ICA
-
 KAIST reveals for the first time the mechanism by which alcohol triggers liver inflammation
<(From left)Dr. Keungmo Yang, Professor Won-Il Jeong, Ph.D candidate Kyurae Kim>
Excessive alcohol consumption causes alcoholic liver disease, and about 20% of these cases progress to alcohol-associated steatohepatitis (ASH), which can lead to liver cirrhosis and liver failure. Early diagnosis and treatment are therefore extremely important. A KAIST research team has identified a new molecular mechanism in which alcohol-damaged liver cells increase reactive oxygen species (ROS), leading to cell death and inflammatory responses. In addition, they discovered that Kupffer cells, immune cells residing in the liver, act as a “dual-function regulator” that can either promote or suppress inflammation through interactions with liver cells.
KAIST (President Kwang-Hyung Lee) announced on the 17th that a research team led by Professor Won-Il Jeong from the Graduate School of Medical Science and Engineering, in collaboration with Professor Won Kim’s team at Seoul National University Boramae Medical Center, has uncovered the molecular pathway of liver damage and inflammation caused by alcohol consumption. This finding offers new clues for the diagnosis and treatment of alcohol-associated liver disease (ALD).
Professor Won-Il Jeong’s research team found that during chronic alcohol intake, expression of the vesicular glutamate transporter VGLUT3 increases, leading to glutamate accumulation in hepatocytes. Subsequent binge drinking causes rapid changes in intracellular calcium levels, which then triggers glutamate* secretion. The secreted glutamate stimulates the glutamate receptor mGluR5 on liver-resident macrophages (Kupffer cells), which induces ROS production and activates a pathological pathway resulting in hepatocyte death and inflammation.
*Glutamate: A type of amino acid involved in intercellular signaling, protein synthesis, and energy metabolism in various tissues including the brain and liver. In excess, it can cause overexcitation and death of nerve cells.
<Figure1. Glutamate accumulation in perivenous hepatocytes through vesicular glutamate transporter 3 after 2-week EtOH intake and its release by binge drinking>
A particularly groundbreaking aspect of this study is that damaged hepatocytes and Kupffer cells can form a "pseudosynapse"—a structure similar to a synapse which is previously thought to occur only in the brain—enabling them to exchange signals. This is the first time such a phenomenon has been identified in the liver.
This pseudosynapse forms when hepatocytes expand (ballooning) due to alcohol, becoming physically attached to Kupffer cells. Simply put, the damaged hepatocytes don’t just die—they send distress signals to nearby immune cells, prompting a response.
This discovery proposes a new paradigm: even in peripheral organs, direct structural contact between cells can allow signal transmission. It also shows that damaged hepatocytes can actively stimulate macrophages and induce regeneration through cell death, revealing the liver’s “autonomous recovery function.”
The team also confirmed in animal models that genetic or pharmacological inhibition of VGLUT3, mGluR5, or the ROS-producing enzyme NOX2 reduces alcohol-induced liver damage. They also confirmed that the same mechanism observed in animal models was present in human patients with ALD by analyzing blood and liver tissue samples.
<Figure2. Binge drinking rapidly alters the intracellular calcium levels to release glutamates and activate mGluR5 of Kupffer cells>
Professor Won-Il Jeong of KAIST said, “These findings may serve as new molecular targets for early diagnosis and treatment of ASH in the future.”
This study was jointly led by Dr. Keungmo Yang (now at Yeouido St. Mary’s Hospital) and Kyurae Kim, a doctoral candidate at KAIST, who served as co–first authors. It was conducted in collaboration with Professor Won Kim’s team at Seoul National University Boramae Medical Center and was published in the journal Nature Communications on July 1.
※ Article Title: Binge drinking triggers VGLUT3-mediated glutamate secretion and subsequent hepatic inflammation by activating mGluR5/NOX2 in Kupffer cells ※ DOI: https://doi.org/10.1038/s41467-025-60820-3
This study was supported by the Ministry of Science and ICT through the National Research Foundation of Korea's Global Leader Program, Mid-Career Researcher Program, and the Bio & Medical Technology Development Program.
2025.07.17 View 74
KAIST reveals for the first time the mechanism by which alcohol triggers liver inflammation
<(From left)Dr. Keungmo Yang, Professor Won-Il Jeong, Ph.D candidate Kyurae Kim>
Excessive alcohol consumption causes alcoholic liver disease, and about 20% of these cases progress to alcohol-associated steatohepatitis (ASH), which can lead to liver cirrhosis and liver failure. Early diagnosis and treatment are therefore extremely important. A KAIST research team has identified a new molecular mechanism in which alcohol-damaged liver cells increase reactive oxygen species (ROS), leading to cell death and inflammatory responses. In addition, they discovered that Kupffer cells, immune cells residing in the liver, act as a “dual-function regulator” that can either promote or suppress inflammation through interactions with liver cells.
KAIST (President Kwang-Hyung Lee) announced on the 17th that a research team led by Professor Won-Il Jeong from the Graduate School of Medical Science and Engineering, in collaboration with Professor Won Kim’s team at Seoul National University Boramae Medical Center, has uncovered the molecular pathway of liver damage and inflammation caused by alcohol consumption. This finding offers new clues for the diagnosis and treatment of alcohol-associated liver disease (ALD).
Professor Won-Il Jeong’s research team found that during chronic alcohol intake, expression of the vesicular glutamate transporter VGLUT3 increases, leading to glutamate accumulation in hepatocytes. Subsequent binge drinking causes rapid changes in intracellular calcium levels, which then triggers glutamate* secretion. The secreted glutamate stimulates the glutamate receptor mGluR5 on liver-resident macrophages (Kupffer cells), which induces ROS production and activates a pathological pathway resulting in hepatocyte death and inflammation.
*Glutamate: A type of amino acid involved in intercellular signaling, protein synthesis, and energy metabolism in various tissues including the brain and liver. In excess, it can cause overexcitation and death of nerve cells.
<Figure1. Glutamate accumulation in perivenous hepatocytes through vesicular glutamate transporter 3 after 2-week EtOH intake and its release by binge drinking>
A particularly groundbreaking aspect of this study is that damaged hepatocytes and Kupffer cells can form a "pseudosynapse"—a structure similar to a synapse which is previously thought to occur only in the brain—enabling them to exchange signals. This is the first time such a phenomenon has been identified in the liver.
This pseudosynapse forms when hepatocytes expand (ballooning) due to alcohol, becoming physically attached to Kupffer cells. Simply put, the damaged hepatocytes don’t just die—they send distress signals to nearby immune cells, prompting a response.
This discovery proposes a new paradigm: even in peripheral organs, direct structural contact between cells can allow signal transmission. It also shows that damaged hepatocytes can actively stimulate macrophages and induce regeneration through cell death, revealing the liver’s “autonomous recovery function.”
The team also confirmed in animal models that genetic or pharmacological inhibition of VGLUT3, mGluR5, or the ROS-producing enzyme NOX2 reduces alcohol-induced liver damage. They also confirmed that the same mechanism observed in animal models was present in human patients with ALD by analyzing blood and liver tissue samples.
<Figure2. Binge drinking rapidly alters the intracellular calcium levels to release glutamates and activate mGluR5 of Kupffer cells>
Professor Won-Il Jeong of KAIST said, “These findings may serve as new molecular targets for early diagnosis and treatment of ASH in the future.”
This study was jointly led by Dr. Keungmo Yang (now at Yeouido St. Mary’s Hospital) and Kyurae Kim, a doctoral candidate at KAIST, who served as co–first authors. It was conducted in collaboration with Professor Won Kim’s team at Seoul National University Boramae Medical Center and was published in the journal Nature Communications on July 1.
※ Article Title: Binge drinking triggers VGLUT3-mediated glutamate secretion and subsequent hepatic inflammation by activating mGluR5/NOX2 in Kupffer cells ※ DOI: https://doi.org/10.1038/s41467-025-60820-3
This study was supported by the Ministry of Science and ICT through the National Research Foundation of Korea's Global Leader Program, Mid-Career Researcher Program, and the Bio & Medical Technology Development Program.
2025.07.17 View 74 -
 A KAIST Team Engineers a Microbial Platform for Efficient Lutein Production
<(From Left) Ph.D. Candidate Hyunmin Eun, Distinguished Professor Sang Yup Lee, , Dr. Cindy Pricilia Surya Prabowo>
The application of systems metabolic engineering strategies, along with the construction of an electron channeling system, has enabled the first gram-per-liter scale production of lutein from Corynebacterium glutamicum, providing a viable alternative to plant-derived lutein production.
A research group at KAIST has successfully engineered a microbial strain capable of producing lutein at industrially relevant levels. The team, led by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering, developed a novel C. glutamicum strain using systems metabolic engineering strategies to overcome the limitations of previous microbial lutein production efforts. This research is expected to be beneficial for the efficient production of other industrially important natural products used in food, pharmaceuticals, and cosmetics.
Lutein is a xanthophyll carotenoid found in egg yolk, fruits, and vegetables, known for its role in protecting our eyes from oxidative stress and reducing the risk of macular degeneration and cataracts. Currently, commercial lutein is predominantly extracted from marigold flowers; however, this approach has several drawbacks, including long cultivation times, high labor costs, and inefficient extraction yields, making it economically unfeasible for large-scale production. These challenges have driven the demand for alternative production methods.
To address these issues, KAIST researchers, including Ph.D. Candidate Hyunmin Eun, Dr. Cindy Pricilia Surya Prabowo, and Distinguished Professor Sang Yup Lee, applied systems metabolic engineering strategies to engineer C. glutamicum, a GRAS (Generally Recognized As Safe) microorganism widely used in industrial fermentation. Unlike Escherichia coli, which was previously explored for microbial lutein production, C. glutamicum lacks endotoxins, making it a safer and more viable option for food and pharmaceutical applications.
The team’s work, entitled “Gram-per-litre scale production of lutein by engineered Corynebacterium,” was published in Nature Synthesis on 04 July , 2025.
This research details the high-level production of lutein using glucose as a renewable carbon source via systems metabolic engineering. The team focused on eliminating metabolic bottlenecks that previously limited microbial lutein synthesis. By employing enzyme scaffold-based electron channeling strategies, the researchers improved metabolic flux towards lutein biosynthesis while minimizing unwanted byproducts.
<Lutein production metabolic pathway engineering>
To enhance productivity, bottleneck enzymes within the metabolic pathway were identified and optimized. It was determined that electron-requiring cytochrome P450 enzymes played a major role in limiting lutein biosynthesis. To overcome this limitation, an electron channeling strategy was implemented, where engineered cytochrome P450 enzymes and their reductase partners were spatially organized on synthetic scaffolds, allowing more efficient electron transfer and significantly increasing lutein production.
The engineered C. glutamicum strain was further optimized in fed-batch fermentation, achieving a record-breaking 1.78 g/L of lutein production within 54 hours, with a content of 19.51 mg/gDCW and a productivity of 32.88 mg/L/h—the highest lutein production performance in any host reported to date. This milestone demonstrates the feasibility of replacing plant-based lutein extraction with microbial fermentation technology.
“We can anticipate that this microbial cell factory-based mass production of lutein will be able to replace the current plant extraction-based process,” said Ph.D. Candidate Hyunmin Eun. He emphasized that the integrated metabolic engineering strategies developed in this study could be broadly applied for the efficient production of other valuable natural products used in pharmaceuticals and nutraceuticals.
<Schematic diagram of microbial-based lutein production platform>
“As maintaining good health in an aging society becomes increasingly important, we expect that the technology and strategies developed here will play pivotal roles in producing other medically and nutritionally significant natural products,” added Distinguished Professor Sang Yup Lee.
This work is supported by the Development of Next-generation Biorefinery Platform Technologies for Leading Bio-based Chemicals Industry project 2022M3J5A1056072 and the Development of Platform Technologies of Microbial Cell Factories for the Next-Generation Biorefineries project 2022M3J5A1056117 from the National Research Foundation supported by the Korean Ministry of Science and ICT.
Source:
Hyunmin Eun (1st), Cindy Pricilia Surya Prabowo (co-1st), and Sang Yup Lee (Corresponding). “Gram-per-litre scale production of lutein by engineered Corynebacterium”. Nature Synthesis (Online published)
For further information:
Sang Yup Lee, Distinguished Professor of Chemical and Biomolecular Engineering, KAIST (leesy@kaist.ac.kr, Tel: +82-42-350-3930)
2025.07.14 View 227
A KAIST Team Engineers a Microbial Platform for Efficient Lutein Production
<(From Left) Ph.D. Candidate Hyunmin Eun, Distinguished Professor Sang Yup Lee, , Dr. Cindy Pricilia Surya Prabowo>
The application of systems metabolic engineering strategies, along with the construction of an electron channeling system, has enabled the first gram-per-liter scale production of lutein from Corynebacterium glutamicum, providing a viable alternative to plant-derived lutein production.
A research group at KAIST has successfully engineered a microbial strain capable of producing lutein at industrially relevant levels. The team, led by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering, developed a novel C. glutamicum strain using systems metabolic engineering strategies to overcome the limitations of previous microbial lutein production efforts. This research is expected to be beneficial for the efficient production of other industrially important natural products used in food, pharmaceuticals, and cosmetics.
Lutein is a xanthophyll carotenoid found in egg yolk, fruits, and vegetables, known for its role in protecting our eyes from oxidative stress and reducing the risk of macular degeneration and cataracts. Currently, commercial lutein is predominantly extracted from marigold flowers; however, this approach has several drawbacks, including long cultivation times, high labor costs, and inefficient extraction yields, making it economically unfeasible for large-scale production. These challenges have driven the demand for alternative production methods.
To address these issues, KAIST researchers, including Ph.D. Candidate Hyunmin Eun, Dr. Cindy Pricilia Surya Prabowo, and Distinguished Professor Sang Yup Lee, applied systems metabolic engineering strategies to engineer C. glutamicum, a GRAS (Generally Recognized As Safe) microorganism widely used in industrial fermentation. Unlike Escherichia coli, which was previously explored for microbial lutein production, C. glutamicum lacks endotoxins, making it a safer and more viable option for food and pharmaceutical applications.
The team’s work, entitled “Gram-per-litre scale production of lutein by engineered Corynebacterium,” was published in Nature Synthesis on 04 July , 2025.
This research details the high-level production of lutein using glucose as a renewable carbon source via systems metabolic engineering. The team focused on eliminating metabolic bottlenecks that previously limited microbial lutein synthesis. By employing enzyme scaffold-based electron channeling strategies, the researchers improved metabolic flux towards lutein biosynthesis while minimizing unwanted byproducts.
<Lutein production metabolic pathway engineering>
To enhance productivity, bottleneck enzymes within the metabolic pathway were identified and optimized. It was determined that electron-requiring cytochrome P450 enzymes played a major role in limiting lutein biosynthesis. To overcome this limitation, an electron channeling strategy was implemented, where engineered cytochrome P450 enzymes and their reductase partners were spatially organized on synthetic scaffolds, allowing more efficient electron transfer and significantly increasing lutein production.
The engineered C. glutamicum strain was further optimized in fed-batch fermentation, achieving a record-breaking 1.78 g/L of lutein production within 54 hours, with a content of 19.51 mg/gDCW and a productivity of 32.88 mg/L/h—the highest lutein production performance in any host reported to date. This milestone demonstrates the feasibility of replacing plant-based lutein extraction with microbial fermentation technology.
“We can anticipate that this microbial cell factory-based mass production of lutein will be able to replace the current plant extraction-based process,” said Ph.D. Candidate Hyunmin Eun. He emphasized that the integrated metabolic engineering strategies developed in this study could be broadly applied for the efficient production of other valuable natural products used in pharmaceuticals and nutraceuticals.
<Schematic diagram of microbial-based lutein production platform>
“As maintaining good health in an aging society becomes increasingly important, we expect that the technology and strategies developed here will play pivotal roles in producing other medically and nutritionally significant natural products,” added Distinguished Professor Sang Yup Lee.
This work is supported by the Development of Next-generation Biorefinery Platform Technologies for Leading Bio-based Chemicals Industry project 2022M3J5A1056072 and the Development of Platform Technologies of Microbial Cell Factories for the Next-Generation Biorefineries project 2022M3J5A1056117 from the National Research Foundation supported by the Korean Ministry of Science and ICT.
Source:
Hyunmin Eun (1st), Cindy Pricilia Surya Prabowo (co-1st), and Sang Yup Lee (Corresponding). “Gram-per-litre scale production of lutein by engineered Corynebacterium”. Nature Synthesis (Online published)
For further information:
Sang Yup Lee, Distinguished Professor of Chemical and Biomolecular Engineering, KAIST (leesy@kaist.ac.kr, Tel: +82-42-350-3930)
2025.07.14 View 227 -
 Professor Jung-woo' Choi ‘s Team Comes in First at the World's Top Acoustic AI Challenge
<Photo1. (From left) Ph.D candidate Yong-hoo Kwon, M.S candidate Do-hwan Kim, Professor Jung-woo Choi, Dr. Dong-heon Lee>
'Acoustic separation and classification technology' is a next-generation artificial intelligence (AI) core technology that enables the early detection of abnormal sounds in areas such as drones, fault detection of factory pipelines, and border surveillance systems, or allows for the separation and editing of spatial audio by sound source when producing AR/VR content.
On the 11th of July, a research team led by Professor Jung-woo Choi of KAIST's Department of Electrical and Electronic Engineering won first place in the 'Spatial Semantic Segmentation of Sound Scenes' task of the 'DCASE2025 Challenge,' the world's most prestigious acoustic detection and analysis competition.
This year’s challenge featured 86 teams competing across six tasks. In this competition, the KAIST research team achieved the best performance in their first-ever participation to Task 4. Professor Jung-woo Choi’s research team consisted of Dr. Dong-heon, Lee, Ph.D. candidate Young-hoo Kwon, and M.S. candidate Do-hwan Kim.
Task 4 titled 'Spatial Semantic Segmentation of Sound Scenes' is a highly demanding task requiring the analysis of spatial information in multi-channel audio signals with overlapping sound sources. The goal was to separate individual sounds and classify them into 18 predefined categories. The research team plans to present their technology at the DCASE workshop in Barcelona this October.
<Figure 1. Example of an acoustic scene with multiple mixed sounds>
Early this year, Dr. Dong-heon Lee developed a state-of-the-art sound source separation AI that combines Transformer and Mamba architectures. During the competition, centered around researcher Young-hoo Kwon, they completed a ‘chain-of-inference architecture' AI model that performs sound source separation and classification again, using the waveforms and types of the initially separated sound sources as clues. This AI model is inspired by human’s auditory scene analysis mechanism that isolates individual sounds by focusing on incomplete clues such as sound type, rhythm, or direction, when listening to complex sounds.
Through this, the team was the only participant to achieve double-digit performance (11 dB) in 'Class-Aware Signal-to-Distortion Ratio Improvement (CA-SDRi)*,' which is the measure for ranking how well the AI separated and classified sounds, proving their technical excellence.
Class-Aware Signal-to-Distortion Ratio Improvement (CA-SDRi): Measures how much clearer (less distorted) the desired sound is separated and classified compared to the original audio, in dB (decibels). A higher number indicates more accurate and cleaner sound separation.
Prof. Jung-woo Choi remarked, "The research team has showcased world-leading acoustic separation AI models for the past three years, and I am delighted that these results have been officially recognized." He added, "I am proud of every member of the research team for winning first place through focused research, despite the significant increase in difficulty and having only a few weeks for development."
<Figure 2. Time-frequency patterns of sound sources separated from a mixed source>
The IEEE DCASE Challenge 2025 was held online, with submissions accepted from April 1 to June 15 and results announced on June 30. Since its launch in 2013, the DCASE Challenge has served as a premier global platform of IEEE Signal Processing Society for showcasing cutting-edge AI models in acoustic signal processing.
This research was supported by the Mid-Career Researcher Support Project and STEAM Research Project of the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology, as well as support from the Future Defense Research Center, funded by the Defense Acquisition Program Administration and the Agency for Defense Development.
2025.07.13 View 69
Professor Jung-woo' Choi ‘s Team Comes in First at the World's Top Acoustic AI Challenge
<Photo1. (From left) Ph.D candidate Yong-hoo Kwon, M.S candidate Do-hwan Kim, Professor Jung-woo Choi, Dr. Dong-heon Lee>
'Acoustic separation and classification technology' is a next-generation artificial intelligence (AI) core technology that enables the early detection of abnormal sounds in areas such as drones, fault detection of factory pipelines, and border surveillance systems, or allows for the separation and editing of spatial audio by sound source when producing AR/VR content.
On the 11th of July, a research team led by Professor Jung-woo Choi of KAIST's Department of Electrical and Electronic Engineering won first place in the 'Spatial Semantic Segmentation of Sound Scenes' task of the 'DCASE2025 Challenge,' the world's most prestigious acoustic detection and analysis competition.
This year’s challenge featured 86 teams competing across six tasks. In this competition, the KAIST research team achieved the best performance in their first-ever participation to Task 4. Professor Jung-woo Choi’s research team consisted of Dr. Dong-heon, Lee, Ph.D. candidate Young-hoo Kwon, and M.S. candidate Do-hwan Kim.
Task 4 titled 'Spatial Semantic Segmentation of Sound Scenes' is a highly demanding task requiring the analysis of spatial information in multi-channel audio signals with overlapping sound sources. The goal was to separate individual sounds and classify them into 18 predefined categories. The research team plans to present their technology at the DCASE workshop in Barcelona this October.
<Figure 1. Example of an acoustic scene with multiple mixed sounds>
Early this year, Dr. Dong-heon Lee developed a state-of-the-art sound source separation AI that combines Transformer and Mamba architectures. During the competition, centered around researcher Young-hoo Kwon, they completed a ‘chain-of-inference architecture' AI model that performs sound source separation and classification again, using the waveforms and types of the initially separated sound sources as clues. This AI model is inspired by human’s auditory scene analysis mechanism that isolates individual sounds by focusing on incomplete clues such as sound type, rhythm, or direction, when listening to complex sounds.
Through this, the team was the only participant to achieve double-digit performance (11 dB) in 'Class-Aware Signal-to-Distortion Ratio Improvement (CA-SDRi)*,' which is the measure for ranking how well the AI separated and classified sounds, proving their technical excellence.
Class-Aware Signal-to-Distortion Ratio Improvement (CA-SDRi): Measures how much clearer (less distorted) the desired sound is separated and classified compared to the original audio, in dB (decibels). A higher number indicates more accurate and cleaner sound separation.
Prof. Jung-woo Choi remarked, "The research team has showcased world-leading acoustic separation AI models for the past three years, and I am delighted that these results have been officially recognized." He added, "I am proud of every member of the research team for winning first place through focused research, despite the significant increase in difficulty and having only a few weeks for development."
<Figure 2. Time-frequency patterns of sound sources separated from a mixed source>
The IEEE DCASE Challenge 2025 was held online, with submissions accepted from April 1 to June 15 and results announced on June 30. Since its launch in 2013, the DCASE Challenge has served as a premier global platform of IEEE Signal Processing Society for showcasing cutting-edge AI models in acoustic signal processing.
This research was supported by the Mid-Career Researcher Support Project and STEAM Research Project of the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology, as well as support from the Future Defense Research Center, funded by the Defense Acquisition Program Administration and the Agency for Defense Development.
2025.07.13 View 69 -
 KAIST Shows That the Brain Can Distinguish Glucose: Clues to Treat Obesity and Diabetes
<(From left)Prof. Greg S.B Suh, Dr. Jieun Kim, Dr. Shinhye Kim, Researcher Wongyo Jeong)
“How does our brain distinguish glucose from the many nutrients absorbed in the gut?” Starting with this question, a KAIST research team has demonstrated that the brain can selectively recognize specific nutrients—particularly glucose—beyond simply detecting total calorie content. This study is expected to offer a new paradigm for appetite control and the treatment of metabolic diseases.
On the 9th, KAIST (President Kwang Hyung Lee) announced that Professor Greg S.B. Suh’s team in the Department of Biological Sciences, in collaboration with Professor Young-Gyun Park’s team (BarNeuro), Professor Seung-Hee Lee’s team (Department of Biological Sciences), and the Albert Einstein College of Medicine in New York, had identified the existence of a gut-brain circuit that allows animals in a hungry state to selectively detect and prefer glucose in the gut.
Organisms derive energy from various nutrients including sugars, proteins, and fats. Previous studies have shown that total caloric information in the gut suppresses hunger neurons in the hypothalamus to regulate appetite. However, the existence of a gut-brain circuit that specifically responds to glucose and corresponding brain cells had not been demonstrated until now.
In this study, the team successfully identified a “gut-brain circuit” that senses glucose—essential for brain function—and regulates food intake behavior for required nutrients.
They further proved, for the first time, that this circuit responds within seconds to not only hunger or external stimuli but also to specific caloric nutrients directly introduced into the small intestine, particularly D-glucose, through the activity of “CRF neurons*” in the brain’s hypothalamus.
*CRF neurons: These neurons secrete corticotropin-releasing factor (CRF) in the hypothalamus and are central to the hypothalamic-pituitary-adrenal (HPA) axis, the body’s core physiological system for responding to stress. CRF neurons are known to regulate neuroendocrine balance in response to stress stimuli.
Using optogenetics to precisely track neural activity in real time, the researchers injected various nutrients—D-glucose, L-glucose, amino acids, and fats—directly into the small intestines of mice and observed the results.
They discovered that among the CRF neurons located in the paraventricular nucleus (PVN)* of the hypothalamus, only those specific to D-glucose showed selective responses. These neurons did not respond—or showed inverse reactions—to other sugars or to proteins and fats. This is the first demonstration that single neurons in the brain can guide nutrient-specific responses depending on gut nutrient influx.
*PVN (Paraventricular Nucleus): A key nucleus within the hypothalamus responsible for maintaining bodily homeostasis.
The team also revealed that glucose-sensing signals in the small intestine are transmitted via the spinal cord to the dorsolateral parabrachial nucleus (PBNdl) of the brain, and from there to CRF neurons in the PVN. In contrast, signals for amino acids and fats are transmitted to the brain through the vagus nerve, a different pathway.
In optogenetic inhibition experiments, suppressing CRF neurons in fasting mice eliminated their preference for glucose, proving that this circuit is essential for glucose-specific nutrient preference.
This study was inspired by Professor Suh’s earlier research at NYU using fruit flies, where he identified “DH44 neurons” that selectively detect glucose and sugar in the gut. Based on the hypothesis that hypothalamic neurons in mammals would show similar functional responses to glucose, the current study was launched.
To test this hypothesis, Dr. Jineun Kim (KAIST Ph.D. graduate, now at Caltech) demonstrated during her doctoral research that hungry mice preferred glucose among various intragastrically infused nutrients and that CRF neurons exhibited rapid and specific responses.
Along with Wongyo Jung (KAIST B.S. graduate, now Ph.D. student at Caltech), they modeled and experimentally confirmed the critical role of CRF neurons. Dr. Shinhye Kim, through collaboration, revealed that specific spinal neurons play a key role in conveying intestinal nutrient information to the brain.
Dr. Jineun Kim and Dr. Shinhye Kim said, “This study started from a simple but fundamental question—‘How does the brain distinguish glucose from various nutrients absorbed in the gut?’ We have shown that spinal-based gut-brain circuits play a central role in energy metabolism and homeostasis by transmitting specific gut nutrient signals to the brain.”
Professor Suh added, “By identifying a gut-brain pathway specialized for glucose, this research offers a new therapeutic target for metabolic diseases such as obesity and diabetes. Our future research will explore similar circuits for sensing other essential nutrients like amino acids and fats and their interaction mechanisms.”
Ph.D. student Jineun Kim, Dr. Shinhye Kim, and student Wongyo Jung (co-first authors) contributed to this study, which was published online in the international journal Neuron on June 20, 2025.
※ Paper Title: Encoding the glucose identity by discrete hypothalamic neurons via the gut-brain axis ※ DOI: https://doi.org/10.1016/j.neuron.2025.05.024
This study was supported by the Samsung Science & Technology Foundation, the National Research Foundation of Korea (NRF) Leader Research Program, the POSCO Cheongam Science Fellowship, the Asan Foundation Biomedical Science Scholarship, the Institute for Basic Science (IBS), and the KAIST KAIX program.
2025.07.09 View 266
KAIST Shows That the Brain Can Distinguish Glucose: Clues to Treat Obesity and Diabetes
<(From left)Prof. Greg S.B Suh, Dr. Jieun Kim, Dr. Shinhye Kim, Researcher Wongyo Jeong)
“How does our brain distinguish glucose from the many nutrients absorbed in the gut?” Starting with this question, a KAIST research team has demonstrated that the brain can selectively recognize specific nutrients—particularly glucose—beyond simply detecting total calorie content. This study is expected to offer a new paradigm for appetite control and the treatment of metabolic diseases.
On the 9th, KAIST (President Kwang Hyung Lee) announced that Professor Greg S.B. Suh’s team in the Department of Biological Sciences, in collaboration with Professor Young-Gyun Park’s team (BarNeuro), Professor Seung-Hee Lee’s team (Department of Biological Sciences), and the Albert Einstein College of Medicine in New York, had identified the existence of a gut-brain circuit that allows animals in a hungry state to selectively detect and prefer glucose in the gut.
Organisms derive energy from various nutrients including sugars, proteins, and fats. Previous studies have shown that total caloric information in the gut suppresses hunger neurons in the hypothalamus to regulate appetite. However, the existence of a gut-brain circuit that specifically responds to glucose and corresponding brain cells had not been demonstrated until now.
In this study, the team successfully identified a “gut-brain circuit” that senses glucose—essential for brain function—and regulates food intake behavior for required nutrients.
They further proved, for the first time, that this circuit responds within seconds to not only hunger or external stimuli but also to specific caloric nutrients directly introduced into the small intestine, particularly D-glucose, through the activity of “CRF neurons*” in the brain’s hypothalamus.
*CRF neurons: These neurons secrete corticotropin-releasing factor (CRF) in the hypothalamus and are central to the hypothalamic-pituitary-adrenal (HPA) axis, the body’s core physiological system for responding to stress. CRF neurons are known to regulate neuroendocrine balance in response to stress stimuli.
Using optogenetics to precisely track neural activity in real time, the researchers injected various nutrients—D-glucose, L-glucose, amino acids, and fats—directly into the small intestines of mice and observed the results.
They discovered that among the CRF neurons located in the paraventricular nucleus (PVN)* of the hypothalamus, only those specific to D-glucose showed selective responses. These neurons did not respond—or showed inverse reactions—to other sugars or to proteins and fats. This is the first demonstration that single neurons in the brain can guide nutrient-specific responses depending on gut nutrient influx.
*PVN (Paraventricular Nucleus): A key nucleus within the hypothalamus responsible for maintaining bodily homeostasis.
The team also revealed that glucose-sensing signals in the small intestine are transmitted via the spinal cord to the dorsolateral parabrachial nucleus (PBNdl) of the brain, and from there to CRF neurons in the PVN. In contrast, signals for amino acids and fats are transmitted to the brain through the vagus nerve, a different pathway.
In optogenetic inhibition experiments, suppressing CRF neurons in fasting mice eliminated their preference for glucose, proving that this circuit is essential for glucose-specific nutrient preference.
This study was inspired by Professor Suh’s earlier research at NYU using fruit flies, where he identified “DH44 neurons” that selectively detect glucose and sugar in the gut. Based on the hypothesis that hypothalamic neurons in mammals would show similar functional responses to glucose, the current study was launched.
To test this hypothesis, Dr. Jineun Kim (KAIST Ph.D. graduate, now at Caltech) demonstrated during her doctoral research that hungry mice preferred glucose among various intragastrically infused nutrients and that CRF neurons exhibited rapid and specific responses.
Along with Wongyo Jung (KAIST B.S. graduate, now Ph.D. student at Caltech), they modeled and experimentally confirmed the critical role of CRF neurons. Dr. Shinhye Kim, through collaboration, revealed that specific spinal neurons play a key role in conveying intestinal nutrient information to the brain.
Dr. Jineun Kim and Dr. Shinhye Kim said, “This study started from a simple but fundamental question—‘How does the brain distinguish glucose from various nutrients absorbed in the gut?’ We have shown that spinal-based gut-brain circuits play a central role in energy metabolism and homeostasis by transmitting specific gut nutrient signals to the brain.”
Professor Suh added, “By identifying a gut-brain pathway specialized for glucose, this research offers a new therapeutic target for metabolic diseases such as obesity and diabetes. Our future research will explore similar circuits for sensing other essential nutrients like amino acids and fats and their interaction mechanisms.”
Ph.D. student Jineun Kim, Dr. Shinhye Kim, and student Wongyo Jung (co-first authors) contributed to this study, which was published online in the international journal Neuron on June 20, 2025.
※ Paper Title: Encoding the glucose identity by discrete hypothalamic neurons via the gut-brain axis ※ DOI: https://doi.org/10.1016/j.neuron.2025.05.024
This study was supported by the Samsung Science & Technology Foundation, the National Research Foundation of Korea (NRF) Leader Research Program, the POSCO Cheongam Science Fellowship, the Asan Foundation Biomedical Science Scholarship, the Institute for Basic Science (IBS), and the KAIST KAIX program.
2025.07.09 View 266 -
 KAIST Develops Novel Candidiasis Treatment Overcoming Side Effects and Resistance
<(From left) Ph. D Candidate Ju Yeon Chung, Prof.Hyun Jung Chung, Ph.D candidate Seungju Yang, Ph.D candidate Ayoung Park, Dr. Yoon-Kyoung Hong from Asan Medical Center, Prof. Yong Pil Chong, Dr. Eunhee Jeon>
Candida, a type of fungus, which can spread throughout the body via the bloodstream, leading to organ damage and sepsis. Recently, the incidence of candidiasis has surged due to the increase in immunosuppressive therapies, medical implants, and transplantation. Korean researchers have successfully developed a next-generation treatment that, unlike existing antifungals, selectively acts only on Candida, achieving both high therapeutic efficacy and low side effects simultaneously.
KAIST (President Kwang Hyung Lee) announced on the 8th that a research team led by Professor Hyun-Jung Chung of the Department of Biological Sciences, in collaboration with Professor Yong Pil Jeong's team at Asan Medical Center, developed a gene-based nanotherapy (FTNx) that simultaneously inhibits two key enzymes in the Candida cell wall.
Current antifungal drugs for Candida have low target selectivity, which can affect human cells. Furthermore, their therapeutic efficacy is gradually decreasing due to the emergence of new resistant strains. Especially for immunocompromised patients, the infection progresses rapidly and has a poor prognosis, making the development of new treatments to overcome the limitations of existing therapies urgent.
The developed treatment can be administered systemically, and by combining gene suppression technology with nanomaterial technology, it effectively overcomes the structural limitations of existing compound-based drugs and successfully achieves selective treatment against only Candida.
The research team created a gold nanoparticle-based complex loaded with short DNA fragments called antisense oligonucleotides (ASO), which simultaneously target two crucial enzymes—β-1,3-glucan synthase (FKS1) and chitin synthase (CHS3)—important for forming the cell wall of the Candida fungus.
By applying a surface coating technology that binds to a specific glycolipid structure (a structure combining sugar and fat) on the Candida cell wall, a targeted delivery device was implemented. This successfully achieved a precise targeting effect, ensuring the complex is not delivered to human cells at all but acts selectively only on Candida.
<Figure 1: Overview of antifungal therapy design and experimental approach>
This complex, after entering Candida cells, cleaves the mRNA produced by the FKS1 and CHS3 genes, thereby inhibiting translation and simultaneously blocking the synthesis of cell wall components β-1,3-glucan and chitin. As a result, the
Candida cell wall loses its structural stability and collapses, suppressing bacterial survival and proliferation.
In fact, experiments using a systemic candidiasis model in mice confirmed the therapeutic effect: a significant reduction in
Candida count in the organs, normalization of immune responses, and a notable increase in survival rates were observed in the treated group.
Professor Hyun-Jung Chung, who led the research, stated, "This study presents a method to overcome the issues of human toxicity and drug resistance spread with existing treatments, marking an important turning point by demonstrating the applicability of gene therapy for systemic infections". She added, "We plan to continue research on optimizing administration methods and verifying toxicity for future clinical application."
This research involved Ju Yeon Chung and Yoon-Kyoung Hong as co-first authors , and was published in the international journal 'Nature Communications' on July 1st.
Paper Title: Effective treatment of systemic candidiasis by synergistic targeting of cell wall synthesis
DOI: 10.1038/s41467-025-60684-7
This research was supported by the Ministry of Health and Welfare and the National Research Foundation of Korea.
2025.07.08 View 270
KAIST Develops Novel Candidiasis Treatment Overcoming Side Effects and Resistance
<(From left) Ph. D Candidate Ju Yeon Chung, Prof.Hyun Jung Chung, Ph.D candidate Seungju Yang, Ph.D candidate Ayoung Park, Dr. Yoon-Kyoung Hong from Asan Medical Center, Prof. Yong Pil Chong, Dr. Eunhee Jeon>
Candida, a type of fungus, which can spread throughout the body via the bloodstream, leading to organ damage and sepsis. Recently, the incidence of candidiasis has surged due to the increase in immunosuppressive therapies, medical implants, and transplantation. Korean researchers have successfully developed a next-generation treatment that, unlike existing antifungals, selectively acts only on Candida, achieving both high therapeutic efficacy and low side effects simultaneously.
KAIST (President Kwang Hyung Lee) announced on the 8th that a research team led by Professor Hyun-Jung Chung of the Department of Biological Sciences, in collaboration with Professor Yong Pil Jeong's team at Asan Medical Center, developed a gene-based nanotherapy (FTNx) that simultaneously inhibits two key enzymes in the Candida cell wall.
Current antifungal drugs for Candida have low target selectivity, which can affect human cells. Furthermore, their therapeutic efficacy is gradually decreasing due to the emergence of new resistant strains. Especially for immunocompromised patients, the infection progresses rapidly and has a poor prognosis, making the development of new treatments to overcome the limitations of existing therapies urgent.
The developed treatment can be administered systemically, and by combining gene suppression technology with nanomaterial technology, it effectively overcomes the structural limitations of existing compound-based drugs and successfully achieves selective treatment against only Candida.
The research team created a gold nanoparticle-based complex loaded with short DNA fragments called antisense oligonucleotides (ASO), which simultaneously target two crucial enzymes—β-1,3-glucan synthase (FKS1) and chitin synthase (CHS3)—important for forming the cell wall of the Candida fungus.
By applying a surface coating technology that binds to a specific glycolipid structure (a structure combining sugar and fat) on the Candida cell wall, a targeted delivery device was implemented. This successfully achieved a precise targeting effect, ensuring the complex is not delivered to human cells at all but acts selectively only on Candida.
<Figure 1: Overview of antifungal therapy design and experimental approach>
This complex, after entering Candida cells, cleaves the mRNA produced by the FKS1 and CHS3 genes, thereby inhibiting translation and simultaneously blocking the synthesis of cell wall components β-1,3-glucan and chitin. As a result, the
Candida cell wall loses its structural stability and collapses, suppressing bacterial survival and proliferation.
In fact, experiments using a systemic candidiasis model in mice confirmed the therapeutic effect: a significant reduction in
Candida count in the organs, normalization of immune responses, and a notable increase in survival rates were observed in the treated group.
Professor Hyun-Jung Chung, who led the research, stated, "This study presents a method to overcome the issues of human toxicity and drug resistance spread with existing treatments, marking an important turning point by demonstrating the applicability of gene therapy for systemic infections". She added, "We plan to continue research on optimizing administration methods and verifying toxicity for future clinical application."
This research involved Ju Yeon Chung and Yoon-Kyoung Hong as co-first authors , and was published in the international journal 'Nature Communications' on July 1st.
Paper Title: Effective treatment of systemic candidiasis by synergistic targeting of cell wall synthesis
DOI: 10.1038/s41467-025-60684-7
This research was supported by the Ministry of Health and Welfare and the National Research Foundation of Korea.
2025.07.08 View 270 -
 KAIST Presents Game-Changing Technology for Intractable Brain Disease Treatment Using Micro OLEDs
<(From left)Professor Kyung Cheol Choi, Hyunjoo J. Lee, Somin Lee from the School of Electrical Engineering>
Optogenetics is a technique that controls neural activity by stimulating neurons expressing light-sensitive proteins with specific wavelengths of light. It has opened new possibilities for identifying causes of brain disorders and developing treatments for intractable neurological diseases. Because this technology requires precise stimulation inside the human brain with minimal damage to soft brain tissue, it must be integrated into a neural probe—a medical device implanted in the brain. KAIST researchers have now proposed a new paradigm for neural probes by integrating micro OLEDs into thin, flexible, implantable medical devices.
KAIST (President Kwang Hyung Lee) announced on the 6th of July that Professor Kyung Cheol Choi and researcher Hyunjoo J. Lee from the School of Electrical Engineering have jointly succeeded in developing an optogenetic neural probe integrated with flexible micro OLEDs.
Optical fibers have been used for decades in optogenetic research to deliver light to deep brain regions from external light sources. Recently, research has focused on flexible optical fibers and ultra-miniaturized neural probes that integrate light sources for single-neuron stimulation.
The research team focused on micro OLEDs due to their high spatial resolution and flexibility, which allow for precise light delivery to small areas of neurons. This enables detailed brain circuit analysis while minimizing side effects and avoiding restrictions on animal movement. Moreover, micro OLEDs offer precise control of light wavelengths and support multi-site stimulation, making them suitable for studying complex brain functions.
However, the device's electrical properties degrade easily in the presence of moisture or water, which limited their use as implantable bioelectronics. Furthermore, optimizing the high-resolution integration process on thin, flexible probes remained a challenge.
To address this, the team enhanced the operational reliability of OLEDs in moist, oxygen-rich environments and minimized tissue damage during implantation. They patterned an ultrathin, flexible encapsulation layer* composed of aluminum oxide and parylene-C (Al₂O₃/parylene-C) at widths of 260–600 micrometers (μm) to maintain biocompatibility.
*Encapsulation layer: A barrier that completely blocks oxygen and water molecules from the external environment, ensuring the longevity and reliability of the device.
When integrating the high-resolution micro OLEDs, the researchers also used parylene-C, the same biocompatible material as the encapsulation layer, to maintain flexibility and safety. To eliminate electrical interference between adjacent OLED pixels and spatially separate them, they introduced a pixel define layer (PDL), enabling the independent operation of eight micro OLEDs.
Furthermore, they precisely controlled the residual stress and thickness in the multilayer film structure of the device, ensuring its flexibility even in biological environments. This optimization allowed for probe insertion without bending or external shuttles or needles, minimizing mechanical stress during implantation.
Advanced Functional Materials-Conceptual diagram of a flexible neural probe for integrated optogenetics (Micro-OLED)>
As a result, the team developed a flexible neural probe with integrated micro OLEDs capable of emitting more than one milliwatt per square millimeter (mW/mm²) at 470 nanometers (nm), the optimal wavelength for activating channelrhodopsin-2. This is a significantly high light output for optogenetics and biomedical stimulation applications.
The ultrathin flexible encapsulation layer exhibited a low water vapor transmission rate of 2.66×10⁻⁵ g/m²/day, allowing the device to maintain functionality for over 10 years. The parylene-C-based barrier also demonstrated excellent performance in biological environments, successfully enabling the independent operation of the integrated OLEDs without electrical interference or bending issues.
Dr. Somin Lee, the lead author from Professor Choi’s lab, stated, “We focused on fine-tuning the integration process of highly flexible, high-resolution micro OLEDs onto thin flexible probes, enhancing their biocompatibility and application potential. This is the first reported development of such flexible OLEDs in a probe format and presents a new paradigm for using flexible OLEDs as implantable medical devices for monitoring and therapy.”
This study, with Dr. Somin Lee as the first author, was published online on March 26 in Advanced Functional Materials (IF 18.5), a leading international journal in the field of nanotechnology, and was selected as the cover article for the upcoming July issue.
※ Title: Advanced Micro-OLED Integration on Thin and Flexible Polymer Neural Probes for Targeted Optogenetic Stimulation ※ DOI: https://doi.org/10.1002/adfm.202420758
The research was supported by the Ministry of Science and ICT and the National Research Foundation of Korea through the Electronic Medicine Technology Development Program (Project title: Development of Core Source Technologies and In Vivo Validation for Brain Cognition and Emotion-Enhancing Light-Stimulating Electronic Medicine).
2025.07.07 View 238
KAIST Presents Game-Changing Technology for Intractable Brain Disease Treatment Using Micro OLEDs
<(From left)Professor Kyung Cheol Choi, Hyunjoo J. Lee, Somin Lee from the School of Electrical Engineering>
Optogenetics is a technique that controls neural activity by stimulating neurons expressing light-sensitive proteins with specific wavelengths of light. It has opened new possibilities for identifying causes of brain disorders and developing treatments for intractable neurological diseases. Because this technology requires precise stimulation inside the human brain with minimal damage to soft brain tissue, it must be integrated into a neural probe—a medical device implanted in the brain. KAIST researchers have now proposed a new paradigm for neural probes by integrating micro OLEDs into thin, flexible, implantable medical devices.
KAIST (President Kwang Hyung Lee) announced on the 6th of July that Professor Kyung Cheol Choi and researcher Hyunjoo J. Lee from the School of Electrical Engineering have jointly succeeded in developing an optogenetic neural probe integrated with flexible micro OLEDs.
Optical fibers have been used for decades in optogenetic research to deliver light to deep brain regions from external light sources. Recently, research has focused on flexible optical fibers and ultra-miniaturized neural probes that integrate light sources for single-neuron stimulation.
The research team focused on micro OLEDs due to their high spatial resolution and flexibility, which allow for precise light delivery to small areas of neurons. This enables detailed brain circuit analysis while minimizing side effects and avoiding restrictions on animal movement. Moreover, micro OLEDs offer precise control of light wavelengths and support multi-site stimulation, making them suitable for studying complex brain functions.
However, the device's electrical properties degrade easily in the presence of moisture or water, which limited their use as implantable bioelectronics. Furthermore, optimizing the high-resolution integration process on thin, flexible probes remained a challenge.
To address this, the team enhanced the operational reliability of OLEDs in moist, oxygen-rich environments and minimized tissue damage during implantation. They patterned an ultrathin, flexible encapsulation layer* composed of aluminum oxide and parylene-C (Al₂O₃/parylene-C) at widths of 260–600 micrometers (μm) to maintain biocompatibility.
*Encapsulation layer: A barrier that completely blocks oxygen and water molecules from the external environment, ensuring the longevity and reliability of the device.
When integrating the high-resolution micro OLEDs, the researchers also used parylene-C, the same biocompatible material as the encapsulation layer, to maintain flexibility and safety. To eliminate electrical interference between adjacent OLED pixels and spatially separate them, they introduced a pixel define layer (PDL), enabling the independent operation of eight micro OLEDs.
Furthermore, they precisely controlled the residual stress and thickness in the multilayer film structure of the device, ensuring its flexibility even in biological environments. This optimization allowed for probe insertion without bending or external shuttles or needles, minimizing mechanical stress during implantation.
Advanced Functional Materials-Conceptual diagram of a flexible neural probe for integrated optogenetics (Micro-OLED)>
As a result, the team developed a flexible neural probe with integrated micro OLEDs capable of emitting more than one milliwatt per square millimeter (mW/mm²) at 470 nanometers (nm), the optimal wavelength for activating channelrhodopsin-2. This is a significantly high light output for optogenetics and biomedical stimulation applications.
The ultrathin flexible encapsulation layer exhibited a low water vapor transmission rate of 2.66×10⁻⁵ g/m²/day, allowing the device to maintain functionality for over 10 years. The parylene-C-based barrier also demonstrated excellent performance in biological environments, successfully enabling the independent operation of the integrated OLEDs without electrical interference or bending issues.
Dr. Somin Lee, the lead author from Professor Choi’s lab, stated, “We focused on fine-tuning the integration process of highly flexible, high-resolution micro OLEDs onto thin flexible probes, enhancing their biocompatibility and application potential. This is the first reported development of such flexible OLEDs in a probe format and presents a new paradigm for using flexible OLEDs as implantable medical devices for monitoring and therapy.”
This study, with Dr. Somin Lee as the first author, was published online on March 26 in Advanced Functional Materials (IF 18.5), a leading international journal in the field of nanotechnology, and was selected as the cover article for the upcoming July issue.
※ Title: Advanced Micro-OLED Integration on Thin and Flexible Polymer Neural Probes for Targeted Optogenetic Stimulation ※ DOI: https://doi.org/10.1002/adfm.202420758
The research was supported by the Ministry of Science and ICT and the National Research Foundation of Korea through the Electronic Medicine Technology Development Program (Project title: Development of Core Source Technologies and In Vivo Validation for Brain Cognition and Emotion-Enhancing Light-Stimulating Electronic Medicine).
2025.07.07 View 238 -
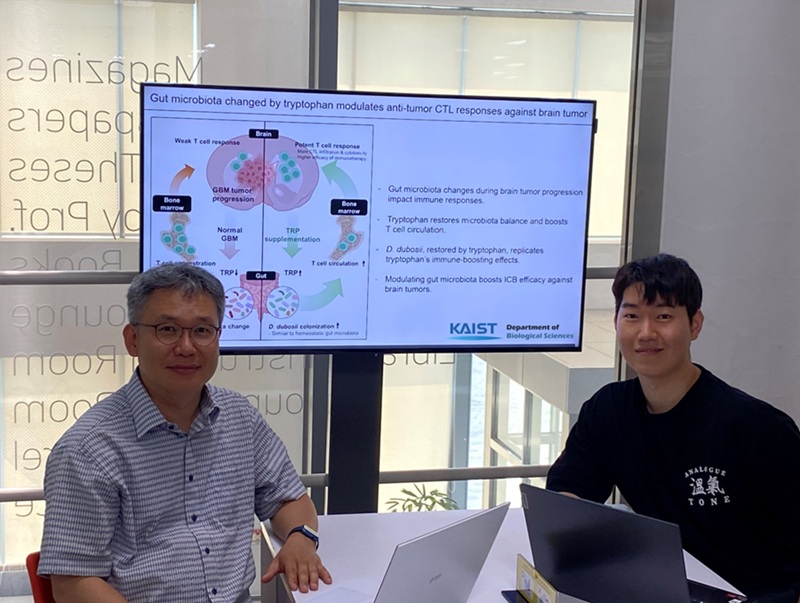 KAIST Enhances Immunotherapy for Difficult-to-Treat Brain Tumors with Gut Microbiota
< Photo 1.(From left) Prof. Heung Kyu Lee, Department of Biological Sciences,
and Dr. Hyeon Cheol Kim>
Advanced treatments, known as immunotherapies that activate T cells—our body's immune cells—to eliminate cancer cells, have shown limited efficacy as standalone therapies for glioblastoma, the most lethal form of brain tumor. This is due to their minimal response to glioblastoma and high resistance to treatment.
Now, a KAIST research team has now demonstrated a new therapeutic strategy that can enhance the efficacy of immunotherapy for brain tumors by utilizing gut microbes and their metabolites. This also opens up possibilities for developing microbiome-based immunotherapy supplements in the future.
KAIST (President Kwang Hyung Lee) announced on July 1 that a research team led by Professor Heung Kyu Lee of the Department of Biological Sciences discovered and demonstrated a method to significantly improve the efficiency of glioblastoma immunotherapy by focusing on changes in the gut microbial ecosystem.
The research team noted that as glioblastoma progresses, the concentration of ‘tryptophan’, an important amino acid in the gut, sharply decreases, leading to changes in the gut microbial ecosystem. They discovered that by supplementing tryptophan to restore microbial diversity, specific beneficial strains activate CD8 T cells (a type of immune cell) and induce their infiltration into tumor tissues. Through a mouse model of glioblastoma, the research team confirmed that tryptophan supplementation enhanced the response of cancer-attacking T cells (especially CD8 T cells), leading to their increased migration to tumor sites such as lymph nodes and the brain.
In this process, they also revealed that ‘Duncaniella dubosii’, a beneficial commensal bacterium present in the gut, plays a crucial role. This bacterium helped T cells effectively redistribute within the body, and survival rates significantly improved when used in combination with immunotherapy (anti-PD-1).
Furthermore, it was demonstrated that even when this commensal bacterium was administered alone to germ-free mice (mice without any commensal microbes), the survival rate for glioblastoma increased. This is because the bacterium utilizes tryptophan to regulate the gut environment, and the metabolites produced in this process strengthen the ability of CD8 T cells to attack cancer cells.
Professor Heung Kyu Lee explained, "This research is a meaningful achievement, showing that even in intractable brain tumors where immune checkpoint inhibitors had no effect, a combined strategy utilizing gut microbes can significantly enhance treatment response."
Dr. Hyeon Cheol Kim of KAIST (currently a postdoctoral researcher at the Institute for Biological Sciences) participated as the first author. The research findings were published online in Cell Reports, an international journal in the life sciences, on June 26.
This research was conducted as part of the Basic Research Program and Bio & Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
※Paper Title: Gut microbiota dysbiosis induced by brain tumor modulates the efficacy of immunotherapy
※DOI: https://doi.org/10.1016/j.celrep.2025.115825
2025.07.02 View 840
KAIST Enhances Immunotherapy for Difficult-to-Treat Brain Tumors with Gut Microbiota
< Photo 1.(From left) Prof. Heung Kyu Lee, Department of Biological Sciences,
and Dr. Hyeon Cheol Kim>
Advanced treatments, known as immunotherapies that activate T cells—our body's immune cells—to eliminate cancer cells, have shown limited efficacy as standalone therapies for glioblastoma, the most lethal form of brain tumor. This is due to their minimal response to glioblastoma and high resistance to treatment.
Now, a KAIST research team has now demonstrated a new therapeutic strategy that can enhance the efficacy of immunotherapy for brain tumors by utilizing gut microbes and their metabolites. This also opens up possibilities for developing microbiome-based immunotherapy supplements in the future.
KAIST (President Kwang Hyung Lee) announced on July 1 that a research team led by Professor Heung Kyu Lee of the Department of Biological Sciences discovered and demonstrated a method to significantly improve the efficiency of glioblastoma immunotherapy by focusing on changes in the gut microbial ecosystem.
The research team noted that as glioblastoma progresses, the concentration of ‘tryptophan’, an important amino acid in the gut, sharply decreases, leading to changes in the gut microbial ecosystem. They discovered that by supplementing tryptophan to restore microbial diversity, specific beneficial strains activate CD8 T cells (a type of immune cell) and induce their infiltration into tumor tissues. Through a mouse model of glioblastoma, the research team confirmed that tryptophan supplementation enhanced the response of cancer-attacking T cells (especially CD8 T cells), leading to their increased migration to tumor sites such as lymph nodes and the brain.
In this process, they also revealed that ‘Duncaniella dubosii’, a beneficial commensal bacterium present in the gut, plays a crucial role. This bacterium helped T cells effectively redistribute within the body, and survival rates significantly improved when used in combination with immunotherapy (anti-PD-1).
Furthermore, it was demonstrated that even when this commensal bacterium was administered alone to germ-free mice (mice without any commensal microbes), the survival rate for glioblastoma increased. This is because the bacterium utilizes tryptophan to regulate the gut environment, and the metabolites produced in this process strengthen the ability of CD8 T cells to attack cancer cells.
Professor Heung Kyu Lee explained, "This research is a meaningful achievement, showing that even in intractable brain tumors where immune checkpoint inhibitors had no effect, a combined strategy utilizing gut microbes can significantly enhance treatment response."
Dr. Hyeon Cheol Kim of KAIST (currently a postdoctoral researcher at the Institute for Biological Sciences) participated as the first author. The research findings were published online in Cell Reports, an international journal in the life sciences, on June 26.
This research was conducted as part of the Basic Research Program and Bio & Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
※Paper Title: Gut microbiota dysbiosis induced by brain tumor modulates the efficacy of immunotherapy
※DOI: https://doi.org/10.1016/j.celrep.2025.115825
2025.07.02 View 840 -
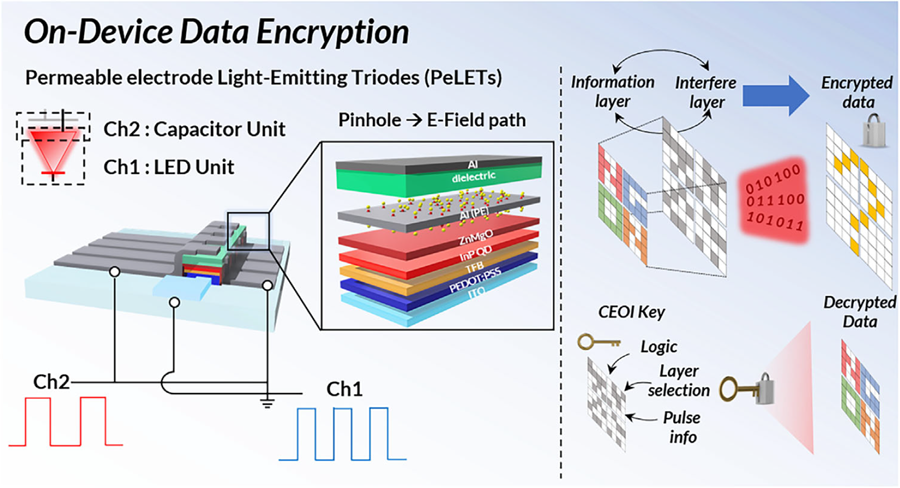 KAIST's Li-Fi - Achieves 100 Times Faster Speed and Enhanced Security of Wi-Fi
- KAIST-KRISS Develop 'On-Device Encryption Optical Transmitter' Based on Eco-Friendly Quantum Dots
- New Li-Fi Platform Technology Achieves High Performance with 17.4% Device Efficiency and 29,000 nit Brightness, Simultaneously Improving Transmission Speed and Security
- Presents New Methodology for High-Speed and Encrypted Communication Through Single-Device-Based Dual-Channel Optical Modulation
< Photo 1. (Front row from left) Seungmin Shin, First Author; Professor Himchan Cho; (Back row from left) Hyungdoh Lee, Seungwoo Lee, Wonbeom Lee; (Top left) Dr. Kyung-geun Lim >
Li-Fi (Light Fidelity) is a wireless communication technology that utilizes the visible light spectrum (400-800 THz), similar to LED light, offering speeds up to 100 times faster than existing Wi-Fi (up to 224 Gbps). While it has fewer limitations in available frequency allocation and less radio interference, it is relatively vulnerable to security breaches as anyone can access it. Korean researchers have now proposed a new Li-Fi platform that overcomes the limitations of conventional optical communication devices and can simultaneously enhance both transmission speed and security.
KAIST (President Kwang Hyung Lee) announced on the 24th that Professor Himchan Cho's research team from the Department of Materials Science and Engineering, in collaboration with Dr. Kyung-geun Lim of the Korea Research Institute of Standards and Science (KRISS, President Ho-Seong Lee) under the National Research Council of Science & Technology (NST, Chairman Young-Sik Kim), has developed 'on-device encryption optical communication device' technology for the utilization of 'Li-Fi,' which is attracting attention as a next-generation ultra-high-speed data communication.
Professor Cho's team created high-efficiency light-emitting triode devices using eco-friendly quantum dots (low-toxicity and sustainable materials). The device developed by the research team is a mechanism that generates light using an electric field. Specifically, the electric field is concentrated in 'tiny holes (pinholes) in the permeable electrode' and transmitted beyond the electrode. This device utilizes this principle to simultaneously process two input data streams.
Using this principle, the research team developed a technology called 'on-device encryption optical transmitter.' The core of this technology is that the device itself converts information into light and simultaneously encrypts it. This means that enhanced security data transmission is possible without the need for complex, separate equipment.
External Quantum Efficiency (EQE) is an indicator of how efficiently electricity is converted into light, with a general commercialization standard of about 20%. The newly developed device recorded an EQE of 17.4%, and its luminance was 29,000 nit, significantly exceeding the maximum brightness of a smartphone OLED screen, which is 2,000 nit, demonstrating a brightness more than 10 times higher.
< Figure 1. Schematic diagram of the device structure developed by the research team and encrypted communication >
Furthermore, to more accurately understand how this device converts information into light, the research team used a method called 'transient electroluminescence analysis.' They analyzed the light-emitting characteristics generated by the device when voltage was instantaneously applied for very short durations (hundreds of nanoseconds = billionths of a second). Through this analysis, they investigated the movement of charges within the device at hundreds of nanoseconds, elucidating the operating mechanism of dual-channel optical modulation implemented within a single device.
Professor Himchan Cho of KAIST stated, "This research overcomes the limitations of existing optical communication devices and proposes a new communication platform that can both increase transmission speed and enhance security."
< Photo 2. Professor Himchan Cho, Department of Materials Science and Engineering >
He added, "This technology, which strengthens security without additional equipment and simultaneously enables encryption and transmission, can be widely applied in various fields where security is crucial in the future."
This research, with Seungmin Shin, a Ph.D. candidate at KAIST's Department of Materials Science and Engineering, participating as the first author, and Professor Himchan Cho and Dr. Kyung-geun Lim of KRISS as co-corresponding authors, was published in the international journal 'Advanced Materials' on May 30th and was selected as an inside front cover paper.※ Paper Title: High-Efficiency Quantum Dot Permeable electrode Light-Emitting Triodes for Visible-Light Communications and On-Device Data Encryption※ DOI: https://doi.org/10.1002/adma.202503189
This research was supported by the National Research Foundation of Korea, the National Research Council of Science & Technology (NST), and the Korea Institute for Advancement of Technology.
2025.06.24 View 1956
KAIST's Li-Fi - Achieves 100 Times Faster Speed and Enhanced Security of Wi-Fi
- KAIST-KRISS Develop 'On-Device Encryption Optical Transmitter' Based on Eco-Friendly Quantum Dots
- New Li-Fi Platform Technology Achieves High Performance with 17.4% Device Efficiency and 29,000 nit Brightness, Simultaneously Improving Transmission Speed and Security
- Presents New Methodology for High-Speed and Encrypted Communication Through Single-Device-Based Dual-Channel Optical Modulation
< Photo 1. (Front row from left) Seungmin Shin, First Author; Professor Himchan Cho; (Back row from left) Hyungdoh Lee, Seungwoo Lee, Wonbeom Lee; (Top left) Dr. Kyung-geun Lim >
Li-Fi (Light Fidelity) is a wireless communication technology that utilizes the visible light spectrum (400-800 THz), similar to LED light, offering speeds up to 100 times faster than existing Wi-Fi (up to 224 Gbps). While it has fewer limitations in available frequency allocation and less radio interference, it is relatively vulnerable to security breaches as anyone can access it. Korean researchers have now proposed a new Li-Fi platform that overcomes the limitations of conventional optical communication devices and can simultaneously enhance both transmission speed and security.
KAIST (President Kwang Hyung Lee) announced on the 24th that Professor Himchan Cho's research team from the Department of Materials Science and Engineering, in collaboration with Dr. Kyung-geun Lim of the Korea Research Institute of Standards and Science (KRISS, President Ho-Seong Lee) under the National Research Council of Science & Technology (NST, Chairman Young-Sik Kim), has developed 'on-device encryption optical communication device' technology for the utilization of 'Li-Fi,' which is attracting attention as a next-generation ultra-high-speed data communication.
Professor Cho's team created high-efficiency light-emitting triode devices using eco-friendly quantum dots (low-toxicity and sustainable materials). The device developed by the research team is a mechanism that generates light using an electric field. Specifically, the electric field is concentrated in 'tiny holes (pinholes) in the permeable electrode' and transmitted beyond the electrode. This device utilizes this principle to simultaneously process two input data streams.
Using this principle, the research team developed a technology called 'on-device encryption optical transmitter.' The core of this technology is that the device itself converts information into light and simultaneously encrypts it. This means that enhanced security data transmission is possible without the need for complex, separate equipment.
External Quantum Efficiency (EQE) is an indicator of how efficiently electricity is converted into light, with a general commercialization standard of about 20%. The newly developed device recorded an EQE of 17.4%, and its luminance was 29,000 nit, significantly exceeding the maximum brightness of a smartphone OLED screen, which is 2,000 nit, demonstrating a brightness more than 10 times higher.
< Figure 1. Schematic diagram of the device structure developed by the research team and encrypted communication >
Furthermore, to more accurately understand how this device converts information into light, the research team used a method called 'transient electroluminescence analysis.' They analyzed the light-emitting characteristics generated by the device when voltage was instantaneously applied for very short durations (hundreds of nanoseconds = billionths of a second). Through this analysis, they investigated the movement of charges within the device at hundreds of nanoseconds, elucidating the operating mechanism of dual-channel optical modulation implemented within a single device.
Professor Himchan Cho of KAIST stated, "This research overcomes the limitations of existing optical communication devices and proposes a new communication platform that can both increase transmission speed and enhance security."
< Photo 2. Professor Himchan Cho, Department of Materials Science and Engineering >
He added, "This technology, which strengthens security without additional equipment and simultaneously enables encryption and transmission, can be widely applied in various fields where security is crucial in the future."
This research, with Seungmin Shin, a Ph.D. candidate at KAIST's Department of Materials Science and Engineering, participating as the first author, and Professor Himchan Cho and Dr. Kyung-geun Lim of KRISS as co-corresponding authors, was published in the international journal 'Advanced Materials' on May 30th and was selected as an inside front cover paper.※ Paper Title: High-Efficiency Quantum Dot Permeable electrode Light-Emitting Triodes for Visible-Light Communications and On-Device Data Encryption※ DOI: https://doi.org/10.1002/adma.202503189
This research was supported by the National Research Foundation of Korea, the National Research Council of Science & Technology (NST), and the Korea Institute for Advancement of Technology.
2025.06.24 View 1956 -
 Distinguished Professor Sang Yup Lee Wins 2025 Global Metabolic Engineering Award
< Distinguished Professor Sang Yup Lee (Senior Vice President for Research) from the Department of Chemical & Biomolecular Engineering >
KAIST announced on the 20th that Professor Sang Yup Lee, who serves as the Vice President for Research and a Distinguished Professor at our university, has been awarded the '2025 Gregory N. Stephanopoulos Award for Metabolic Engineering' by the International Metabolic Engineering Society (IMES). Professor Lee delivered his award lecture at the 16th Metabolic Engineering Conference (ME16), held in Copenhagen, Denmark, from June 15th to 19th.
This award was established through contributions from the American Institute of Chemical Engineers (AIChE) Foundation, as well as fellow colleagues and acquaintances, to honor the achievements of Dr. Gregory Stephanopoulos, widely recognized as one of the pioneers of metabolic engineering. Presented biennially, the award recognizes scientists who have successfully commercialized fundamental research in metabolic engineering or have made outstanding contributions to the quantitative analysis, design, and modeling of metabolic pathways.
Professor Sang Yup Lee boasts an impressive record of over 770 journal papers and more than 860 patents. His groundbreaking research in metabolic engineering and biochemical engineering is highly acclaimed globally.
Throughout his 31 years as a professor at KAIST, Professor Lee has developed various metabolic engineering-based technologies and strategies. These advancements have been transferred to industries, facilitating the production of bulk chemicals, polymers, natural products, pharmaceuticals, and health functional foods. He has also founded companies and actively engages in advisory roles with various enterprises.
The International Metabolic Engineering Society (IMES) defines metabolic engineering as the manipulation of metabolic pathways in microorganisms or cells to produce useful substances (such as pharmaceuticals, biofuels, and chemical products). It utilizes tools like systems biology, synthetic biology, and computational modeling with the aim of enhancing the economic viability and sustainability of bio-based processes.
Furthermore, Professor Lee previously received the Merck Metabolic Engineering Award, a prominent international award in the field, in 2008. In 2018, he was honored with the Eni Award, often referred to as the Nobel Prize in energy, presented by the President of Italy.
Professor Sang Yup Lee remarked, "Metabolic engineering is a discipline that leads the current and future of biotechnology. It is a tremendous honor to receive this meaningful award at a time when the transition to a bio-based economy is accelerating. Together with my students and fellow researchers, we have generated numerous patents and transferred technologies to industry, and also established startups in the fields of biofuels, wound healing, and cosmetics. I will continue to pursue research that encompasses both fundamental research and technological commercialization."
The 'International Metabolic Engineering Society (IMES)' is a specialized society under the American Institute of Chemical Engineers. Its mission is to enable the production of various bio-based products, including pharmaceuticals, food additives, chemicals, and fuels, through metabolic engineering. The society hosts the Metabolic Engineering Conference biennially, offering researchers opportunities for knowledge exchange and collaboration.
2025.06.20 View 1658
Distinguished Professor Sang Yup Lee Wins 2025 Global Metabolic Engineering Award
< Distinguished Professor Sang Yup Lee (Senior Vice President for Research) from the Department of Chemical & Biomolecular Engineering >
KAIST announced on the 20th that Professor Sang Yup Lee, who serves as the Vice President for Research and a Distinguished Professor at our university, has been awarded the '2025 Gregory N. Stephanopoulos Award for Metabolic Engineering' by the International Metabolic Engineering Society (IMES). Professor Lee delivered his award lecture at the 16th Metabolic Engineering Conference (ME16), held in Copenhagen, Denmark, from June 15th to 19th.
This award was established through contributions from the American Institute of Chemical Engineers (AIChE) Foundation, as well as fellow colleagues and acquaintances, to honor the achievements of Dr. Gregory Stephanopoulos, widely recognized as one of the pioneers of metabolic engineering. Presented biennially, the award recognizes scientists who have successfully commercialized fundamental research in metabolic engineering or have made outstanding contributions to the quantitative analysis, design, and modeling of metabolic pathways.
Professor Sang Yup Lee boasts an impressive record of over 770 journal papers and more than 860 patents. His groundbreaking research in metabolic engineering and biochemical engineering is highly acclaimed globally.
Throughout his 31 years as a professor at KAIST, Professor Lee has developed various metabolic engineering-based technologies and strategies. These advancements have been transferred to industries, facilitating the production of bulk chemicals, polymers, natural products, pharmaceuticals, and health functional foods. He has also founded companies and actively engages in advisory roles with various enterprises.
The International Metabolic Engineering Society (IMES) defines metabolic engineering as the manipulation of metabolic pathways in microorganisms or cells to produce useful substances (such as pharmaceuticals, biofuels, and chemical products). It utilizes tools like systems biology, synthetic biology, and computational modeling with the aim of enhancing the economic viability and sustainability of bio-based processes.
Furthermore, Professor Lee previously received the Merck Metabolic Engineering Award, a prominent international award in the field, in 2008. In 2018, he was honored with the Eni Award, often referred to as the Nobel Prize in energy, presented by the President of Italy.
Professor Sang Yup Lee remarked, "Metabolic engineering is a discipline that leads the current and future of biotechnology. It is a tremendous honor to receive this meaningful award at a time when the transition to a bio-based economy is accelerating. Together with my students and fellow researchers, we have generated numerous patents and transferred technologies to industry, and also established startups in the fields of biofuels, wound healing, and cosmetics. I will continue to pursue research that encompasses both fundamental research and technological commercialization."
The 'International Metabolic Engineering Society (IMES)' is a specialized society under the American Institute of Chemical Engineers. Its mission is to enable the production of various bio-based products, including pharmaceuticals, food additives, chemicals, and fuels, through metabolic engineering. The society hosts the Metabolic Engineering Conference biennially, offering researchers opportunities for knowledge exchange and collaboration.
2025.06.20 View 1658 -
 KAIST Develops Glare-Free, Heat-Blocking 'Smart Window'... Applicable to Buildings and Vehicles
• Professor Hong Chul Moon of the Department of Chemical and Biomolecular Engineering develops RECM, a next-generation smart window technology, expecting cooling energy savings and effective indoor thermal management.
• When using the developed RECM, a significantly superior temperature reduction effect is observed compared to conventional windows.
• With a 'pedestrian-friendly smart window' design that eliminates glare by suppressing external reflections, it is expected to be adapted in architectural structures, transportation, and more.
< (From left) First author Hoy Jung Jo, Professor Hong Chul Moon >
In the building sector, which accounts for approximately 40% of global energy consumption, heat ingress through windows has been identified as a primary cause of wasted heating and cooling energy. Our research team has successfully developed a 'pedestrian-friendly smart window' technology capable of not only reducing heating and cooling energy in urban buildings but also resolving the persistent issue of 'light pollution' in urban living.
On the 17th of June, Professor Hong Chul Moon's research team at KAIST's Department of Chemical and Biomolecular Engineering announced the development of a 'smart window technology' that allows users to control the light and heat entering through windows according to their intent, and effectively neutralize glare from external sources.
Recently, 'active smart window' technology, which enables free adjustment of light and heat based on user operation, has garnered significant attention. Unlike conventional windows that passively react to changes in temperature or light, this is a next-generation window system that can be controlled in real-time via electrical signals.
The next-generation smart window technology developed by the research team, RECM (Reversible Electrodeposition and Electrochromic Mirror), is a smart window system based on a single-structured *electrochromic device that can actively control the transmittance of visible light and near-infrared (heat).
*Electrochromic device: A device whose optical properties change in response to an electrical signal.
In particular, by effectively suppressing the glare phenomenon caused by external reflected light—a problem previously identified in traditional metal *deposition smart windows—through the combined application of electrochromic materials, a 'pedestrian-friendly smart window' suitable for building facades has been realized.
*Deposition: A process involving the electrochemical reaction to coat metal ions, such as Ag+, onto an electrode surface in solid form.
The RECM system developed in this study operates in three modes depending on voltage control.
Mode I (Transparent Mode) is advantageous for allowing sunlight to enter the indoor space during winter, as it transmits both light and heat like ordinary glass.
In Mode II (Colored Mode), *Prussian Blue (PB) and **DHV+• chemical species are formed through a redox (oxidation-reduction) reaction, causing the window to turn a deep blue color. In this state, light is absorbed, and only a portion of the heat is transmitted, allowing for privacy while enabling appropriate indoor temperature control.
*Prussian Blue: An electrochromic material that transitions between colorless and blue upon electrical stimulation.
**DHV+•: A radical state colored molecule generated upon electrical stimulation.
Mode III (Colored and Deposition Mode) involves the reduction and deposition of silver (Ag+) ions on the electrode surface, reflecting both light and heat. Concurrently, the colored material absorbs the reflected light, effectively blocking glare for external pedestrians.
The research team validated the practical indoor temperature reduction effect of the RECM technology through experiments utilizing a miniature model house. When a conventional glass window was installed, the indoor temperature rose to 58.7°C within 45 minutes. Conversely, when RECM was operated in Mode III, the temperature reached 31.5°C, demonstrating a temperature reduction effect of approximately 27.2°C.
Furthermore, since each state transition is achievable solely by electrical signals, it is regarded as an active smart technology capable of instantaneous response according to season, time, and intended use.
< Figure 1. Operation mechanism of the RECM smart window. The RECM system can switch among three states—transparent, colored, and colored & deposition—via electrical stimulation. At -1.6 V, DHV•+ and Prussian Blue (PB) are formed, blocking visible light to provide privacy protection and heat blocking. At -2.0 V, silver (Ag) is deposited on the electrode surface, reflecting light and heat, while DHV•+ and Prussian Blue absorb reflected light, effectively suppressing external glare. Through this mechanism, it functions as an active smart window that simultaneously controls light, heat, and glare. >
Professor Hong Chul Moon of KAIST, the corresponding author of this study, stated, "This research goes beyond existing smart window technologies limited to visible light control, presenting a truly smart window platform that comprehensively considers not only active indoor thermal control but also the visual safety of pedestrians." He added, "Various applications are anticipated, from urban buildings to vehicles and trains."
< Figure 2. Analysis of glare suppression effect of conventional reflective smart windows and RECM. This figure presents the results comparing the glare phenomenon occurring during silver (Ag) deposition between conventional reflective smart windows and RECM Mode III. Conventional reflective devices resulted in strong reflected light on the desk surface due to their high reflectivity. In contrast, RECM Mode III, where the colored material absorbed reflected light, showed a 33% reduction in reflected light intensity, and no reflected light was observed from outside. This highlights the RECM system's distinctiveness and practicality as a 'pedestrian-friendly smart window' optimized for dense urban environments, extending beyond just heat blocking. >
The findings of this research were published on June 13, 2025, in Volume 10, Issue 6 of 'ACS Energy Letters'. The listed authors for this publication are Hoy Jung Jo, Yeon Jae Jang, Hyeon-Don Kim, Kwang-Seop Kim, and Hong Chul Moon.
※ Paper Title: Glare-Free, Energy-Efficient Smart Windows: A Pedestrian-Friendly System with Dynamically Tunable Light and Heat Regulation
※ DOI: 10.1021/acsenergylett.5c00637
< Figure 3. Temperature reduction performance verification in a miniature model house. The actual heat blocking effect was evaluated by applying RECM devices to a model building. Under identical conditions, the indoor temperature with ordinary glass rose to 58.7°C, whereas with RECM in Mode III, it reached 31.5°C, demonstrating a maximum temperature reduction effect of 27.2°C. The indoor temperature difference was also visually confirmed through thermal images, which proves the potential for indoor temperature control in urban buildings. >
This research was supported by the Nano & Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT and the internal research program of the Korea Institute of Machinery and Materials.
2025.06.20 View 3567
KAIST Develops Glare-Free, Heat-Blocking 'Smart Window'... Applicable to Buildings and Vehicles
• Professor Hong Chul Moon of the Department of Chemical and Biomolecular Engineering develops RECM, a next-generation smart window technology, expecting cooling energy savings and effective indoor thermal management.
• When using the developed RECM, a significantly superior temperature reduction effect is observed compared to conventional windows.
• With a 'pedestrian-friendly smart window' design that eliminates glare by suppressing external reflections, it is expected to be adapted in architectural structures, transportation, and more.
< (From left) First author Hoy Jung Jo, Professor Hong Chul Moon >
In the building sector, which accounts for approximately 40% of global energy consumption, heat ingress through windows has been identified as a primary cause of wasted heating and cooling energy. Our research team has successfully developed a 'pedestrian-friendly smart window' technology capable of not only reducing heating and cooling energy in urban buildings but also resolving the persistent issue of 'light pollution' in urban living.
On the 17th of June, Professor Hong Chul Moon's research team at KAIST's Department of Chemical and Biomolecular Engineering announced the development of a 'smart window technology' that allows users to control the light and heat entering through windows according to their intent, and effectively neutralize glare from external sources.
Recently, 'active smart window' technology, which enables free adjustment of light and heat based on user operation, has garnered significant attention. Unlike conventional windows that passively react to changes in temperature or light, this is a next-generation window system that can be controlled in real-time via electrical signals.
The next-generation smart window technology developed by the research team, RECM (Reversible Electrodeposition and Electrochromic Mirror), is a smart window system based on a single-structured *electrochromic device that can actively control the transmittance of visible light and near-infrared (heat).
*Electrochromic device: A device whose optical properties change in response to an electrical signal.
In particular, by effectively suppressing the glare phenomenon caused by external reflected light—a problem previously identified in traditional metal *deposition smart windows—through the combined application of electrochromic materials, a 'pedestrian-friendly smart window' suitable for building facades has been realized.
*Deposition: A process involving the electrochemical reaction to coat metal ions, such as Ag+, onto an electrode surface in solid form.
The RECM system developed in this study operates in three modes depending on voltage control.
Mode I (Transparent Mode) is advantageous for allowing sunlight to enter the indoor space during winter, as it transmits both light and heat like ordinary glass.
In Mode II (Colored Mode), *Prussian Blue (PB) and **DHV+• chemical species are formed through a redox (oxidation-reduction) reaction, causing the window to turn a deep blue color. In this state, light is absorbed, and only a portion of the heat is transmitted, allowing for privacy while enabling appropriate indoor temperature control.
*Prussian Blue: An electrochromic material that transitions between colorless and blue upon electrical stimulation.
**DHV+•: A radical state colored molecule generated upon electrical stimulation.
Mode III (Colored and Deposition Mode) involves the reduction and deposition of silver (Ag+) ions on the electrode surface, reflecting both light and heat. Concurrently, the colored material absorbs the reflected light, effectively blocking glare for external pedestrians.
The research team validated the practical indoor temperature reduction effect of the RECM technology through experiments utilizing a miniature model house. When a conventional glass window was installed, the indoor temperature rose to 58.7°C within 45 minutes. Conversely, when RECM was operated in Mode III, the temperature reached 31.5°C, demonstrating a temperature reduction effect of approximately 27.2°C.
Furthermore, since each state transition is achievable solely by electrical signals, it is regarded as an active smart technology capable of instantaneous response according to season, time, and intended use.
< Figure 1. Operation mechanism of the RECM smart window. The RECM system can switch among three states—transparent, colored, and colored & deposition—via electrical stimulation. At -1.6 V, DHV•+ and Prussian Blue (PB) are formed, blocking visible light to provide privacy protection and heat blocking. At -2.0 V, silver (Ag) is deposited on the electrode surface, reflecting light and heat, while DHV•+ and Prussian Blue absorb reflected light, effectively suppressing external glare. Through this mechanism, it functions as an active smart window that simultaneously controls light, heat, and glare. >
Professor Hong Chul Moon of KAIST, the corresponding author of this study, stated, "This research goes beyond existing smart window technologies limited to visible light control, presenting a truly smart window platform that comprehensively considers not only active indoor thermal control but also the visual safety of pedestrians." He added, "Various applications are anticipated, from urban buildings to vehicles and trains."
< Figure 2. Analysis of glare suppression effect of conventional reflective smart windows and RECM. This figure presents the results comparing the glare phenomenon occurring during silver (Ag) deposition between conventional reflective smart windows and RECM Mode III. Conventional reflective devices resulted in strong reflected light on the desk surface due to their high reflectivity. In contrast, RECM Mode III, where the colored material absorbed reflected light, showed a 33% reduction in reflected light intensity, and no reflected light was observed from outside. This highlights the RECM system's distinctiveness and practicality as a 'pedestrian-friendly smart window' optimized for dense urban environments, extending beyond just heat blocking. >
The findings of this research were published on June 13, 2025, in Volume 10, Issue 6 of 'ACS Energy Letters'. The listed authors for this publication are Hoy Jung Jo, Yeon Jae Jang, Hyeon-Don Kim, Kwang-Seop Kim, and Hong Chul Moon.
※ Paper Title: Glare-Free, Energy-Efficient Smart Windows: A Pedestrian-Friendly System with Dynamically Tunable Light and Heat Regulation
※ DOI: 10.1021/acsenergylett.5c00637
< Figure 3. Temperature reduction performance verification in a miniature model house. The actual heat blocking effect was evaluated by applying RECM devices to a model building. Under identical conditions, the indoor temperature with ordinary glass rose to 58.7°C, whereas with RECM in Mode III, it reached 31.5°C, demonstrating a maximum temperature reduction effect of 27.2°C. The indoor temperature difference was also visually confirmed through thermal images, which proves the potential for indoor temperature control in urban buildings. >
This research was supported by the Nano & Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT and the internal research program of the Korea Institute of Machinery and Materials.
2025.06.20 View 3567 -
 Simultaneous Analysis of 21 Chemical Reactions... AI to Transform New Drug Development
< Photo 1. (From left) Professor Hyunwoo Kim and students Donghun Kim and Gyeongseon Choi in the Integrated M.S./Ph.D. program of the Department of Chemistry >
Thalidomide, a drug once used to alleviate morning sickness in pregnant women, exhibits distinct properties due to its optical isomers* in the body: one isomer has a sedative effect, while the other causes severe side effects like birth defects. As this example illustrates, precise organic synthesis techniques, which selectively synthesize only the desired optical isomer, are crucial in new drug development. Overcoming the traditional methods that struggled with simultaneously analyzing multiple reactants, our research team has developed the world's first technology to precisely analyze 21 types of reactants simultaneously. This breakthrough is expected to make a significant contribution to new drug development utilizing AI and robots.
*Optical Isomers: A pair of molecules with the same chemical formula that are mirror images of each other and cannot be superimposed due to their asymmetric structure. This is analogous to a left and right hand, which are similar in form but cannot be perfectly overlaid.
KAIST's Professor Hyunwoo Kim's research team in the Department of Chemistry announced on the 16th that they have developed an innovative optical isomer analysis technology suitable for the era of AI-driven autonomous synthesis*. This research is the world's first technology to precisely analyze asymmetric catalytic reactions involving multiple reactants simultaneously using high-resolution fluorine nuclear magnetic resonance spectroscopy (19F NMR). It is expected to make groundbreaking contributions to various fields, including new drug development and catalyst optimization.
*AI-driven Autonomous Synthesis: An advanced technology that automates and optimizes chemical substance synthesis processes using artificial intelligence (AI). It is gaining attention as a core element for realizing automated and intelligent research environments in future laboratories. AI predicts and adjusts experimental conditions, interprets results, and designs subsequent experiments independently, minimizing human intervention in repetitive experiments and significantly increasing research efficiency and innovativeness.
Currently, while autonomous synthesis systems can automate everything from reaction design to execution, reaction analysis still relies on individual processing using traditional equipment. This leads to slower speeds and bottlenecks, making it unsuitable for high-speed repetitive experiments.
Furthermore, multi-substrate simultaneous screening techniques proposed in the 1990s garnered attention as a strategy to maximize reaction analysis efficiency. However, limitations of existing chromatography-based analysis methods restricted the number of applicable substrates. In asymmetric synthesis reactions, which selectively synthesize only the desired optical isomer, simultaneously analyzing more than 10 types of substrates was nearly impossible.
< Figure 1. Conventional organic reaction evaluation methods follow a process of deriving optimal reaction conditions using a single substrate, then expanding the substrate scope one by one under those conditions, leaving potential reaction areas unexplored. To overcome this, high-throughput screening is introduced to broadly explore catalyst reactivity for various substrates. When combined with multi-substrate screening, this approach allows for a much broader and more systematic understanding of reaction scope and trends. >
To overcome these limitations, the research team developed a 19F NMR-based multi-substrate simultaneous screening technology. This method involves performing asymmetric catalytic reactions with multiple reactants in a single reaction vessel, introducing a fluorine functional group into the products, and then applying their self-developed chiral cobalt reagent to clearly quantify all optical isomers using 19F NMR.
Utilizing the excellent resolution and sensitivity of 19F NMR, the research team successfully performed asymmetric synthesis reactions of 21 substrates simultaneously in a single reaction vessel and quantitatively measured the product yield and optical isomer ratio without any separate purification steps.
Professor Hyunwoo Kim stated, "While anyone can perform asymmetric synthesis reactions with multiple substrates in one reactor, accurately analyzing all the products has been a challenging problem to solve until now. We expect that achieving world-class multi-substrate screening analysis technology will greatly contribute to enhancing the analytical capabilities of AI-driven autonomous synthesis platforms."
< Figure 2. A method for analyzing multi-substrate asymmetric catalytic reactions, where different substrates react simultaneously in a single reactor, using fluorine nuclear magnetic resonance has been implemented. By utilizing the characteristics of fluorine nuclear magnetic resonance, which has a clean background signal and a wide chemical shift range, the reactivity of each substrate can be quantitatively analyzed. It is also shown that the optical activity of all reactants can be simultaneously measured using a cobalt metal complex. >
He further added, "This research provides a technology that can rapidly verify the efficiency and selectivity of asymmetric catalytic reactions essential for new drug development, and it is expected to be utilized as a core analytical tool for AI-driven autonomous research."
< Figure 3. It can be seen that in a multi-substrate reductive amination reaction using a total of 21 substrates, the yield and optical activity of the reactants according to the catalyst system were simultaneously measured using a fluorine nuclear magnetic resonance-based analysis platform. The yield of each reactant is indicated by color saturation, and the optical activity by numbers. >
Donghun Kim (first author, Integrated M.S./Ph.D. program) and Gyeongseon Choi (second author, Integrated M.S./Ph.D. program) from the KAIST Department of Chemistry participated in this research. The study was published online in the Journal of the American Chemical Society on May 27, 2025.※ Paper Title: One-pot Multisubstrate Screening for Asymmetric Catalysis Enabled by 19F NMR-based Simultaneous Chiral Analysis※ DOI: 10.1021/jacs.5c03446
This research was supported by the National Research Foundation of Korea's Mid-Career Researcher Program, the Asymmetric Catalytic Reaction Design Center, and the KAIST KC30 Project.
< Figure 4. Conceptual diagram of performing multi-substrate screening reactions and utilizing fluorine nuclear magnetic resonance spectroscopy. >
2025.06.16 View 2332
Simultaneous Analysis of 21 Chemical Reactions... AI to Transform New Drug Development
< Photo 1. (From left) Professor Hyunwoo Kim and students Donghun Kim and Gyeongseon Choi in the Integrated M.S./Ph.D. program of the Department of Chemistry >
Thalidomide, a drug once used to alleviate morning sickness in pregnant women, exhibits distinct properties due to its optical isomers* in the body: one isomer has a sedative effect, while the other causes severe side effects like birth defects. As this example illustrates, precise organic synthesis techniques, which selectively synthesize only the desired optical isomer, are crucial in new drug development. Overcoming the traditional methods that struggled with simultaneously analyzing multiple reactants, our research team has developed the world's first technology to precisely analyze 21 types of reactants simultaneously. This breakthrough is expected to make a significant contribution to new drug development utilizing AI and robots.
*Optical Isomers: A pair of molecules with the same chemical formula that are mirror images of each other and cannot be superimposed due to their asymmetric structure. This is analogous to a left and right hand, which are similar in form but cannot be perfectly overlaid.
KAIST's Professor Hyunwoo Kim's research team in the Department of Chemistry announced on the 16th that they have developed an innovative optical isomer analysis technology suitable for the era of AI-driven autonomous synthesis*. This research is the world's first technology to precisely analyze asymmetric catalytic reactions involving multiple reactants simultaneously using high-resolution fluorine nuclear magnetic resonance spectroscopy (19F NMR). It is expected to make groundbreaking contributions to various fields, including new drug development and catalyst optimization.
*AI-driven Autonomous Synthesis: An advanced technology that automates and optimizes chemical substance synthesis processes using artificial intelligence (AI). It is gaining attention as a core element for realizing automated and intelligent research environments in future laboratories. AI predicts and adjusts experimental conditions, interprets results, and designs subsequent experiments independently, minimizing human intervention in repetitive experiments and significantly increasing research efficiency and innovativeness.
Currently, while autonomous synthesis systems can automate everything from reaction design to execution, reaction analysis still relies on individual processing using traditional equipment. This leads to slower speeds and bottlenecks, making it unsuitable for high-speed repetitive experiments.
Furthermore, multi-substrate simultaneous screening techniques proposed in the 1990s garnered attention as a strategy to maximize reaction analysis efficiency. However, limitations of existing chromatography-based analysis methods restricted the number of applicable substrates. In asymmetric synthesis reactions, which selectively synthesize only the desired optical isomer, simultaneously analyzing more than 10 types of substrates was nearly impossible.
< Figure 1. Conventional organic reaction evaluation methods follow a process of deriving optimal reaction conditions using a single substrate, then expanding the substrate scope one by one under those conditions, leaving potential reaction areas unexplored. To overcome this, high-throughput screening is introduced to broadly explore catalyst reactivity for various substrates. When combined with multi-substrate screening, this approach allows for a much broader and more systematic understanding of reaction scope and trends. >
To overcome these limitations, the research team developed a 19F NMR-based multi-substrate simultaneous screening technology. This method involves performing asymmetric catalytic reactions with multiple reactants in a single reaction vessel, introducing a fluorine functional group into the products, and then applying their self-developed chiral cobalt reagent to clearly quantify all optical isomers using 19F NMR.
Utilizing the excellent resolution and sensitivity of 19F NMR, the research team successfully performed asymmetric synthesis reactions of 21 substrates simultaneously in a single reaction vessel and quantitatively measured the product yield and optical isomer ratio without any separate purification steps.
Professor Hyunwoo Kim stated, "While anyone can perform asymmetric synthesis reactions with multiple substrates in one reactor, accurately analyzing all the products has been a challenging problem to solve until now. We expect that achieving world-class multi-substrate screening analysis technology will greatly contribute to enhancing the analytical capabilities of AI-driven autonomous synthesis platforms."
< Figure 2. A method for analyzing multi-substrate asymmetric catalytic reactions, where different substrates react simultaneously in a single reactor, using fluorine nuclear magnetic resonance has been implemented. By utilizing the characteristics of fluorine nuclear magnetic resonance, which has a clean background signal and a wide chemical shift range, the reactivity of each substrate can be quantitatively analyzed. It is also shown that the optical activity of all reactants can be simultaneously measured using a cobalt metal complex. >
He further added, "This research provides a technology that can rapidly verify the efficiency and selectivity of asymmetric catalytic reactions essential for new drug development, and it is expected to be utilized as a core analytical tool for AI-driven autonomous research."
< Figure 3. It can be seen that in a multi-substrate reductive amination reaction using a total of 21 substrates, the yield and optical activity of the reactants according to the catalyst system were simultaneously measured using a fluorine nuclear magnetic resonance-based analysis platform. The yield of each reactant is indicated by color saturation, and the optical activity by numbers. >
Donghun Kim (first author, Integrated M.S./Ph.D. program) and Gyeongseon Choi (second author, Integrated M.S./Ph.D. program) from the KAIST Department of Chemistry participated in this research. The study was published online in the Journal of the American Chemical Society on May 27, 2025.※ Paper Title: One-pot Multisubstrate Screening for Asymmetric Catalysis Enabled by 19F NMR-based Simultaneous Chiral Analysis※ DOI: 10.1021/jacs.5c03446
This research was supported by the National Research Foundation of Korea's Mid-Career Researcher Program, the Asymmetric Catalytic Reaction Design Center, and the KAIST KC30 Project.
< Figure 4. Conceptual diagram of performing multi-substrate screening reactions and utilizing fluorine nuclear magnetic resonance spectroscopy. >
2025.06.16 View 2332 -
 KAIST Holds a Ceremony to Declare their Renewed Commitment for Ethical Management
KAIST held a ceremony to declare their renewed "Commitment for Ethical Management" to raise awareness and solidify the commitment its members to faithfully fulfill ethical responsibilities and duties.
Last March, the university established the 'Special Committee for Ethical Management,' chaired by the Provost, and under the leadership of this committee, a new 'Code of Ethics' and 'Code of Conduct' were prepared, containing ethical standards that members must adhere to across all areas of education, research, and administration.
< Photo 1. Attendees pledge to practice ethics during the declaration for the ethical management. >
This ceremony was arranged as an occasion for the president, key executives, and representatives from each university constituent to share the purpose and direction of the newly established ethical standards and to pledge their commitment to practicing them.
The Ethical Management Declaration consisted of: ▲ a progress report by the KAIST Special Committee for Ethical Management, ▲ a commemorative address by the president, ▲ an oath of the Code of Ethics and Code of Conduct, and ▲ the presentation of the 'Excellent Ethics Professor Award' organized by the Graduate Student Human Rights Center. Attendees shared the values and meaning of ethical management pursued by KAIST.
Particularly at this ceremony, six representatives – faculty, staff, and students – selected to reflect KAIST's values encompassing diversity in position, role, gender, and future generations, took the oath for the Code of Ethics and Code of Conduct.
< Photo 2. Attendees pledge to practice ethics during the Ethical Management Declaration. >
Also introduced at the ceremony was the "Ethical Excellence Award for Professors". It is an award that was organized by the Graduate Student Human Rights Center under the KAIST Student Council to recognize the faculty members for their outstanding ethical conduct in the laboratory setting. The 2025 recipients of the newly established award were the honored at the declaration ceremony for added significance.
Taking this declaration ceremony as an example, KAIST plans to actively encourage each departments, divisions and offices to also hold ethical management declarations of their own to establish a trustworthy, healthy, and transparent organizational culture through the daily practice of ethical responsibilities, and to continuously spread the practice of ethical management among all members.
President Kwang Hyung Lee emphasized, "Adhering to research and social ethics must be the foundation for KAIST to become a university trusted globally," and expressed, "I hope this ceremony serves as a turning point for all members to more faithfully practice their ethical responsibilities and duties."
2025.06.16 View 1240
KAIST Holds a Ceremony to Declare their Renewed Commitment for Ethical Management
KAIST held a ceremony to declare their renewed "Commitment for Ethical Management" to raise awareness and solidify the commitment its members to faithfully fulfill ethical responsibilities and duties.
Last March, the university established the 'Special Committee for Ethical Management,' chaired by the Provost, and under the leadership of this committee, a new 'Code of Ethics' and 'Code of Conduct' were prepared, containing ethical standards that members must adhere to across all areas of education, research, and administration.
< Photo 1. Attendees pledge to practice ethics during the declaration for the ethical management. >
This ceremony was arranged as an occasion for the president, key executives, and representatives from each university constituent to share the purpose and direction of the newly established ethical standards and to pledge their commitment to practicing them.
The Ethical Management Declaration consisted of: ▲ a progress report by the KAIST Special Committee for Ethical Management, ▲ a commemorative address by the president, ▲ an oath of the Code of Ethics and Code of Conduct, and ▲ the presentation of the 'Excellent Ethics Professor Award' organized by the Graduate Student Human Rights Center. Attendees shared the values and meaning of ethical management pursued by KAIST.
Particularly at this ceremony, six representatives – faculty, staff, and students – selected to reflect KAIST's values encompassing diversity in position, role, gender, and future generations, took the oath for the Code of Ethics and Code of Conduct.
< Photo 2. Attendees pledge to practice ethics during the Ethical Management Declaration. >
Also introduced at the ceremony was the "Ethical Excellence Award for Professors". It is an award that was organized by the Graduate Student Human Rights Center under the KAIST Student Council to recognize the faculty members for their outstanding ethical conduct in the laboratory setting. The 2025 recipients of the newly established award were the honored at the declaration ceremony for added significance.
Taking this declaration ceremony as an example, KAIST plans to actively encourage each departments, divisions and offices to also hold ethical management declarations of their own to establish a trustworthy, healthy, and transparent organizational culture through the daily practice of ethical responsibilities, and to continuously spread the practice of ethical management among all members.
President Kwang Hyung Lee emphasized, "Adhering to research and social ethics must be the foundation for KAIST to become a university trusted globally," and expressed, "I hope this ceremony serves as a turning point for all members to more faithfully practice their ethical responsibilities and duties."
2025.06.16 View 1240