Yonsei+University
-
 KAIST Develops Virtual Staining Technology for 3D Histopathology
Moving beyond traditional methods of observing thinly sliced and stained cancer tissues, a collaborative international research team led by KAIST has successfully developed a groundbreaking technology. This innovation uses advanced optical techniques combined with an artificial intelligence-based deep learning algorithm to create realistic, virtually stained 3D images of cancer tissue without the need for serial sectioning nor staining. This breakthrough is anticipated to pave the way for next-generation non-invasive pathological diagnosis.
< Photo 1. (From left) Juyeon Park (Ph.D. Candidate, Department of Physics), Professor YongKeun Park (Department of Physics) (Top left) Professor Su-Jin Shin (Gangnam Severance Hospital), Professor Tae Hyun Hwang (Vanderbilt University School of Medicine) >
KAIST (President Kwang Hyung Lee) announced on the 26th that a research team led by Professor YongKeun Park of the Department of Physics, in collaboration with Professor Su-Jin Shin's team at Yonsei University Gangnam Severance Hospital, Professor Tae Hyun Hwang's team at Mayo Clinic, and Tomocube's AI research team, has developed an innovative technology capable of vividly displaying the 3D structure of cancer tissues without separate staining.
For over 200 years, conventional pathology has relied on observing cancer tissues under a microscope, a method that only shows specific cross-sections of the 3D cancer tissue. This has limited the ability to understand the three-dimensional connections and spatial arrangements between cells.
To overcome this, the research team utilized holotomography (HT), an advanced optical technology, to measure the 3D refractive index information of tissues. They then integrated an AI-based deep learning algorithm to successfully generate virtual H&E* images.* H&E (Hematoxylin & Eosin): The most widely used staining method for observing pathological tissues. Hematoxylin stains cell nuclei blue, and eosin stains cytoplasm pink.
The research team quantitatively demonstrated that the images generated by this technology are highly similar to actual stained tissue images. Furthermore, the technology exhibited consistent performance across various organs and tissues, proving its versatility and reliability as a next-generation pathological analysis tool.
< Figure 1. Comparison of conventional 3D tissue pathology procedure and the 3D virtual H&E staining technology proposed in this study. The traditional method requires preparing and staining dozens of tissue slides, while the proposed technology can reduce the number of slides by up to 10 times and quickly generate H&E images without the staining process. >
Moreover, by validating the feasibility of this technology through joint research with hospitals and research institutions in Korea and the United States, utilizing Tomocube's holotomography equipment, the team demonstrated its potential for full-scale adoption in real-world pathological research settings.
Professor YongKeun Park stated, "This research marks a major advancement by transitioning pathological analysis from conventional 2D methods to comprehensive 3D imaging. It will greatly enhance biomedical research and clinical diagnostics, particularly in understanding cancer tumor boundaries and the intricate spatial arrangements of cells within tumor microenvironments."
< Figure 2. Results of AI-based 3D virtual H&E staining and quantitative analysis of pathological tissue. The virtually stained images enabled 3D reconstruction of key pathological features such as cell nuclei and glandular lumens. Based on this, various quantitative indicators, including cell nuclear distribution, volume, and surface area, could be extracted. >
This research, with Juyeon Park, a student of the Integrated Master’s and Ph.D. Program at KAIST, as the first author, was published online in the prestigious journal Nature Communications on May 22.
(Paper title: Revealing 3D microanatomical structures of unlabeled thick cancer tissues using holotomography and virtual H&E staining.
[https://doi.org/10.1038/s41467-025-59820-0]
This study was supported by the Leader Researcher Program of the National Research Foundation of Korea, the Global Industry Technology Cooperation Center Project of the Korea Institute for Advancement of Technology, and the Korea Health Industry Development Institute.
2025.05.26 View 2686
KAIST Develops Virtual Staining Technology for 3D Histopathology
Moving beyond traditional methods of observing thinly sliced and stained cancer tissues, a collaborative international research team led by KAIST has successfully developed a groundbreaking technology. This innovation uses advanced optical techniques combined with an artificial intelligence-based deep learning algorithm to create realistic, virtually stained 3D images of cancer tissue without the need for serial sectioning nor staining. This breakthrough is anticipated to pave the way for next-generation non-invasive pathological diagnosis.
< Photo 1. (From left) Juyeon Park (Ph.D. Candidate, Department of Physics), Professor YongKeun Park (Department of Physics) (Top left) Professor Su-Jin Shin (Gangnam Severance Hospital), Professor Tae Hyun Hwang (Vanderbilt University School of Medicine) >
KAIST (President Kwang Hyung Lee) announced on the 26th that a research team led by Professor YongKeun Park of the Department of Physics, in collaboration with Professor Su-Jin Shin's team at Yonsei University Gangnam Severance Hospital, Professor Tae Hyun Hwang's team at Mayo Clinic, and Tomocube's AI research team, has developed an innovative technology capable of vividly displaying the 3D structure of cancer tissues without separate staining.
For over 200 years, conventional pathology has relied on observing cancer tissues under a microscope, a method that only shows specific cross-sections of the 3D cancer tissue. This has limited the ability to understand the three-dimensional connections and spatial arrangements between cells.
To overcome this, the research team utilized holotomography (HT), an advanced optical technology, to measure the 3D refractive index information of tissues. They then integrated an AI-based deep learning algorithm to successfully generate virtual H&E* images.* H&E (Hematoxylin & Eosin): The most widely used staining method for observing pathological tissues. Hematoxylin stains cell nuclei blue, and eosin stains cytoplasm pink.
The research team quantitatively demonstrated that the images generated by this technology are highly similar to actual stained tissue images. Furthermore, the technology exhibited consistent performance across various organs and tissues, proving its versatility and reliability as a next-generation pathological analysis tool.
< Figure 1. Comparison of conventional 3D tissue pathology procedure and the 3D virtual H&E staining technology proposed in this study. The traditional method requires preparing and staining dozens of tissue slides, while the proposed technology can reduce the number of slides by up to 10 times and quickly generate H&E images without the staining process. >
Moreover, by validating the feasibility of this technology through joint research with hospitals and research institutions in Korea and the United States, utilizing Tomocube's holotomography equipment, the team demonstrated its potential for full-scale adoption in real-world pathological research settings.
Professor YongKeun Park stated, "This research marks a major advancement by transitioning pathological analysis from conventional 2D methods to comprehensive 3D imaging. It will greatly enhance biomedical research and clinical diagnostics, particularly in understanding cancer tumor boundaries and the intricate spatial arrangements of cells within tumor microenvironments."
< Figure 2. Results of AI-based 3D virtual H&E staining and quantitative analysis of pathological tissue. The virtually stained images enabled 3D reconstruction of key pathological features such as cell nuclei and glandular lumens. Based on this, various quantitative indicators, including cell nuclear distribution, volume, and surface area, could be extracted. >
This research, with Juyeon Park, a student of the Integrated Master’s and Ph.D. Program at KAIST, as the first author, was published online in the prestigious journal Nature Communications on May 22.
(Paper title: Revealing 3D microanatomical structures of unlabeled thick cancer tissues using holotomography and virtual H&E staining.
[https://doi.org/10.1038/s41467-025-59820-0]
This study was supported by the Leader Researcher Program of the National Research Foundation of Korea, the Global Industry Technology Cooperation Center Project of the Korea Institute for Advancement of Technology, and the Korea Health Industry Development Institute.
2025.05.26 View 2686 -
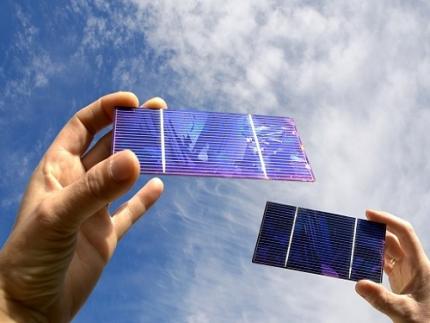 KAIST Researchers Introduce New and Improved, Next-Generation Perovskite Solar Cell
- KAIST-Yonsei university researchers developed innovative dipole technology to maximize near-infrared photon harvesting efficiency
- Overcoming the shortcoming of existing perovskite solar cells that cannot utilize approximately 52% of total solar energy
- Development of next-generation solar cell technology with high efficiency and high stability that can absorb near-infrared light beyond the existing visible light range with a perovskite-dipole-organic semiconductor hybrid structure
< Photo. (From left) Professor Jung-Yong Lee, Ph.D. candidate Min-Ho Lee, and Master’s candidate Min Seok Kim of the School of Electrical Engineering >
Existing perovskite solar cells, which have the problem of not being able to utilize approximately 52% of total solar energy, have been developed by a Korean research team as an innovative technology that maximizes near-infrared light capture performance while greatly improving power conversion efficiency. This greatly increases the possibility of commercializing next-generation solar cells and is expected to contribute to important technological advancements in the global solar cell market.
The research team of Professor Jung-Yong Lee of the School of Electrical Engineering at KAIST (President Kwang-Hyung Lee) and Professor Woojae Kim of the Department of Chemistry at Yonsei University announced on October 31st that they have developed a high-efficiency and high-stability organic-inorganic hybrid solar cell production technology that maximizes near-infrared light capture beyond the existing visible light range.
The research team suggested and advanced a hybrid next-generation device structure with organic photo-semiconductors that complements perovskite materials limited to visible light absorption and expands the absorption range to near-infrared.
In addition, they revealed the electronic structure problem that mainly occurs in the structure and announced a high-performance solar cell device that dramatically solved this problem by introducing a dipole layer*.
*Dipole layer: A thin material layer that controls the energy level within the device to facilitate charge transport and forms an interface potential difference to improve device performance.
Existing lead-based perovskite solar cells have a problem in that their absorption spectrum is limited to the visible light region with a wavelength of 850 nanometers (nm) or less, which prevents them from utilizing approximately 52% of the total solar energy.
To solve this problem, the research team designed a hybrid device that combined an organic bulk heterojunction (BHJ) with perovskite and implemented a solar cell that can absorb up to the near-infrared region.
In particular, by introducing a sub-nanometer dipole interface layer, they succeeded in alleviating the energy barrier between the perovskite and the organic bulk heterojunction (BHJ), suppressing charge accumulation, maximizing the contribution to the near-infrared, and improving the current density (JSC) to 4.9 mA/cm².
The key achievement of this study is that the power conversion efficiency (PCE) of the hybrid device has been significantly increased from 20.4% to 24.0%. In particular, this study achieved a high internal quantum efficiency (IQE) compared to previous studies, reaching 78% in the near-infrared region.
< Figure. The illustration of the mechanism of improving the electronic structure and charge transfer capability through Perovskite/organic hybrid device structure and dipole interfacial layers (DILs). The proposed dipole interfacial layer forms a strong interfacial dipole, effectively reducing the energy barrier between the perovskite and organic bulk heterojunction (BHJ), and suppressing hole accumulation. This technology improves near-infrared photon harvesting and charge transfer, and as a result, the power conversion efficiency of the solar cell increases to 24.0%. In addition, it achieves excellent stability by maintaining performance for 1,200 hours even in an extremely humid environment. >
In addition, this device showed high stability, showing excellent results of maintaining more than 80% of the initial efficiency in the maximum output tracking for more than 800 hours even under extreme humidity conditions.
Professor Jung-Yong Lee said, “Through this study, we have effectively solved the charge accumulation and energy band mismatch problems faced by existing perovskite/organic hybrid solar cells, and we will be able to significantly improve the power conversion efficiency while maximizing the near-infrared light capture performance, which will be a new breakthrough that can solve the mechanical-chemical stability problems of existing perovskites and overcome the optical limitations.”
This study, in which KAIST School of Electrical Engineering Ph.D. candidate Min-Ho Lee and Master's candidate Min Seok Kim participated as co-first authors, was published in the September 30th online edition of the international academic journal Advanced Materials. (Paper title: Suppressing Hole Accumulation Through Sub-Nanometer Dipole Interfaces in Hybrid Perovskite/Organic Solar Cells for Boosting Near-Infrared Photon Harvesting).
This study was conducted with the support of the National Research Foundation of Korea.
2024.10.31 View 6829
KAIST Researchers Introduce New and Improved, Next-Generation Perovskite Solar Cell
- KAIST-Yonsei university researchers developed innovative dipole technology to maximize near-infrared photon harvesting efficiency
- Overcoming the shortcoming of existing perovskite solar cells that cannot utilize approximately 52% of total solar energy
- Development of next-generation solar cell technology with high efficiency and high stability that can absorb near-infrared light beyond the existing visible light range with a perovskite-dipole-organic semiconductor hybrid structure
< Photo. (From left) Professor Jung-Yong Lee, Ph.D. candidate Min-Ho Lee, and Master’s candidate Min Seok Kim of the School of Electrical Engineering >
Existing perovskite solar cells, which have the problem of not being able to utilize approximately 52% of total solar energy, have been developed by a Korean research team as an innovative technology that maximizes near-infrared light capture performance while greatly improving power conversion efficiency. This greatly increases the possibility of commercializing next-generation solar cells and is expected to contribute to important technological advancements in the global solar cell market.
The research team of Professor Jung-Yong Lee of the School of Electrical Engineering at KAIST (President Kwang-Hyung Lee) and Professor Woojae Kim of the Department of Chemistry at Yonsei University announced on October 31st that they have developed a high-efficiency and high-stability organic-inorganic hybrid solar cell production technology that maximizes near-infrared light capture beyond the existing visible light range.
The research team suggested and advanced a hybrid next-generation device structure with organic photo-semiconductors that complements perovskite materials limited to visible light absorption and expands the absorption range to near-infrared.
In addition, they revealed the electronic structure problem that mainly occurs in the structure and announced a high-performance solar cell device that dramatically solved this problem by introducing a dipole layer*.
*Dipole layer: A thin material layer that controls the energy level within the device to facilitate charge transport and forms an interface potential difference to improve device performance.
Existing lead-based perovskite solar cells have a problem in that their absorption spectrum is limited to the visible light region with a wavelength of 850 nanometers (nm) or less, which prevents them from utilizing approximately 52% of the total solar energy.
To solve this problem, the research team designed a hybrid device that combined an organic bulk heterojunction (BHJ) with perovskite and implemented a solar cell that can absorb up to the near-infrared region.
In particular, by introducing a sub-nanometer dipole interface layer, they succeeded in alleviating the energy barrier between the perovskite and the organic bulk heterojunction (BHJ), suppressing charge accumulation, maximizing the contribution to the near-infrared, and improving the current density (JSC) to 4.9 mA/cm².
The key achievement of this study is that the power conversion efficiency (PCE) of the hybrid device has been significantly increased from 20.4% to 24.0%. In particular, this study achieved a high internal quantum efficiency (IQE) compared to previous studies, reaching 78% in the near-infrared region.
< Figure. The illustration of the mechanism of improving the electronic structure and charge transfer capability through Perovskite/organic hybrid device structure and dipole interfacial layers (DILs). The proposed dipole interfacial layer forms a strong interfacial dipole, effectively reducing the energy barrier between the perovskite and organic bulk heterojunction (BHJ), and suppressing hole accumulation. This technology improves near-infrared photon harvesting and charge transfer, and as a result, the power conversion efficiency of the solar cell increases to 24.0%. In addition, it achieves excellent stability by maintaining performance for 1,200 hours even in an extremely humid environment. >
In addition, this device showed high stability, showing excellent results of maintaining more than 80% of the initial efficiency in the maximum output tracking for more than 800 hours even under extreme humidity conditions.
Professor Jung-Yong Lee said, “Through this study, we have effectively solved the charge accumulation and energy band mismatch problems faced by existing perovskite/organic hybrid solar cells, and we will be able to significantly improve the power conversion efficiency while maximizing the near-infrared light capture performance, which will be a new breakthrough that can solve the mechanical-chemical stability problems of existing perovskites and overcome the optical limitations.”
This study, in which KAIST School of Electrical Engineering Ph.D. candidate Min-Ho Lee and Master's candidate Min Seok Kim participated as co-first authors, was published in the September 30th online edition of the international academic journal Advanced Materials. (Paper title: Suppressing Hole Accumulation Through Sub-Nanometer Dipole Interfaces in Hybrid Perovskite/Organic Solar Cells for Boosting Near-Infrared Photon Harvesting).
This study was conducted with the support of the National Research Foundation of Korea.
2024.10.31 View 6829 -
 Former Minister of Science and Technology Woo Sik Kim Elected as New Chairman of Board of Trustees
Dr. Woo Sik Kim, former Minister of Science and Technology and Deputy Prime Minister, was elected as the new chairman of the KAIST Board of Trustees on March 26. Dr. Kim will succeed Chairman Jang-Mu Lee, whose three-year term expired last month.
Dr. Kim is a chemical engineering professor who spent most of his academic career at Yonsei University from 1968. In 2000, he held the office of president of Yonsei University for four years before moving to the Presidential Office of President Roh Moo-Hyun as his chief of staff in 2004. After serving in the Blue House for two years, he served as the Minister of Science and Technology from 2006 to 2008.
An emeritus fellow of the National Academy of Engineering of Korea (NAEK), Chairman Kim also taught at KAIST as an invited distinguished professor from 2008 to 2010. He is currently the chairman of the Creativity Engineering Institute (CEI).
(END)
2020.04.06 View 14270
Former Minister of Science and Technology Woo Sik Kim Elected as New Chairman of Board of Trustees
Dr. Woo Sik Kim, former Minister of Science and Technology and Deputy Prime Minister, was elected as the new chairman of the KAIST Board of Trustees on March 26. Dr. Kim will succeed Chairman Jang-Mu Lee, whose three-year term expired last month.
Dr. Kim is a chemical engineering professor who spent most of his academic career at Yonsei University from 1968. In 2000, he held the office of president of Yonsei University for four years before moving to the Presidential Office of President Roh Moo-Hyun as his chief of staff in 2004. After serving in the Blue House for two years, he served as the Minister of Science and Technology from 2006 to 2008.
An emeritus fellow of the National Academy of Engineering of Korea (NAEK), Chairman Kim also taught at KAIST as an invited distinguished professor from 2008 to 2010. He is currently the chairman of the Creativity Engineering Institute (CEI).
(END)
2020.04.06 View 14270 -
 Accurate Detection of Low-Level Somatic Mutation in Intractable Epilepsy
KAIST medical scientists have developed an advanced method for perfectly detecting low-level somatic mutation in patients with intractable epilepsy. Their study showed that deep sequencing replicates of major focal epilepsy genes accurately and efficiently identified low-level somatic mutations in intractable epilepsy.
According to the study, their diagnostic method could increase the accuracy up to 100%, unlike the conventional sequencing analysis, which stands at about 30% accuracy. This work was published in Acta Neuropathologica.
Epilepsy is a neurological disorder common in children. Approximately one third of child patients are diagnosed with intractable epilepsy despite adequate anti-epileptic medication treatment.
Somatic mutations in mTOR pathway genes, SLC35A2, and BRAF are the major genetic causes of intractable epilepsies. A clinical trial to target Focal Cortical Dysplasia type II (FCDII), the mTOR inhibitor is underway at Severance Hospital, their collaborator in Seoul, Korea. However, it is difficult to detect such somatic mutations causing intractable epilepsy because their mutational burden is less than 5%, which is similar to the level of sequencing artifacts. In the clinical field, this has remained a standing challenge for the genetic diagnosis of somatic mutations in intractable epilepsy.
Professor Jeong Ho Lee’s team at the Graduate School of Medical Science and Engineering analyzed paired brain and peripheral tissues from 232 intractable epilepsy patients with various brain pathologies at Severance Hospital using deep sequencing and extracted the major focal epilepsy genes.
They narrowed down target genes to eight major focal epilepsy genes, eliminating almost all of the false positive calls using deep targeted sequencing. As a result, the advanced method robustly increased the accuracy and enabled them to detect low-level somatic mutations in unmatched Formalin Fixed Paraffin Embedded (FFPE) brain samples, the most clinically relevant samples.
Professor Lee conducted this study in collaboration with Professor Dong Suk Kim and Hoon-Chul Kang at Severance Hospital of Yonsei University. He said, “This advanced method of genetic analysis will improve overall patient care by providing more comprehensive genetic counseling and informing decisions on alternative treatments.”
Professor Lee has investigated low-level somatic mutations arising in the brain for a decade. He is developing innovative diagnostics and therapeutics for untreatable brain disorders including intractable epilepsy and glioblastoma at a tech-startup called SoVarGen. “All of the technologies we used during the research were transferred to the company. This research gave us very good momentum to reach the next phase of our startup,” he remarked.
The work was supported by grants from the Suh Kyungbae Foundation, a National Research Foundation of Korea grant funded by the Ministry of Science and ICT, the Korean Health Technology R&D Project from the Ministry of Health & Welfare, and the Netherlands Organization for Health Research and Development.
(Figure: Landscape of somatic and germline mutations identified in intractable epilepsy patients. a Signaling pathways for all of the mutated genes identified in this study. Bold: somatic mutation, Regular: germline mutation. b The distribution of variant allelic frequencies (VAFs) of identified somatic mutations. c The detecting rate and types of identified mutations according to histopathology. Yellow: somatic mutations, green: two-hit mutations, grey: germline mutations.)
2019.08.14 View 30287
Accurate Detection of Low-Level Somatic Mutation in Intractable Epilepsy
KAIST medical scientists have developed an advanced method for perfectly detecting low-level somatic mutation in patients with intractable epilepsy. Their study showed that deep sequencing replicates of major focal epilepsy genes accurately and efficiently identified low-level somatic mutations in intractable epilepsy.
According to the study, their diagnostic method could increase the accuracy up to 100%, unlike the conventional sequencing analysis, which stands at about 30% accuracy. This work was published in Acta Neuropathologica.
Epilepsy is a neurological disorder common in children. Approximately one third of child patients are diagnosed with intractable epilepsy despite adequate anti-epileptic medication treatment.
Somatic mutations in mTOR pathway genes, SLC35A2, and BRAF are the major genetic causes of intractable epilepsies. A clinical trial to target Focal Cortical Dysplasia type II (FCDII), the mTOR inhibitor is underway at Severance Hospital, their collaborator in Seoul, Korea. However, it is difficult to detect such somatic mutations causing intractable epilepsy because their mutational burden is less than 5%, which is similar to the level of sequencing artifacts. In the clinical field, this has remained a standing challenge for the genetic diagnosis of somatic mutations in intractable epilepsy.
Professor Jeong Ho Lee’s team at the Graduate School of Medical Science and Engineering analyzed paired brain and peripheral tissues from 232 intractable epilepsy patients with various brain pathologies at Severance Hospital using deep sequencing and extracted the major focal epilepsy genes.
They narrowed down target genes to eight major focal epilepsy genes, eliminating almost all of the false positive calls using deep targeted sequencing. As a result, the advanced method robustly increased the accuracy and enabled them to detect low-level somatic mutations in unmatched Formalin Fixed Paraffin Embedded (FFPE) brain samples, the most clinically relevant samples.
Professor Lee conducted this study in collaboration with Professor Dong Suk Kim and Hoon-Chul Kang at Severance Hospital of Yonsei University. He said, “This advanced method of genetic analysis will improve overall patient care by providing more comprehensive genetic counseling and informing decisions on alternative treatments.”
Professor Lee has investigated low-level somatic mutations arising in the brain for a decade. He is developing innovative diagnostics and therapeutics for untreatable brain disorders including intractable epilepsy and glioblastoma at a tech-startup called SoVarGen. “All of the technologies we used during the research were transferred to the company. This research gave us very good momentum to reach the next phase of our startup,” he remarked.
The work was supported by grants from the Suh Kyungbae Foundation, a National Research Foundation of Korea grant funded by the Ministry of Science and ICT, the Korean Health Technology R&D Project from the Ministry of Health & Welfare, and the Netherlands Organization for Health Research and Development.
(Figure: Landscape of somatic and germline mutations identified in intractable epilepsy patients. a Signaling pathways for all of the mutated genes identified in this study. Bold: somatic mutation, Regular: germline mutation. b The distribution of variant allelic frequencies (VAFs) of identified somatic mutations. c The detecting rate and types of identified mutations according to histopathology. Yellow: somatic mutations, green: two-hit mutations, grey: germline mutations.)
2019.08.14 View 30287 -
 Potential Drug to Cure Ciliopathies
(from left: Professor Joon Kim and PhD candidate Yong Joon Kim)
Ciliopathies are rare disorders involving functional and structural abnormalities of cilia. Although they are rare, they may reach 1 in 1,000 births. Unfortunately, there are no small-molecule drugs for treating ciliary defects. A KAIST research team conducted successful research that introduces a potential treatment that will be a foundation for developing drugs to treat the disease as well as a platform for developing small-molecule drugs for similar genetic disorders.
It was found that mutations in genes required for the formation or function of primary cilia cause ciliopathies and they result in cerebellar disorders, kidney dysfunction, and retinal degeneration.
Primary cilia are cell organelles playing a crucial role in the human body. They participate in intercellular signal transduction during embryonic development and allow retinal photoreceptor cells to function.
Currently, there are no approved drugs available for treating most ciliopathies. In fact, this is the case for most of the rare genetic disorders involving functional abnormalities through genetic mutation, and gene therapy is usually the only treatment available.
To tackle this issue, a team led by Professor Joon Kim from the Graduate School of Medical Science and Engineering and Ho Jeong Kwon from Yonsei University constructed a cell that mimics a gene-mutated CEP290, one of the main causes of ciliopathies, through genome editing. They then used cell-based compound library screening to obtain a natural small-molecule compound capable of relieving defects in ciliogenesis, the production of cilia.
The CEP290 protein forms a complex with a ciliopathy protein called NPHP5 to support the function of the ciliary transition zone. In cases where the CEP290 protein is not formed due to a genetic mutation, NPHP5 will not function normally. Here, the compound was confirmed to partially restore the function of the complex by normalizing the function of NPHP5.
The team also identified that the compound is capable of retarding retinal degeneration by injecting the compound into animal models.
As a result, they discovered a lead compound for developing medication to treat ciliopathy patients involving retinal degeneration. Hence, the findings imply that chemical compounds that target other proteins interacting with the disease protein can mitigate shortages of a disease protein in recessive genetic disorders.
PhD candidate Yong Joon Kim stated, “This study shows how genetic disorders caused by genetic mutation can be treated with small-molecule drugs.”
Professor Kim said, “Since the efficacy of the candidate drug has been verified through animal testing, a follow-up study will also be conducted to demonstrate the effect on humans.”
This research was published in the Journal of Clinical Investigation on July 23.
Figure 1. Identification of compounds that rescue ciliogenesis defects caused by CEP290 knockout
Figure 2. Eupatilin injection ameliorates M-opsin trafficking and electrophysiological response of cone photoreceptors in rd16 mice
2018.08.30 View 8878
Potential Drug to Cure Ciliopathies
(from left: Professor Joon Kim and PhD candidate Yong Joon Kim)
Ciliopathies are rare disorders involving functional and structural abnormalities of cilia. Although they are rare, they may reach 1 in 1,000 births. Unfortunately, there are no small-molecule drugs for treating ciliary defects. A KAIST research team conducted successful research that introduces a potential treatment that will be a foundation for developing drugs to treat the disease as well as a platform for developing small-molecule drugs for similar genetic disorders.
It was found that mutations in genes required for the formation or function of primary cilia cause ciliopathies and they result in cerebellar disorders, kidney dysfunction, and retinal degeneration.
Primary cilia are cell organelles playing a crucial role in the human body. They participate in intercellular signal transduction during embryonic development and allow retinal photoreceptor cells to function.
Currently, there are no approved drugs available for treating most ciliopathies. In fact, this is the case for most of the rare genetic disorders involving functional abnormalities through genetic mutation, and gene therapy is usually the only treatment available.
To tackle this issue, a team led by Professor Joon Kim from the Graduate School of Medical Science and Engineering and Ho Jeong Kwon from Yonsei University constructed a cell that mimics a gene-mutated CEP290, one of the main causes of ciliopathies, through genome editing. They then used cell-based compound library screening to obtain a natural small-molecule compound capable of relieving defects in ciliogenesis, the production of cilia.
The CEP290 protein forms a complex with a ciliopathy protein called NPHP5 to support the function of the ciliary transition zone. In cases where the CEP290 protein is not formed due to a genetic mutation, NPHP5 will not function normally. Here, the compound was confirmed to partially restore the function of the complex by normalizing the function of NPHP5.
The team also identified that the compound is capable of retarding retinal degeneration by injecting the compound into animal models.
As a result, they discovered a lead compound for developing medication to treat ciliopathy patients involving retinal degeneration. Hence, the findings imply that chemical compounds that target other proteins interacting with the disease protein can mitigate shortages of a disease protein in recessive genetic disorders.
PhD candidate Yong Joon Kim stated, “This study shows how genetic disorders caused by genetic mutation can be treated with small-molecule drugs.”
Professor Kim said, “Since the efficacy of the candidate drug has been verified through animal testing, a follow-up study will also be conducted to demonstrate the effect on humans.”
This research was published in the Journal of Clinical Investigation on July 23.
Figure 1. Identification of compounds that rescue ciliogenesis defects caused by CEP290 knockout
Figure 2. Eupatilin injection ameliorates M-opsin trafficking and electrophysiological response of cone photoreceptors in rd16 mice
2018.08.30 View 8878 -
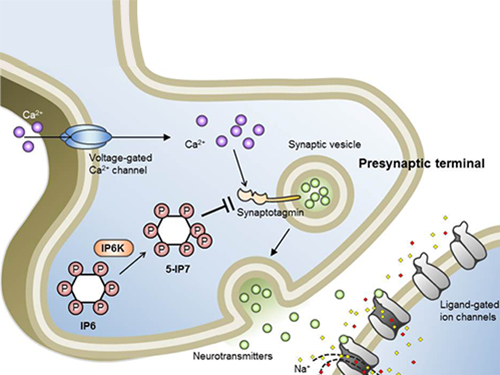 Professor Seyun Kim Identifies a Neuron Signal Controlling Molecule
A research team led by Professor Seyun Kim of the Department of Biological Sciences at KAIST has identified inositol pyrophosphates as the molecule that strongly controls neuron signaling via synaptotagmin.
Professors Tae-Young Yoon of Yonsei University’s Y-IBS and Sung-Hyun Kim of Kyung Hee University’s Department of Biomedical Science also joined the team.
The results were published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS) on June 30, 2016.
This interdisciplinary research project was conducted by six research teams from four different countries and covered a wide scope of academic fields, from neurobiology to super resolution optic imaging.
Inositol pyrophosphates such as 5-diphosphoinositol pentakisphos-phate (5-IP7), which naturally occur in corns and beans, are essential metabolites in the body. In particular, inositol hexakisphosphate (IP6) has anti-cancer properties and is thought to have an important role in cell signaling.
Inositol pentakisphosphate (IP7) differs from IP6 by having an additional phosphate group, which was first discovered 20 years ago. IP7 has recently been identified as playing a key role in diabetes and obesity.
Psychopathy and neurodegenerative diseases are known to result from the disrupted balance of inositol pyrophosphates. However, the role and the mechanism of action of IP7 in brain neurons and nerve transmission remained unknown.
Professor Kim’s team has worked on inositol pyrophosphates for several years and discovered that very small quantities of IP7 control cell-signaling transduction. Professor Yoon of Yonsei University identified IP7 as a much stronger inhibitor of neuron signaling compared to IP6. In particular, IP7 directly suppresses synaptotagmin, one of the key proteins in neuron signaling. Moreover, Professor Kim of Kyung Hee University observed IP7 inhibition in sea horse neurons.
Together, the joint research team identified inositol pyrophosphates as the key switch metabolite of brain-signaling transduction.
The researchers hope that future research on synaptotagmin and IP7 will reveal the mechanism of neuron-signal transduction and thus enable the treatment of neurological disorders.
These research findings were the result of cooperation of various science and technology institutes: KAIST, Yonsei-IBS (Institute for Basic Science), Kyung Hee University, Sungkyunkwan University, KIST, University of Zurich in Switzerland, and Albert-Ludwigs-University Freiburg in Germany.
Schematic Image of Controlling the Synaptic Exocytotic Pathway by 5-IP7 , Helping the Understanding of the Signaling Mechanisms of Inositol Pyrophosphates
2016.07.21 View 12895
Professor Seyun Kim Identifies a Neuron Signal Controlling Molecule
A research team led by Professor Seyun Kim of the Department of Biological Sciences at KAIST has identified inositol pyrophosphates as the molecule that strongly controls neuron signaling via synaptotagmin.
Professors Tae-Young Yoon of Yonsei University’s Y-IBS and Sung-Hyun Kim of Kyung Hee University’s Department of Biomedical Science also joined the team.
The results were published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS) on June 30, 2016.
This interdisciplinary research project was conducted by six research teams from four different countries and covered a wide scope of academic fields, from neurobiology to super resolution optic imaging.
Inositol pyrophosphates such as 5-diphosphoinositol pentakisphos-phate (5-IP7), which naturally occur in corns and beans, are essential metabolites in the body. In particular, inositol hexakisphosphate (IP6) has anti-cancer properties and is thought to have an important role in cell signaling.
Inositol pentakisphosphate (IP7) differs from IP6 by having an additional phosphate group, which was first discovered 20 years ago. IP7 has recently been identified as playing a key role in diabetes and obesity.
Psychopathy and neurodegenerative diseases are known to result from the disrupted balance of inositol pyrophosphates. However, the role and the mechanism of action of IP7 in brain neurons and nerve transmission remained unknown.
Professor Kim’s team has worked on inositol pyrophosphates for several years and discovered that very small quantities of IP7 control cell-signaling transduction. Professor Yoon of Yonsei University identified IP7 as a much stronger inhibitor of neuron signaling compared to IP6. In particular, IP7 directly suppresses synaptotagmin, one of the key proteins in neuron signaling. Moreover, Professor Kim of Kyung Hee University observed IP7 inhibition in sea horse neurons.
Together, the joint research team identified inositol pyrophosphates as the key switch metabolite of brain-signaling transduction.
The researchers hope that future research on synaptotagmin and IP7 will reveal the mechanism of neuron-signal transduction and thus enable the treatment of neurological disorders.
These research findings were the result of cooperation of various science and technology institutes: KAIST, Yonsei-IBS (Institute for Basic Science), Kyung Hee University, Sungkyunkwan University, KIST, University of Zurich in Switzerland, and Albert-Ludwigs-University Freiburg in Germany.
Schematic Image of Controlling the Synaptic Exocytotic Pathway by 5-IP7 , Helping the Understanding of the Signaling Mechanisms of Inositol Pyrophosphates
2016.07.21 View 12895