Transformation
-
 KAIST-NYU Digital Governance Forum Held
KAIST (President Kwang Hyung Lee) held the 'KAIST-NYU Digital Governance Forum' at the Korea Press Center in the morning of October 28th, 2022.
This forum was held in continuation to discuss the objectives of the 'Digital Vision Forum' that was hosted by New York University (NYU) back in September in the United States, and is the first public event to be held through joint efforts by KAIST and NYU since the signage of the 'KAIST-NYU Joint Campus' was presented at the New York forum.
< Signage of KAIST-NYU Joint Campus >
This forum was promoted based on the consensus of the two universities to create an international forum of solidarity to solve global challenges and seek new governance in the era of digital transformation.
Digital innovation technology is expected to bring economic and industrial benefits as well as political, social and ethical risks such as accelerating the digital divide, among others. In particular, in a time of global digital transformation, as the competition for digital and AI supremacy based on technology nationalism catches fire, there is an emergent need for a global governance system in which digital innovation and the value of freedom co-exist. With the consensus formed through this forum with NYU, KAIST plans to focus on detailing the vision for future digital cooperation that encompasses various stakeholders in our society.
To this end, President Kwang Hyung Lee of KAIST and President Andrew Hamilton of NYU led the forum with keynote addresses with President Hamilton taking part virtually, followed by NYU Professor Matthew Liao, a world-renowned scholar specialized in the ethics in the field of science and technology, and Jason Allford, Special Representative of the World Bank Group to Korea, presenting on relevant topics for discussion.
From KAIST, Professor Kyung Ryul Park of the Graduate School of Science and Technology Policy and Director So Young Kim of the Korea Policy Center for the Fourth Industrial Revolution, followed with their presentations. A panel discussion on governance in the period of digital transformation was also held, led by Professor Dongman Lee, the Dean of the College of Engineering.
To kick things off, Professor Matthew Liao of NYU proposed a normative system that can harmonize technology and social ethics while explaining various ethical issues following the technological development of artificial intelligence.
Jason Allford, Special Representative of the World Bank Group to Korea, outlined the changing roles of government in the digital era from the perspective of transparency and government efficiency and explained global development strategies through various cases of digital innovations by international organizations.
Professor Kyung Ryul Park of the Graduate School of Science and Technology Policy at KAIST emphasized that the core of new digital governance is not only innovative technology but also the participation and harmony of various stakeholders at home at abroad and brought up the importance of multi-dimensional international solidarity based on digital transformation that goes beyond the flat ‘technological geopolitics.’
Professor So Young Kim, the Director of the Korea Policy Center for the Fourth Industrial Revolution at KAIST, commented on the current government's digital platform strategy and emphasized the need for a leading digital transformation strategy that goes beyond the governance of the existing government.
Edward Mermelstein, the Commissioner for International Affairs of New York City, said, “The City of New York, shall also provide active support for the cooperative governance initiative organized by KAIST in Korea. As the conversation progresses further, we can draw up plans to organize international organizations to support the effort, likely to be named ‘Digitization for Good’, and we can go on to consider future collaboration,” to express the city’s willingness and anticipation for active cooperation.
Andrew Hamilton, the President of NYU, said "NYU is thrilled by the partnership we are embarking upon with KAIST, which goes hand in hand with our global tradition, and is based upon our bedrock commitment to the free movement of people and ideas.” He added that “As data-driven software, AI, and social networks become even more essential parts of our daily lives, I am confident that today’s discussions will lead to new and promising insights.”
President Kwang Hyung Lee of KAIST said, “It is significant that we are to cooperate with New York University to prepare a venue to assess the changes of the forth coming era at a time in which digital technology, government platforms, and public data are attracting attention as a medium that can create various social and economic value.”
President Lee added, “KAIST and NYU, the two institutions in cross-continental partnership to lead innovations in higher education via the creation of a joint campus, have joined forces to host this forum to create an opportunity to envision the future of a cooperative governance that is inclusive of key players like the government, businesses, the civil societies, academia, and international organizations.”
The 'KAIST-NYU Digital Governance Forum' was broadcast live on KAIST’s Official YouTube Channel from 9:30 am on the 28th of October (Korea Standard Time) with simultaneous interpretation provided in both Korean and English. A recording of the video is available online for everyone to watch free of charge.
KAIST’s YouTube Channel: https://www.youtube.com/c/KAISTofficial
Forum Recording with English interpretation: https://youtu.be/Vs31i7BtfEw
2022.10.28 View 6769
KAIST-NYU Digital Governance Forum Held
KAIST (President Kwang Hyung Lee) held the 'KAIST-NYU Digital Governance Forum' at the Korea Press Center in the morning of October 28th, 2022.
This forum was held in continuation to discuss the objectives of the 'Digital Vision Forum' that was hosted by New York University (NYU) back in September in the United States, and is the first public event to be held through joint efforts by KAIST and NYU since the signage of the 'KAIST-NYU Joint Campus' was presented at the New York forum.
< Signage of KAIST-NYU Joint Campus >
This forum was promoted based on the consensus of the two universities to create an international forum of solidarity to solve global challenges and seek new governance in the era of digital transformation.
Digital innovation technology is expected to bring economic and industrial benefits as well as political, social and ethical risks such as accelerating the digital divide, among others. In particular, in a time of global digital transformation, as the competition for digital and AI supremacy based on technology nationalism catches fire, there is an emergent need for a global governance system in which digital innovation and the value of freedom co-exist. With the consensus formed through this forum with NYU, KAIST plans to focus on detailing the vision for future digital cooperation that encompasses various stakeholders in our society.
To this end, President Kwang Hyung Lee of KAIST and President Andrew Hamilton of NYU led the forum with keynote addresses with President Hamilton taking part virtually, followed by NYU Professor Matthew Liao, a world-renowned scholar specialized in the ethics in the field of science and technology, and Jason Allford, Special Representative of the World Bank Group to Korea, presenting on relevant topics for discussion.
From KAIST, Professor Kyung Ryul Park of the Graduate School of Science and Technology Policy and Director So Young Kim of the Korea Policy Center for the Fourth Industrial Revolution, followed with their presentations. A panel discussion on governance in the period of digital transformation was also held, led by Professor Dongman Lee, the Dean of the College of Engineering.
To kick things off, Professor Matthew Liao of NYU proposed a normative system that can harmonize technology and social ethics while explaining various ethical issues following the technological development of artificial intelligence.
Jason Allford, Special Representative of the World Bank Group to Korea, outlined the changing roles of government in the digital era from the perspective of transparency and government efficiency and explained global development strategies through various cases of digital innovations by international organizations.
Professor Kyung Ryul Park of the Graduate School of Science and Technology Policy at KAIST emphasized that the core of new digital governance is not only innovative technology but also the participation and harmony of various stakeholders at home at abroad and brought up the importance of multi-dimensional international solidarity based on digital transformation that goes beyond the flat ‘technological geopolitics.’
Professor So Young Kim, the Director of the Korea Policy Center for the Fourth Industrial Revolution at KAIST, commented on the current government's digital platform strategy and emphasized the need for a leading digital transformation strategy that goes beyond the governance of the existing government.
Edward Mermelstein, the Commissioner for International Affairs of New York City, said, “The City of New York, shall also provide active support for the cooperative governance initiative organized by KAIST in Korea. As the conversation progresses further, we can draw up plans to organize international organizations to support the effort, likely to be named ‘Digitization for Good’, and we can go on to consider future collaboration,” to express the city’s willingness and anticipation for active cooperation.
Andrew Hamilton, the President of NYU, said "NYU is thrilled by the partnership we are embarking upon with KAIST, which goes hand in hand with our global tradition, and is based upon our bedrock commitment to the free movement of people and ideas.” He added that “As data-driven software, AI, and social networks become even more essential parts of our daily lives, I am confident that today’s discussions will lead to new and promising insights.”
President Kwang Hyung Lee of KAIST said, “It is significant that we are to cooperate with New York University to prepare a venue to assess the changes of the forth coming era at a time in which digital technology, government platforms, and public data are attracting attention as a medium that can create various social and economic value.”
President Lee added, “KAIST and NYU, the two institutions in cross-continental partnership to lead innovations in higher education via the creation of a joint campus, have joined forces to host this forum to create an opportunity to envision the future of a cooperative governance that is inclusive of key players like the government, businesses, the civil societies, academia, and international organizations.”
The 'KAIST-NYU Digital Governance Forum' was broadcast live on KAIST’s Official YouTube Channel from 9:30 am on the 28th of October (Korea Standard Time) with simultaneous interpretation provided in both Korean and English. A recording of the video is available online for everyone to watch free of charge.
KAIST’s YouTube Channel: https://www.youtube.com/c/KAISTofficial
Forum Recording with English interpretation: https://youtu.be/Vs31i7BtfEw
2022.10.28 View 6769 -
 Every Moment of Ultrafast Chemical Bonding Now Captured on Film
- The emerging moment of bond formation, two separate bonding steps, and subsequent vibrational motions were visualized. -
< Emergence of molecular vibrations and the evolution to covalent bonds observed in the research. Video Credit: KEK IMSS >
A team of South Korean researchers led by Professor Hyotcherl Ihee from the Department of Chemistry at KAIST reported the direct observation of the birthing moment of chemical bonds by tracking real-time atomic positions in the molecule. Professor Ihee, who also serves as Associate Director of the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science (IBS), conducted this study in collaboration with scientists at the Institute of Materials Structure Science of High Energy Accelerator Research Organization (KEK IMSS, Japan), RIKEN (Japan), and Pohang Accelerator Laboratory (PAL, South Korea). This work was published in Nature on June 24.
Targeted cancer drugs work by striking a tight bond between cancer cell and specific molecular targets that are involved in the growth and spread of cancer. Detailed images of such chemical bonding sites or pathways can provide key information necessary for maximizing the efficacy of oncogene treatments. However, atomic movements in a molecule have never been captured in the middle of the action, not even for an extremely simple molecule such as a triatomic molecule, made of only three atoms.
Professor Ihee's group and their international collaborators finally succeeded in capturing the ongoing reaction process of the chemical bond formation in the gold trimer. "The femtosecond-resolution images revealed that such molecular events took place in two separate stages, not simultaneously as previously assumed," says Professor Ihee, the corresponding author of the study. "The atoms in the gold trimer complex atoms remain in motion even after the chemical bonding is complete. The distance between the atoms increased and decreased periodically, exhibiting the molecular vibration. These visualized molecular vibrations allowed us to name the characteristic motion of each observed vibrational mode." adds Professor Ihee.
Atoms move extremely fast at a scale of femtosecond (fs) ― quadrillionths (or millionths of a billionth) of a second. Its movement is minute in the level of angstrom equal to one ten-billionth of a meter. They are especially elusive during the transition state where reaction intermediates are transitioning from reactants to products in a flash. The KAIST-IBS research team made this experimentally challenging task possible by using femtosecond x-ray liquidography (solution scattering). This experimental technique combines laser photolysis and x-ray scattering techniques. When a laser pulse strikes the sample, X-rays scatter and initiate the chemical bond formation reaction in the gold trimer complex. Femtosecond x-ray pulses obtained from a special light source called an x-ray free-electron laser (XFEL) were used to interrogate the bond-forming process. The experiments were performed at two XFEL facilities (4th generation linear accelerator) that are PAL-XFEL in South Korea and SACLA in Japan, and this study was conducted in collaboration with researchers from KEK IMSS, PAL, RIKEN, and the Japan Synchrotron Radiation Research Institute (JASRI).
Scattered waves from each atom interfere with each other and thus their x-ray scattering images are characterized by specific travel directions. The KAIST-IBS research team traced real-time positions of the three gold atoms over time by analyzing x-ray scattering images, which are determined by a three-dimensional structure of a molecule. Structural changes in the molecule complex resulted in multiple characteristic scattering images over time. When a molecule is excited by a laser pulse, multiple vibrational quantum states are simultaneously excited. The superposition of several excited vibrational quantum states is called a wave packet. The researchers tracked the wave packet in three-dimensional nuclear coordinates and found that the first half round of chemical bonding was formed within 35 fs after photoexcitation. The second half of the reaction followed within 360 fs to complete the entire reaction dynamics.
They also accurately illustrated molecular vibration motions in both temporal- and spatial-wise. This is quite a remarkable feat considering that such an ultrafast speed and a minute length of motion are quite challenging conditions for acquiring precise experimental data.
In this study, the KAIST-IBS research team improved upon their 2015 study published by Nature. In the previous study in 2015, the speed of the x-ray camera (time resolution) was limited to 500 fs, and the molecular structure had already changed to be linear with two chemical bonds within 500 fs. In this study, the progress of the bond formation and bent-to-linear structural transformation could be observed in real time, thanks to the improvement time resolution down to 100 fs. Thereby, the asynchronous bond formation mechanism in which two chemical bonds are formed in 35 fs and 360 fs, respectively, and the bent-to-linear transformation completed in 335 fs were visualized. In short, in addition to observing the beginning and end of chemical reactions, they reported every moment of the intermediate, ongoing rearrangement of nuclear configurations with dramatically improved experimental and analytical methods.
They will push this method of 'real-time tracking of atomic positions in a molecule and molecular vibration using femtosecond x-ray scattering' to reveal the mechanisms of organic and inorganic catalytic reactions and reactions involving proteins in the human body. "By directly tracking the molecular vibrations and real-time positions of all atoms in a molecule in the middle of reaction, we will be able to uncover mechanisms of various unknown organic and inorganic catalytic reactions and biochemical reactions," notes Dr. Jong Goo Kim, the lead author of the study.
Publications:
Kim, J. G., et al. (2020) ‘Mapping the emergence of molecular vibrations mediating bond formation’. Nature. Volume 582. Page 520-524. Available online at https://doi.org/10.1038/s41586-020-2417-3
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.06.24 View 17152
Every Moment of Ultrafast Chemical Bonding Now Captured on Film
- The emerging moment of bond formation, two separate bonding steps, and subsequent vibrational motions were visualized. -
< Emergence of molecular vibrations and the evolution to covalent bonds observed in the research. Video Credit: KEK IMSS >
A team of South Korean researchers led by Professor Hyotcherl Ihee from the Department of Chemistry at KAIST reported the direct observation of the birthing moment of chemical bonds by tracking real-time atomic positions in the molecule. Professor Ihee, who also serves as Associate Director of the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science (IBS), conducted this study in collaboration with scientists at the Institute of Materials Structure Science of High Energy Accelerator Research Organization (KEK IMSS, Japan), RIKEN (Japan), and Pohang Accelerator Laboratory (PAL, South Korea). This work was published in Nature on June 24.
Targeted cancer drugs work by striking a tight bond between cancer cell and specific molecular targets that are involved in the growth and spread of cancer. Detailed images of such chemical bonding sites or pathways can provide key information necessary for maximizing the efficacy of oncogene treatments. However, atomic movements in a molecule have never been captured in the middle of the action, not even for an extremely simple molecule such as a triatomic molecule, made of only three atoms.
Professor Ihee's group and their international collaborators finally succeeded in capturing the ongoing reaction process of the chemical bond formation in the gold trimer. "The femtosecond-resolution images revealed that such molecular events took place in two separate stages, not simultaneously as previously assumed," says Professor Ihee, the corresponding author of the study. "The atoms in the gold trimer complex atoms remain in motion even after the chemical bonding is complete. The distance between the atoms increased and decreased periodically, exhibiting the molecular vibration. These visualized molecular vibrations allowed us to name the characteristic motion of each observed vibrational mode." adds Professor Ihee.
Atoms move extremely fast at a scale of femtosecond (fs) ― quadrillionths (or millionths of a billionth) of a second. Its movement is minute in the level of angstrom equal to one ten-billionth of a meter. They are especially elusive during the transition state where reaction intermediates are transitioning from reactants to products in a flash. The KAIST-IBS research team made this experimentally challenging task possible by using femtosecond x-ray liquidography (solution scattering). This experimental technique combines laser photolysis and x-ray scattering techniques. When a laser pulse strikes the sample, X-rays scatter and initiate the chemical bond formation reaction in the gold trimer complex. Femtosecond x-ray pulses obtained from a special light source called an x-ray free-electron laser (XFEL) were used to interrogate the bond-forming process. The experiments were performed at two XFEL facilities (4th generation linear accelerator) that are PAL-XFEL in South Korea and SACLA in Japan, and this study was conducted in collaboration with researchers from KEK IMSS, PAL, RIKEN, and the Japan Synchrotron Radiation Research Institute (JASRI).
Scattered waves from each atom interfere with each other and thus their x-ray scattering images are characterized by specific travel directions. The KAIST-IBS research team traced real-time positions of the three gold atoms over time by analyzing x-ray scattering images, which are determined by a three-dimensional structure of a molecule. Structural changes in the molecule complex resulted in multiple characteristic scattering images over time. When a molecule is excited by a laser pulse, multiple vibrational quantum states are simultaneously excited. The superposition of several excited vibrational quantum states is called a wave packet. The researchers tracked the wave packet in three-dimensional nuclear coordinates and found that the first half round of chemical bonding was formed within 35 fs after photoexcitation. The second half of the reaction followed within 360 fs to complete the entire reaction dynamics.
They also accurately illustrated molecular vibration motions in both temporal- and spatial-wise. This is quite a remarkable feat considering that such an ultrafast speed and a minute length of motion are quite challenging conditions for acquiring precise experimental data.
In this study, the KAIST-IBS research team improved upon their 2015 study published by Nature. In the previous study in 2015, the speed of the x-ray camera (time resolution) was limited to 500 fs, and the molecular structure had already changed to be linear with two chemical bonds within 500 fs. In this study, the progress of the bond formation and bent-to-linear structural transformation could be observed in real time, thanks to the improvement time resolution down to 100 fs. Thereby, the asynchronous bond formation mechanism in which two chemical bonds are formed in 35 fs and 360 fs, respectively, and the bent-to-linear transformation completed in 335 fs were visualized. In short, in addition to observing the beginning and end of chemical reactions, they reported every moment of the intermediate, ongoing rearrangement of nuclear configurations with dramatically improved experimental and analytical methods.
They will push this method of 'real-time tracking of atomic positions in a molecule and molecular vibration using femtosecond x-ray scattering' to reveal the mechanisms of organic and inorganic catalytic reactions and reactions involving proteins in the human body. "By directly tracking the molecular vibrations and real-time positions of all atoms in a molecule in the middle of reaction, we will be able to uncover mechanisms of various unknown organic and inorganic catalytic reactions and biochemical reactions," notes Dr. Jong Goo Kim, the lead author of the study.
Publications:
Kim, J. G., et al. (2020) ‘Mapping the emergence of molecular vibrations mediating bond formation’. Nature. Volume 582. Page 520-524. Available online at https://doi.org/10.1038/s41586-020-2417-3
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.06.24 View 17152 -
 In Jin Cho Earned the Best Poster Prize at ME Summit 2017
In Jin Cho, a Ph.D. student in the Department of Chemical and Biomolecular Engineering at KAIST received the best poster prize at the International Metabolic Engineering Summit 2017 held on October 24 in Beijing, China.
The International Metabolic Engineering Summit is a global conference where scientists and corporate researchers in the field of metabolic engineering present their latest research outcomes and build networks.
At this year’s summit, about 500 researchers from around the world participated in active academic exchanges, including giving keynote speeches and presenting posters.
During the poster session, the summit selects one person for the KeAi-synthetic and Systems Biotechnology Poster Award, two for Microbial Cell Factories Poster Awards, and three for Biotechnology Journal Poster Awards among the posters presented by graduate students, post-doctoral fellows and researchers. Cho received the KeAi-synthetic and Systems Biotechnology Poster Award. Her winning poster is on the biotransformation of p-xylene to terephthalic acid using engineered Escherichia coli.
Terephthalic acid is generally produced by p-xylene oxidation; however, this process requires a high temperature and pressure as well as a toxic catalyst during the reaction process.
Cho and Ziwei Luo, a Ph.D. student at KAIST, co-conducted the research and developed a successful biological conversion process. Compared to the existing chemical process, it does not require a high temperature and pressure; and it is environmentally friendly with a relatively high conversion rate of approximately 97%.
Cho’s advisor, Distinguished Professor Sang Yup Lee said, “Further research on glucose-derived terephthalic acid will enable us to produce biomass-based eco-friendly terephthalic acid through engineered Escherichia coli.”
2017.10.31 View 9668
In Jin Cho Earned the Best Poster Prize at ME Summit 2017
In Jin Cho, a Ph.D. student in the Department of Chemical and Biomolecular Engineering at KAIST received the best poster prize at the International Metabolic Engineering Summit 2017 held on October 24 in Beijing, China.
The International Metabolic Engineering Summit is a global conference where scientists and corporate researchers in the field of metabolic engineering present their latest research outcomes and build networks.
At this year’s summit, about 500 researchers from around the world participated in active academic exchanges, including giving keynote speeches and presenting posters.
During the poster session, the summit selects one person for the KeAi-synthetic and Systems Biotechnology Poster Award, two for Microbial Cell Factories Poster Awards, and three for Biotechnology Journal Poster Awards among the posters presented by graduate students, post-doctoral fellows and researchers. Cho received the KeAi-synthetic and Systems Biotechnology Poster Award. Her winning poster is on the biotransformation of p-xylene to terephthalic acid using engineered Escherichia coli.
Terephthalic acid is generally produced by p-xylene oxidation; however, this process requires a high temperature and pressure as well as a toxic catalyst during the reaction process.
Cho and Ziwei Luo, a Ph.D. student at KAIST, co-conducted the research and developed a successful biological conversion process. Compared to the existing chemical process, it does not require a high temperature and pressure; and it is environmentally friendly with a relatively high conversion rate of approximately 97%.
Cho’s advisor, Distinguished Professor Sang Yup Lee said, “Further research on glucose-derived terephthalic acid will enable us to produce biomass-based eco-friendly terephthalic acid through engineered Escherichia coli.”
2017.10.31 View 9668 -
 From Pencil Lead to Batteries: the Unlimited Transformation of Carbon
Those materials, like lead or diamond, made completely up of Carbon are being used in numerous ways as materials or parts. Especially with the discovery of carbon nanotubes, graphemes, and other carbon based materials in nanoscale, the carbon based materials are receiving a lot of interest in both fields of research and industry.
The carbon nanotubes and graphemes are considered as the ‘dream material’ and have a structure of a cross section of a bee hive. Such structure allows the material to have strength higher than that of a diamond and still be able to bend, be transparent and also conduct electricity. However the problem up till now was that these carbon structures appeared in layers and in bunches and were therefore hard to separate to individual layers or tubes.
Professor Kim Sang Wook’s research team developed the technology that can assemble the grapheme and carbon nanotubes in a three dimensional manner.
The team was able to assemble the grapheme ad carbon nanotubes in an entirely new three dimensional structure. In addition, the team was able to efficiently extract single layered grapheme from cheap pencil lead.
Professor Kim is scheduled to give a guest lecture in the “Materials Research Society” in San Francisco and the paper was published in ‘Advanced Functional Materials’ magazine as an ‘Invited Feature Article’.
2011.05.11 View 11593
From Pencil Lead to Batteries: the Unlimited Transformation of Carbon
Those materials, like lead or diamond, made completely up of Carbon are being used in numerous ways as materials or parts. Especially with the discovery of carbon nanotubes, graphemes, and other carbon based materials in nanoscale, the carbon based materials are receiving a lot of interest in both fields of research and industry.
The carbon nanotubes and graphemes are considered as the ‘dream material’ and have a structure of a cross section of a bee hive. Such structure allows the material to have strength higher than that of a diamond and still be able to bend, be transparent and also conduct electricity. However the problem up till now was that these carbon structures appeared in layers and in bunches and were therefore hard to separate to individual layers or tubes.
Professor Kim Sang Wook’s research team developed the technology that can assemble the grapheme and carbon nanotubes in a three dimensional manner.
The team was able to assemble the grapheme ad carbon nanotubes in an entirely new three dimensional structure. In addition, the team was able to efficiently extract single layered grapheme from cheap pencil lead.
Professor Kim is scheduled to give a guest lecture in the “Materials Research Society” in San Francisco and the paper was published in ‘Advanced Functional Materials’ magazine as an ‘Invited Feature Article’.
2011.05.11 View 11593 -
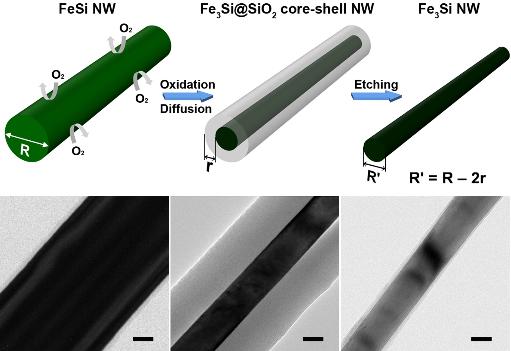 Nanowire crystal transformation method was newly developed by a KAIST research team.
Figure 1
Schematic illustration of NW crystal transformation process. FeSi is converted to Fe3Si by high-temperature thermal annealing in diluted O2 condition and subsequent wet etching by 5% HF.
Figure 2
Low-resolution TEM images of FeSi; Fe3Si@SiO2 core—shell; Fe3Si NW after shell-etching; and Scale bars are 20 nm
Professor Bongsoo Kim of the Department of Chemistry, KAIST, and his research team succeeded to fabricate Heusler alloy Fe3Si nanowires by a diffusion-driven crystal structure transformation method from paramagnetic FeSi nanowires. This methodology is also applied to Co2Si nanowires in order to obtain metal-rich nanowires (Co) as another evidence of the structural transformation process. The newly developed nanowire crystal transformation method, Professor Kim said, would be valuable as a general method to fabricate metal-rich silicide nanowires that are otherwise difficult to synthesize.
Metal silicide nanowires are potentially useful in a wide array of fields including nao-optics, information technology, biosensors, and medicine. Chemical synthesis of these nanowires, however, is challenging due to the complex phase behavior of silicides.
The metal silicide nanowires are grown on a silicon substrate covered with a thin layer of silicon oxide via a simple chemical vapor deposition (CVD) process using single or multiple source precursors. Alternatively, the nanowires can be grown on the thin silicon oxide film via a chemical vapor transport (CVT) process using solid metal silicide precursors.
The CVT-based method has been highly effective for the syntheses of metal silicide NWs, but changing the composition of metal silicide NWs in a wider range, especially achieving a composition of a metal to silicon, has been quite difficult.
Thus, developing efficient and reliable synthetic methods to adjust flexibly the elemental compositions in metal silicide NWs can be valuable for the fabrication of practical spintronic and neonelectronic devices.
Professor Kim expliained, “The key concept underlying this work is metal-enrichment of metal silicide NWs by thermal diffusion. This conversion method could prove highly valuable, since novel metal-rich silicide NWs that are difficult to synthesize but possess interesting physical properties can be fabricated from other metal silicide NWs.”
The research result was published in Nanao Letters, a leading peer-reviewed journal, and posted online in early August 2010.
2010.08.25 View 11674
Nanowire crystal transformation method was newly developed by a KAIST research team.
Figure 1
Schematic illustration of NW crystal transformation process. FeSi is converted to Fe3Si by high-temperature thermal annealing in diluted O2 condition and subsequent wet etching by 5% HF.
Figure 2
Low-resolution TEM images of FeSi; Fe3Si@SiO2 core—shell; Fe3Si NW after shell-etching; and Scale bars are 20 nm
Professor Bongsoo Kim of the Department of Chemistry, KAIST, and his research team succeeded to fabricate Heusler alloy Fe3Si nanowires by a diffusion-driven crystal structure transformation method from paramagnetic FeSi nanowires. This methodology is also applied to Co2Si nanowires in order to obtain metal-rich nanowires (Co) as another evidence of the structural transformation process. The newly developed nanowire crystal transformation method, Professor Kim said, would be valuable as a general method to fabricate metal-rich silicide nanowires that are otherwise difficult to synthesize.
Metal silicide nanowires are potentially useful in a wide array of fields including nao-optics, information technology, biosensors, and medicine. Chemical synthesis of these nanowires, however, is challenging due to the complex phase behavior of silicides.
The metal silicide nanowires are grown on a silicon substrate covered with a thin layer of silicon oxide via a simple chemical vapor deposition (CVD) process using single or multiple source precursors. Alternatively, the nanowires can be grown on the thin silicon oxide film via a chemical vapor transport (CVT) process using solid metal silicide precursors.
The CVT-based method has been highly effective for the syntheses of metal silicide NWs, but changing the composition of metal silicide NWs in a wider range, especially achieving a composition of a metal to silicon, has been quite difficult.
Thus, developing efficient and reliable synthetic methods to adjust flexibly the elemental compositions in metal silicide NWs can be valuable for the fabrication of practical spintronic and neonelectronic devices.
Professor Kim expliained, “The key concept underlying this work is metal-enrichment of metal silicide NWs by thermal diffusion. This conversion method could prove highly valuable, since novel metal-rich silicide NWs that are difficult to synthesize but possess interesting physical properties can be fabricated from other metal silicide NWs.”
The research result was published in Nanao Letters, a leading peer-reviewed journal, and posted online in early August 2010.
2010.08.25 View 11674