generate electricity with high efficiency using hydrogen, a clean energy source, and are expected to play an important part in the upcoming hydrogen society. The recent development of an excellent universal electrode material that is applicable to all next-generation fuel cells and can withstand 700 hours of operation has therefore garnered a great deal of attention.
On August 9, a joint research team led by Prof. WooChul Jung from the KAIST Department of Materials Science and Engineering, Prof. Kang Taek Lee from the KAIST Department of Mechanical Engineering, and Prof. Jun Hyuk Kim from the Department of Chemical Engineering at Hongik University announced the development of an electrode material that is applicable to both oxygen- and proton-conducting solid oxide cells.
Depending on the type of ion conducted by the electrolyte, ceramic fuel cells are categorized into either solid oxide fuel cells (SOFC) or protonic ceramic fuel cells (PCFC). As they can both convert between electricity and hydrogen production, fuel cells can be categorized into a total of four device types. These devices are applicable in hydrogen fuel cell vehicles, hydrogen charging stations, and power generation systems, and are henceforth emerging as core next-generation technologies for a carbon-neutral society.
However, these devices have a chronic problem where the speed of their slowest reaction would decrease with a drop of driving temperature, which greatly reduces device efficiency. Various studies have been conducted to solve this, but most reported that electrode materials have low catalytic activity and their applications are limited to specific devices, which limits them from being used as SOFCs that require reversible power conversion and hydrogen production.
< Figure 1. Schematic diagram of high-performance oxygen ion conductive solid oxide fuel cell (SOFC) and proton conductive ceramic fuel cell (PCFC) operates with the new universal electrodes >
To solve this issue, the research team doped a perovskite oxide material with Ta5+, a high valence ion that did not receive much attention in the field. Through this, the team successfully stabilized what is usually a highly unstable crystal structure, and confirmed that catalytic activity improved by 100 times.
The electrode material developed by the team was applied to all four of the mentioned device types. Furthermore, their efficiencies were greater than any of the devices reported thus far, and showed excellent performance by stably running for much longer (700 hours) compared to existing materials that deteriorated within the first 100 hours of operation.
< Figure 2. (a) Power conversion and hydrogen production performance chart for the protonic ceramic fuel cell (PCFC) with the new universal electrodes (b) and performance comparison with other reported devices >
This research, in which KAIST’s Ph.D. candidates Dongyeon Kim and Sejong Ahn, and Professor Jun Hyuk Kim from Hongik University contributed as co-first authors, was published in the internationally renowned Energy & Environmental Science under the title, "Oxygen-Electrode for Reversible Solid Oxide Electrochemical Cells at Reduced Temperatures".
Prof. WooChul Jung said, “We broke free from the idea that we must develop a completely new material to solve an existing problem, and instead suggested a way to control the crystal structure of a lesser-known material to develop a high-efficiency fuel cell, and that’s what makes these results more significant.”
Prof. Kang Taek Lee added, “Unlike previously reported materials that could only be applied to one device type at a time, our material has the flexibility of being applicable to all four. We therefore look forward to its contribution in the commercialization of eco-friendly energy technology including fuel cells and water-splitting equipment for hydrogen production.”
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Ministry of Science and ICT.
2023.08.22 View 8740 -
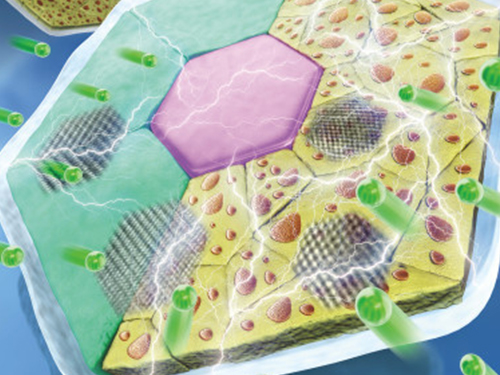 High-Performance Sodium Ion Batteries Using Copper Sulfide
(Prof.Yuk and his two PhD candidates Parks)
Researchers presented a new strategy for extending sodium ion batteries’ cyclability using copper sulfide as the electrode material. This strategy has led to high-performance conversion reactions and is expected to advance the commercialization of sodium ion batteries as they emerge as an alternative to lithium ion batteries.
Professor Jong Min Yuk’s team confirmed the stable sodium storage mechanism using copper sulfide, a superior electrode material that is pulverization-tolerant and induces capacity recovery. Their findings suggest that when employing copper sulfide, sodium ion batteries will have a lifetime of more than five years with one charge per a day. Even better, copper sulfide, composed of abundant natural materials such as copper and sulfur, has better cost competitiveness than lithium ion batteries, which use lithium and cobalt.
Intercalation-type materials such as graphite, which serve as commercialized anode materials in lithium ion batteries, have not been viable for high-capacity sodium storage due to their insufficient interlayer spacing. Thus, conversion and alloying reactions type materials have been explored to meet higher capacity in the anode part. However, those materials generally bring up large volume expansions and abrupt crystallographic changes, which lead to severe capacity degradation.
The team confirmed that semi-coherent phase interfaces and grain boundaries in conversion reactions played key roles in enabling pulverization-tolerant conversion reactions and capacity recovery, respectively.
Most of conversion and alloying reactions type battery materials usually experience severe capacity degradations due to having completely different crystal structures and large volume expansion before and after the reactions. However, copper sulfides underwent a gradual crystallographic change to make the semi-coherent interfaces, which eventually prevented the pulverization of particles. Based on this unique mechanism, the team confirmed that copper sulfide exhibits a high capacity and high cycling stability regardless of its size and morphology.
Professor Yuk said, “Sodium ion batteries employing copper sulfide can advance sodium ion batteries, which could contribute to the development of low-cost energy storage systems and address the micro-dust issue”
This study was posted in Advanced Science on April 26 online and selected as the inside back cover for June issue.
(Figure: Schematic model demonstrating grain boundaries and phase interfaces formations.)
2019.07.15 View 29165
High-Performance Sodium Ion Batteries Using Copper Sulfide
(Prof.Yuk and his two PhD candidates Parks)
Researchers presented a new strategy for extending sodium ion batteries’ cyclability using copper sulfide as the electrode material. This strategy has led to high-performance conversion reactions and is expected to advance the commercialization of sodium ion batteries as they emerge as an alternative to lithium ion batteries.
Professor Jong Min Yuk’s team confirmed the stable sodium storage mechanism using copper sulfide, a superior electrode material that is pulverization-tolerant and induces capacity recovery. Their findings suggest that when employing copper sulfide, sodium ion batteries will have a lifetime of more than five years with one charge per a day. Even better, copper sulfide, composed of abundant natural materials such as copper and sulfur, has better cost competitiveness than lithium ion batteries, which use lithium and cobalt.
Intercalation-type materials such as graphite, which serve as commercialized anode materials in lithium ion batteries, have not been viable for high-capacity sodium storage due to their insufficient interlayer spacing. Thus, conversion and alloying reactions type materials have been explored to meet higher capacity in the anode part. However, those materials generally bring up large volume expansions and abrupt crystallographic changes, which lead to severe capacity degradation.
The team confirmed that semi-coherent phase interfaces and grain boundaries in conversion reactions played key roles in enabling pulverization-tolerant conversion reactions and capacity recovery, respectively.
Most of conversion and alloying reactions type battery materials usually experience severe capacity degradations due to having completely different crystal structures and large volume expansion before and after the reactions. However, copper sulfides underwent a gradual crystallographic change to make the semi-coherent interfaces, which eventually prevented the pulverization of particles. Based on this unique mechanism, the team confirmed that copper sulfide exhibits a high capacity and high cycling stability regardless of its size and morphology.
Professor Yuk said, “Sodium ion batteries employing copper sulfide can advance sodium ion batteries, which could contribute to the development of low-cost energy storage systems and address the micro-dust issue”
This study was posted in Advanced Science on April 26 online and selected as the inside back cover for June issue.
(Figure: Schematic model demonstrating grain boundaries and phase interfaces formations.)
2019.07.15 View 29165